The Dietary Antioxidant Piceatannol Inhibits Adipogenesis of Human Adipose Mesenchymal Stem Cells and Limits Glucose Transport and Lipogenic Activities in Adipocytes
Abstract
:1. Introduction
2. Results
2.1. Piceatannol Impairs Lipid Accumulation and Inhibits Lipogenesis in Mouse Adipocytes
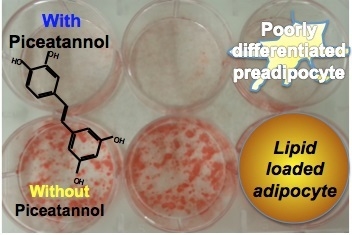
2.2. Piceatannol Impairs Adipogenesis in Human Preadipocytes
2.3. Piceatannol Induces Desensitization to Insulin Activation of Glucose Uptake and Down-Regulates Lipogenic Enzymes in Human Adipocytes
2.4. Piceatannol Dose-Dependently Limits Hydrogen Peroxide Detection in Human Adipose Tissue Preparations
3. Discussion
4. Materials and Methods
4.1. Patients and Human Adipose Tissue Samples
4.2. Cell Cultures and Treatments
4.3. Assessment of Cell Viability, Lipid Accumulation, and Expression of Lipogenic Enzymes
4.4. Glucose Transport and Incorporation into Lipids
4.5. Hydrogen Peroxide Detection in Adipose Tissue Homogenates
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Quintela, A.; Carpéné, C.; Fernandez, M.; Aguirre, L.; Milton-Laskibar, I.; Contreras, J.; Portillo, M.P. Anti-obesity effects of resveratrol: Comparison between animal models and humans. J. Physiol. Biochem. 2016, 73, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhou, R.; Wang, B.; Mi, M.T. Effect of resveratrol on glucose control and insulin sensitivity: A meta-analysis of 11 randomized controlled trials. Am. J. Clin. Nutr. 2014, 99, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Christenson, J.; Whitby, S.J.; Mellor, D.; Thomas, J.; McKune, A.; Roach, P.D.; Naumovski, N. The Effects of Resveratrol Supplementation in Overweight and Obese Humans: A Systematic Review of Randomized Trials. Metab. Syndr. Relat. Disord. 2016, 14, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F. The resveratrol fiasco. Pharmacol. Res. 2014, 90, 87. [Google Scholar] [CrossRef] [PubMed]
- Carpéné, C.; Gomez-Zorita, S.; Deleruyelle, S.; Carpéné, M.A. Novel strategies for preventing diabetes and obesity complications with natural polyphenols. Curr. Med. Chem. 2015, 22, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, J.; Kim, K.H. The Therapeutic Potential of Piceatannol, a Natural Stilbene, in Metabolic Diseases: A Review. J. Med. Food 2017, 20, 427–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.L.; Chan, S.W. A review of the pharmacological effects of piceatannol on cardiovascular diseases. Phytother. Res. 2014, 28, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.Y.; Seo, S.G.; Heo, Y.S.; Yue, S.; Cheng, J.X.; Lee, K.W.; Kim, K.H. Piceatannol, natural polyphenolic stilbene, inhibits adipogenesis via modulation of mitotic clonal expansion and insulin receptor-dependent insulin signaling in early phase of differentiation. J. Biol. Chem. 2012, 287, 11566–11578. [Google Scholar] [CrossRef] [PubMed]
- Hijona, E.; Aguirre, L.; Perez-Matute, P.; Villanueva-Millan, M.J.; Mosqueda-Solis, A.; Hasnaoui, M.; Nepveu, F.; Senard, J.M.; Bujanda, L.; Aldamiz-Echevarria, L.; et al. Limited beneficial effects of piceatannol supplementation on obesity complications in the obese Zucker rat: Gut microbiota, metabolic, endocrine, and cardiac aspects. J. Physiol. Biochem. 2016, 72, 567–582. [Google Scholar] [CrossRef] [PubMed]
- Mosqueda-Solis, A.; Lasa, A.; Gomez-Zorita, S.; Eseberri, I.; Pico, C.; Portillo, M.P. Screening of potential anti-adipogenic effects of phenolic compounds showing different chemical structure in 3T3-L1 preadipocytes. Food Funct. 2017, 8, 3576–3586. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ordemann, J.; Muller, J.M.; Dubiel, W. The COP9 signalosome, cullin 3 and Keap1 supercomplex regulates CHOP stability and adipogenesis. Biol. Open 2012, 1, 705–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Beek, E.A.; Bakker, A.H.; Kruyt, P.M.; Vink, C.; Saris, W.H.; Franssen-van Hal, N.L.; Keijer, J. Comparative expression analysis of isolated human adipocytes and the human adipose cell lines LiSa-2 and PAZ6. Int. J. Obes. (Lond.) 2008, 32, 912–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, H.; Kehinde, O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell 1975, 5, 19–27. [Google Scholar] [CrossRef]
- Krieger-Brauer, H.I.; Medda, P.K.; Kather, H. Insulin-induced activation of NADPH-dependent H2O2 generation in human adipocyte plasma membranes is mediated by Galphai2. J. Biol. Chem. 1997, 272, 10135–10143. [Google Scholar] [CrossRef] [PubMed]
- Green, A.; Bustillos, D.P.; Misbin, R.I. β-Hydroxybutyrate increases the insulin sensitivity of adipocyte glucose transport at a postreceptor level. Diabetes 1984, 33, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Morin, N.; Lizcano, J.M.; Fontana, E.; Marti, L.; Smih, F.; Rouet, P.; Prévot, D.; Zorzano, A.; Unzeta, M.; Carpéné, C. Semicarbazide-sensitive amine oxidase substrates stimulate glucose transport and inhibit lipolysis in human adipocytes. J. Pharmacol. Exp. Ther. 2001, 297, 563–572. [Google Scholar] [PubMed]
- Gomez-Zorita, S.; Belles, C.; Briot, A.; Fernandez-Quintela, A.; Portillo, M.P.; Carpéné, C. Pterostilbene Inhibits Lipogenic Activity similar to Resveratrol or Caffeine but Differently Modulates Lipolysis in Adipocytes. Phytother. Res. 2017, 31, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Setoguchi, Y.; Oritani, Y.; Ito, R.; Inagaki, H.; Maruki-Uchida, H.; Ichiyanagi, T.; Ito, T. Absorption and metabolism of piceatannol in rats. J. Agric. Food Chem. 2014, 62, 2541–2548. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Ogura, Y.; Maruki-Uchida, H.; Sai, M.; Suzuki, T.; Kanasaki, K.; Hara, Y.; Seto, H.; Kuroshima, Y.; Monno, I.; et al. The Effect of Piceatannol from Passion Fruit (Passiflora edulis) Seeds on Metabolic Health in Humans. Nutrients 2017, 9, 1142. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, P.; Chang, Q.; Wang, J.; Liu, J.; Lv, Y.; Wang, T.T.Y.; Gao, B.; Zhang, Y.; Yu, L.L. Inhibitory Effect of Piceatannol on TNF-alpha-Mediated Inflammation and Insulin Resistance in 3T3-L1 Adipocytes. J. Agric. Food Chem. 2017, 65, 4634–4641. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Bouzar, C.; Cottet-Rousselle, C.; Zagotta, I.; Lamarche, F.; Wabitsch, M.; Tokarska-Schlattner, M.; Fischer-Posovszky, P.; Schlattner, U.; Rousseau, D. Resveratrol inhibits lipogenesis of 3T3-L1 and SGBS cells by inhibition of insulin signaling and mitochondrial mass increase. Biochim. Biophys. Acta 2016, 1857, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Gerszon, J.; Serafin, E.; Buczkowski, A.; Michlewska, S.; Bielnicki, J.A.; Rodacka, A. Functional consequences of piceatannol binding to glyceraldehyde-3-phosphate dehydrogenase. PLoS ONE 2018, 13, e0190656. [Google Scholar] [CrossRef] [PubMed]
- Sueishi, Y.; Nii, R.; Kakizaki, N. Resveratrol analogues like piceatannol are potent antioxidants as quantitatively demonstrated through the high scavenging ability against reactive oxygen species and methyl radical. Bioorg. Med. Chem. Lett. 2017, 27, 5203–5206. [Google Scholar] [CrossRef] [PubMed]
- Roupe, K.A.; Yanez, J.A.; Teng, X.W.; Davies, N.M. Pharmacokinetics of selected stilbenes: Rhapontigenin, piceatannol and pinosylvin in rats. J. Pharm. Pharmacol. 2006, 58, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Les, F.; Deleruyelle, S.; Cassagnes, L.E.; Boutin, J.A.; Balogh, B.; Arbones-Mainar, J.M.; Biron, S.; Marceau, P.; Richard, D.; Nepveu, F.; et al. Piceatannol and resveratrol share inhibitory effects on hydrogen peroxide release, monoamine oxidase and lipogenic activities in adipose tissue, but differ in their antilipolytic properties. Chem. Biol. Interact. 2016, 258, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Pizzinat, N.; Marti, L.; Remaury, A.; Leger, F.; Langin, D.; Lafontan, M.; Carpéné, C.; Parini, A. High expression of monoamine oxidases in human white adipose tissue: Evidence for their involvement in noradrenaline clearance. Biochem. Pharmacol. 1999, 58, 1735–1742. [Google Scholar] [CrossRef]
- Wabitsch, M.; Bruderlein, S.; Melzner, I.; Braun, M.; Mechtersheimer, G.; Moller, P. LiSa-2, a novel human liposarcoma cell line with a high capacity for terminal adipose differentiation. Int. J. Cancer 2000, 88, 889–894. [Google Scholar] [CrossRef] [Green Version]
- Shen, P.; Yue, Y.; Kim, K.H.; Park, Y. Piceatannol Reduces Fat Accumulation in Caenorhabditis elegans. J. Med. Food 2017, 20, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.-C.; Lin, Y.-H.; Chen, H.-J.; Chou, S.-C.; Cheng, A.-C.; Kalyanam, N.; Ho, C.-T.; Pan, M.-H. Piceatannol exerts anti-obesity effects in C57BL/6 mice through modulating adipogenic proteins and gut microbiota. Molecules 2016, 21, 1419. [Google Scholar] [CrossRef] [PubMed]
- Ishihata, A.; Maruki-Uchida, H.; Gotoh, N.; Kanno, S.; Aso, Y.; Togashi, S.; Sai, M.; Ito, T.; Katano, Y. Vascular- and hepato-protective effects of passion fruit seed extract containing piceatannol in chronic high-fat diet-fed rats. Food Funct. 2016, 7, 4075–4081. [Google Scholar] [CrossRef] [PubMed]
- Perez-Diaz, S.; Garcia-Rodriguez, B.; Gonzalez-Irazabal, Y.; Valero, M.; Lagos-Lizan, J.; Arbones-Mainar, J.M. Knockdown of PTRF ameliorates adipocyte differentiation and functionality of human mesenchymal stem cells. Am. J. Physiol. Cell Physiol. 2017, 312, C83–C91. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Zorita, S.; Lasa, A.; Abendano, N.; Fernandez-Quintela, A.; Mosqueda-Solis, A.; Garcia-Sobreviela, M.P.; Arbones-Mainar, J.M.; Portillo, M.P. Phenolic compounds apigenin, hesperidin and kaempferol reduce in vitro lipid accumulation in human adipocytes. J. Transl. Med. 2017, 15, 237. [Google Scholar] [CrossRef] [PubMed]
- Les, F.; Carpéné, C.; Arbones-Mainar, J.M.; Decaunes, P.; Valero, M.S.; Lopez, V. Pomegranate juice and its main polyphenols exhibit direct effects on amine oxidases from human adipose tissue and inhibit lipid metabolism in adipocytes. J. Funct. Foods 2017, 33, 323–331. [Google Scholar] [CrossRef]
- Carpéné, C.; Mercader, J.; Le Gonidec, S.; Schaak, S.; Mialet-Perez, J.; Zakaroff-Girard, A.; Galitzky, J. Body fat reduction without cardiovascular changes in mice after oral treatment by the MAO inhibitor phenelzine. Br. J. Pharmacol. 2018, 175, 2428–2440. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Panchuk-Voloshina, N. A one-step fluorometric method for the continuous measurement of monoamine oxidase activity. Anal. Biochem. 1997, 253, 169–174. [Google Scholar] [CrossRef] [PubMed]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carpéné, C.; Pejenaute, H.; Del Moral, R.; Boulet, N.; Hijona, E.; Andrade, F.; Villanueva-Millán, M.J.; Aguirre, L.; Arbones-Mainar, J.M. The Dietary Antioxidant Piceatannol Inhibits Adipogenesis of Human Adipose Mesenchymal Stem Cells and Limits Glucose Transport and Lipogenic Activities in Adipocytes. Int. J. Mol. Sci. 2018, 19, 2081. https://doi.org/10.3390/ijms19072081
Carpéné C, Pejenaute H, Del Moral R, Boulet N, Hijona E, Andrade F, Villanueva-Millán MJ, Aguirre L, Arbones-Mainar JM. The Dietary Antioxidant Piceatannol Inhibits Adipogenesis of Human Adipose Mesenchymal Stem Cells and Limits Glucose Transport and Lipogenic Activities in Adipocytes. International Journal of Molecular Sciences. 2018; 19(7):2081. https://doi.org/10.3390/ijms19072081
Chicago/Turabian StyleCarpéné, Christian, Héctor Pejenaute, Raquel Del Moral, Nathalie Boulet, Elizabeth Hijona, Fernando Andrade, Maria Jesùs Villanueva-Millán, Leixuri Aguirre, and José Miguel Arbones-Mainar. 2018. "The Dietary Antioxidant Piceatannol Inhibits Adipogenesis of Human Adipose Mesenchymal Stem Cells and Limits Glucose Transport and Lipogenic Activities in Adipocytes" International Journal of Molecular Sciences 19, no. 7: 2081. https://doi.org/10.3390/ijms19072081
APA StyleCarpéné, C., Pejenaute, H., Del Moral, R., Boulet, N., Hijona, E., Andrade, F., Villanueva-Millán, M. J., Aguirre, L., & Arbones-Mainar, J. M. (2018). The Dietary Antioxidant Piceatannol Inhibits Adipogenesis of Human Adipose Mesenchymal Stem Cells and Limits Glucose Transport and Lipogenic Activities in Adipocytes. International Journal of Molecular Sciences, 19(7), 2081. https://doi.org/10.3390/ijms19072081