Non-Covalent Associates of siRNAs and AuNPs Enveloped with Lipid Layer and Doped with Amphiphilic Peptide for Efficient siRNA Delivery
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Core Formation Conditions
2.2. Assemblage of Multilayer Nanoconstruction
2.2.1. The Synthesis of the pH-Sensitive Lipidoid
2.2.2. Preparation of Enveloped Cores
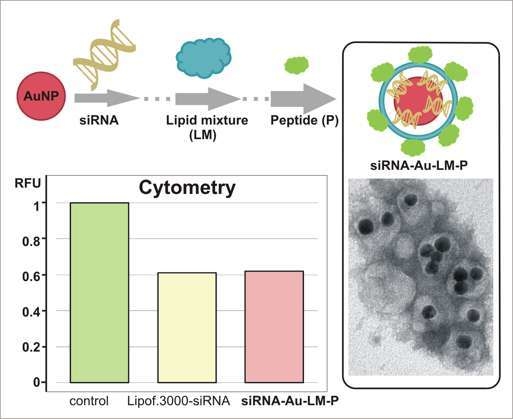
2.3. Ability of Obtained Nanoconstruction Penetrate a Cell and Influence on GFP Expression
3. Materials and Methods
3.1. Reagents
3.2. Synthesis of Lipids
3.2.1. Compound 1: 2-Dodecylamino-4,6-dichloro-1,3,5-triazine
3.2.2. Compound 2: 2-Dodecylamino-4-oleylamino-6-chloro-1,3,5-triazine
3.2.3. Lipidoid 3: 2-[[4-Dodecylamino-6-oleylamino-1,3,5-triazine-2yl]-(2-hydroxyethyl)amino]ethanol
3.3. Preparation of Stearyl-Modified Peptide (RL)4G-NH2
3.3.1. Stearic Acid N-Hydroxysuccinimide Ester
3.3.2. Synthesis of Stearyl-Modified Peptide (RL)4G-NH2 (P)
3.4. Synthesis of siRNA
3.5. Deprotection, Purification and Annealing of siRNAs
3.6. Preparation of Citrate AuNPs
Preparation of Non-Covalent Associate of siRNA and AuNPs
- (I)
- High concentration samples: 5 µL, 100 nM AuNPs, 20 µM siRNA and 10 mM NaCl.
- (II)
- Medium concentration samples: 160 µL, 3 nM AuNPs, 0.6 µM siRNA and 10–40 mM NaCl.
- (III)
- Low concentration samples: 1000 µL, 0.5 nM AuNPs, 0.1 µM siRNA and 0.05 mM NaCl.
3.7. Fluorescence Intensity Measurements and Calculation of Density of siRNA on the AuNP Surface
3.8. Preparation of Lipid-Enveloped siRNA-AuNPs
3.9. Characterization of Nanospecies (The Term Nanospecies Is Used for Designation of All Particles, without Emphasis at Their Structure)
3.10. Transmission Electron Microscopy
3.11. Cells
3.12. Fluorescent Confocal Microscopy
3.13. Flow Cytometry
3.14. Statistical Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Du, B.; Tian, L.; Gu, X.; Li, D.; Wang, E.; Wang, J. Anionic Lipid, pH-Sensitive Liposome-Gold Nanoparticle Hybrids for Gene Delivery—Quantitative Research of the Mechanism. Small 2015, 11, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Rhim, W.K.; Kim, J.S.; Nam, J.M. Lipid-gold-nanoparticle hybrid-based gene delivery. Small 2008, 4, 1651–1655. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, L.; Xie, Y.; Wang, N.; Tang, R.; Zheng, W.; Jiang, X. Genome Editing for Cancer Therapy: Delivery of Cas9 Protein/sgRNA Plasmid via a Gold Nanocluster/Lipid Core-Shell Nanocarrier. Adv. Sci. 2017, 4, 1700175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMahon, K.M.; Mutharasan, R.K.; Tripathy, S.; Veliceasa, D.; Bobeica, M.; Shumaker, D.K.; Luthi, A.J.; Helfand, B.T.; Ardehali, H.; Mirkin, C.A.; et al. Biomimetic high density lipoprotein nanoparticles for nucleic acid delivery. Nano Lett. 2011, 11, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.H.; Bae, K.H.; Jo, S.D.; Kim, J.S.; Park, T.G. Cationic lipid-coated gold nanoparticles as efficient and non-cytotoxic intracellular siRNA delivery vehicles. Pharm. Res. 2012, 29, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Wanga, M.; Petersen, N.O. Characterization of phospholipid encapsulated gold nanoparticles: A versatile platform to study drug delivery and cellular uptake mechanisms. Can. J. Chem. 2015, 93, 265–271. [Google Scholar] [CrossRef]
- Cutler, J.I.; Zhang, K.; Zheng, D.; Auyeung, E.; Prigodich, A.E.; Mirkin, C.A. Polyvalent Nucleic Acid Nanostructures. J. Am. Chem. Soc. 2011, 133, 9254–9257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Venkatesulu, B.P.; Mahadevan, L.S.; Aliru, M.L.; Singh, A.; Prasad, P.N.; Krishnan, S. Gold-Small Interfering RNA as Optically Responsive Nanostructures for Cancer Theranostics. J. Biomed. Nanotechnol. 2018, 14, 809–828. [Google Scholar] [CrossRef] [PubMed]
- Epanchintseva, A.; Dolodoev, A.; Grigor’eva, A.; Chelobanov, B.; Pyshnyi, D.; Ryabchikova, E.; Pyshnaya, I. Non-covalent binding of nucleic acids with gold nanoparticles provides their stability and effective desorption in environment mimicking biological media. Nanotechnology 2018, 29, 355601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanki, K.; Zeng, X.; Justesen, S.; Tejlmann, S.; Falkenberg, E.; van Driessche, E.; Nielsen, H.M.; Franzyk, H.; Foged, C. Engineering of small interfering RNA-loaded lipidoid-poly(DL-lactic-coglycolic acid) hybrid nanoparticles for highly efficient and safe gene silencing: A quality by design-based approach. Eur. J. Pharm. Biopharm. 2017, 120, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Akinc, A.; Goldberg, M.; Qin, J.; Dorkin, J.R.; Gamba-Vitalo, C.; Maier, M.; Jayaprakash, K.N.; Jayaraman, M.; Rajeev, K.G.; Manoharan, M.; et al. Development of Lipidoid–siRNA Formulations for Systemic Delivery to the Liver. Mol. Ther. 2009, 17, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mangala, L.S.; Rodriguez-Aguayo, C.; Kong, X.; Lopez-Berestein, G.; Sood, A.K. RNA interference-based therapy and its delivery systems. Cancer Metastasis Rev. 2018, 37, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Wittrup, A.; Ai, A.; Liu, X.; Hamar, P.; Trifonova, R.; Charisse, K.; Manoharan, M.; Kirchhausen, T.; Lieberman, J. Visualizing lipid-formulated siRNA release from endosomes and target gene knockdown. Nat. Biotechnol. 2015, 33, 870–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, K.; Huang, L. Achieving efficient RNAi therapy: Progress and challenges. Acta Pharm. Sin. B 2013, 3, 213–225. [Google Scholar] [CrossRef]
- Epanchintseva, A.; Vorobjev, P.; Pyshnyi, D.; Pyshnaya, I. Fast and Strong Adsorption of Native Oligonucleotides on Citrate-Coated Gold Nanoparticles. Langmuir 2018, 34, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Demers, L.M.; Östblom, M.; Zhang, H.; Jang, N.; Liedberg, B.; Mirkin, C.A. Thermal Desorption Behavior and Binding Properties of DNA Bases and Nucleosides on Gold. J. Am. Chem. Soc. 2002, 124, 11248–11249. [Google Scholar] [CrossRef] [PubMed]
- Rapino, S.; Zerbetto, F. Modeling the Stability and the Motion of DNA Nucleobases on the Gold Surface. Langmuir 2005, 21, 2512–2518. [Google Scholar] [CrossRef] [PubMed]
- Ramsing, N.B.; Rippe, K.; Jovin, T.M. Helix-Coil Transition of Parallel-Stranded DNA. Thermodynamics of Hairpin and Linear Duplex Oligonucleotides. Biochemistry 1989, 28, 9528–9535. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Li, F.; Wan, Y.; Wei, M.; Liu, H.; Su, Y.; Chen, N.; Huang, Q.; Fan, C. Designed Diblock Oligonucleotide for the Synthesis of Spatially Isolated and Highly Hybridizable Functionalization of DNA–Gold Nanoparticle Nanoconjugates. J. Am. Chem. Soc. 2012, 134, 11876–11879. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, Y.; Shao, H.; Wang, Z.; Wang, X.; Jiang, X. Label-Free Colorimetric Detection of Cadmium Ions in Rice Samples Using Gold Nanoparticles. Anal. Chem. 2014, 86, 8530–8534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnaby, S.N.; Lee, A.; Mirkin, C.A. Probing the inherent stability of siRNA immobilized on nanoparticle constructs. Proc. Natl. Acad. Sci. USA 2014, 111, 9739–9744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giljohann, D.A.; Seferos, D.S.; Prigodich, A.E.; Patel, P.C.; Mirkin, C.A. Gene Regulation with Polyvalent siRNA-Nanoparticle Conjugates. J. Am. Chem. Soc. 2009, 131, 2072–2073. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.; Wong, J.H.; Cheung, R.C.F.; Zuo, T.; Ng, T.B. Therapeutic potentials of short interfering RNAs. Appl. Microbiol. Biotechnol. 2017, 101, 7091–7111. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Qiao, W.; Luo, L.; Peng, H. Design and surface/interfacial properties of asymmetric triazine carboxyl betaine surfactants. J. Surfactants Deterg. 2014, 17, 629–636. [Google Scholar] [CrossRef]
- Klausner, R.D.; Kleinfeld, A.M.; Hoover, R.L.; Karnovsky, M.J. Lipid domains in membranes. Evidence derived from structural perturbations induced by free fatty acids and lifetime heterogeneity analysis. J. Biol. Chem. 1980, 255, 1286–1295. [Google Scholar] [PubMed]
- Ibarguren, M.; López, D.J.; Escribá, P.V. The effect of natural and synthetic fatty acids on membrane structure, microdomain organization, cellular functions and human health. Biochim. Biophys. Acta Biomembr. 2014, 1838, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Vasilev, K.; Kreiter, M.; Mittler, S.; Knoll, W. Surface Modification of Citrate-Reduced Colloidal Gold Nanoparticles with 2-Mercaptosuccinic Acid. Langmuir 2003, 19, 9518–9525. [Google Scholar] [CrossRef]
- Lu, W.; Wang, L.; Li, J.; Zhao, Y.; Zhou, Z.; Shi, J.; Zuo, X.; Pan, D. Quantitative investigation of the poly-adenine DNA dissociation from the surface of gold nanoparticles. Sci. Rep. 2015, 5, 10158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zatsepin, T.S.; Kotelevtsev, Y.V.; Koteliansky, V. Lipid nanoparticles for targeted siRNA delivery—Going from bench to bedside. Int. J. Nanomed. 2016, 11, 3077–3086. [Google Scholar] [CrossRef]
- Yamada, Y.; Akita, H.; Kamiya, H.; Kogure, K.; Yamamoto, T.; Shinobara, Y.; Yamashita, K.; Kobayashi, H.; Kikuchi, H.; Harashima, H. MITO-Porter: A liposome-based carrier system for delivery of macromoleculesinto mitochondria via membrane fusion. Biochim. Biophys. Acta Biomembr. 2008, 1778, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.S.; Ferré, S. Amazing stability of the arginine-phosphate electrostatic interaction. J. Proteom. Res. 2005, 4, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, T.J. Hydration properties of lamellar and non-lamellar phases of phosphatidylcholine and phospatidylethanolamine. Chem. Phys. Lipids 1996, 81, 117–131. [Google Scholar] [CrossRef]
- Marrink, S.-J.; Mark, A.E. Molecular View of Hexagonal Phase Formation in Phospholipid Membranes. Biophys. J. 2004, 87, 3894–3900. [Google Scholar] [CrossRef] [PubMed]
- Agardan, N.B.M.; Sarisozen, C.; Torchilin, V.P. Redox-triggered intracellular siRNA delivery. Chem. Commun. 2018, 54, 6368–6371. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Munye, M.M.; Tagalakis, A.D.; Manunta, M.D.I.; Hart, S.L. The Role of the helper lipid on the DNA transfection efficiency of lipopolyplex formulations. Sci. Rep. 2014, 4, 7107. [Google Scholar] [CrossRef] [PubMed]
- Marks, J.R.; Placone, J.; Hristova, K.; Wimley, W.C. Spontaneous membrane-translocating peptides by orthogonal high-throughput screening. J. Am. Chem. Soc. 2011, 133, 8995–9004. [Google Scholar] [CrossRef] [PubMed]
- Dowdy, S.F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 2017, 35, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Lapidot, Y.; Rappoport, S.; Wolman, Y. Use of esters of N-hydroxysuccinimide in the synthesis of N-acylamino acids. J. Lipid Res. 1967, 8, 142–145. [Google Scholar] [PubMed]
- Bellon, L. Oligoribonucleotides with 2′-O-(tert-Butyldimethylsilyl) Groups. Curr. Protoc. Nucleic Acid Chem. 2000, 1, 3.6.1–3.6.13. [Google Scholar] [CrossRef]
- Tschuch, C.; Schulz, A.; Pscherer, A.; Werft, W.; Benner, A.; Hotz-Wagenblatt, A.; Barrionuevo, L.S.; Lichter, P.; Mertens, D. Off-target effects of siRNA specific for GFP. BMC Mol. Biol. 2008, 9, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storhoff, J.J.; Elghanian, R.; Mucic, R.C.; Mirkin, C.A.; Letsinger, R.L. One-pot colorimetric differentiation of polynucleotides with single base imperfections using gold nanoparticle probes. J. Am. Chem. Soc. 1998, 120, 1959–1964. [Google Scholar] [CrossRef]












| Sample | AuNPs (M) | siRNA (M) | Ratio siRNA/AuNPs |
|---|---|---|---|
| I | 10−7 | 2 × 10−5 | 200 |
| II | 3 × 10−9 | 6 × 10−7 | 200 |
| III | 5 × 10−10 | 10−7 | 200 |
| Designation | Sequence |
|---|---|
| ORN1 | 5′-CAA-GCU-GAC-CCU-GAA-GUU-CTT-3′ |
| ORN2 | 5′-GAA-CUU-CAG-GGU-CAG-CUU-GTT-3′ |
| ORN3 | 5′-Cy5.5-CAA-GCU-GAC-CCU-GAA-GUU-CTT-3′ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poletaeva, J.; Dovydenko, I.; Epanchintseva, A.; Korchagina, K.; Pyshnyi, D.; Apartsin, E.; Ryabchikova, E.; Pyshnaya, I. Non-Covalent Associates of siRNAs and AuNPs Enveloped with Lipid Layer and Doped with Amphiphilic Peptide for Efficient siRNA Delivery. Int. J. Mol. Sci. 2018, 19, 2096. https://doi.org/10.3390/ijms19072096
Poletaeva J, Dovydenko I, Epanchintseva A, Korchagina K, Pyshnyi D, Apartsin E, Ryabchikova E, Pyshnaya I. Non-Covalent Associates of siRNAs and AuNPs Enveloped with Lipid Layer and Doped with Amphiphilic Peptide for Efficient siRNA Delivery. International Journal of Molecular Sciences. 2018; 19(7):2096. https://doi.org/10.3390/ijms19072096
Chicago/Turabian StylePoletaeva, Julia, Ilya Dovydenko, Anna Epanchintseva, Kseniya Korchagina, Dmitrii Pyshnyi, Evgeny Apartsin, Elena Ryabchikova, and Inna Pyshnaya. 2018. "Non-Covalent Associates of siRNAs and AuNPs Enveloped with Lipid Layer and Doped with Amphiphilic Peptide for Efficient siRNA Delivery" International Journal of Molecular Sciences 19, no. 7: 2096. https://doi.org/10.3390/ijms19072096
APA StylePoletaeva, J., Dovydenko, I., Epanchintseva, A., Korchagina, K., Pyshnyi, D., Apartsin, E., Ryabchikova, E., & Pyshnaya, I. (2018). Non-Covalent Associates of siRNAs and AuNPs Enveloped with Lipid Layer and Doped with Amphiphilic Peptide for Efficient siRNA Delivery. International Journal of Molecular Sciences, 19(7), 2096. https://doi.org/10.3390/ijms19072096