Level-Specific Differences in Systemic Expression of Pro- and Anti-Inflammatory Cytokines and Chemokines after Spinal Cord Injury
Abstract
:1. Introduction
2. Results
2.1. Level-Specific Differences in Plasma Protein Levels after Cervical and Thoracic Laminectomy
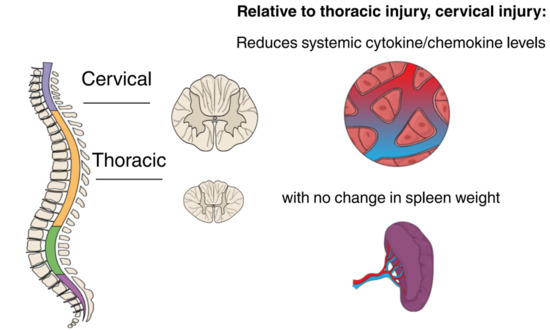
2.2. Level-Specific Differences in Plasma Protein Levels after Cervical and Thoracic SCI
2.3. Temporal Expression Patterns of Plasma Proteins after SCI
2.4. Functional Classification of Serum Protein after SCI
2.5. Spleen Weight
3. Discussion
4. Materials and Methods
4.1. Clip-Compression SCI and Spleen Weight
4.2. Neurobehavioural Assessments
4.3. Blood Collection and High-Throughput ELISA
4.4. Clustering and Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BBB | Basso, Beattie and Bresnahan |
| BMS | Basso Mouse Scale |
| BSCB | Blood-spinal-cord-barrier |
| PBS | Phosphate buffer solution |
| SCI | Spinal cord injury |
| GMCSF | Granulocyte-macrophage colony-stimulating factor |
| IL1a | Interleukin 1 alpha |
| IL1b | Interleukin 1 beta |
| IL2 | Interleukin-2 |
| IL17A | Interleukin-17A |
| IL18 | Interleukin-18 |
| MCP1 | Monocyte chemoattractant protein-1 |
| IP10 | Interferon gamma-induced protein 10 |
| LIX | Lipopolysaccharide-induced CXC chemokine |
| MIP2 | macrophage inflammatory protein 2 |
| RANTES | regulated on activation, normal T cell expressed and secreted |
| GCSF | Granulocyte-colony stimulating factor |
| IL4 | Interleukin-4 |
| IL10 | Interleukin-10 |
| IL12 | Interleukin-12 |
| IL5 | Interleukin-5 |
| IFNg | Interferon gamma |
| VEGF | Vascular endothelial growth factor |
References
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef] [PubMed]
- Tator, C.H.; Fehlings, M.G. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J. Neurosurg. 1991, 75, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Tator, C.H.; Koyanagi, I. Vascular mechanisms in the pathophysiology of human spinal cord injury. J. Neurosurg. 1997, 86, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Figley, S.A.; Khosravi, R.; Legasto, J.M.; Tseng, Y.-F.; Fehlings, M.G. Characterization of vascular disruption and blood-spinal cord barrier permeability following traumatic spinal cord injury. J. Neurotrauma 2014, 31, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, J.D.; Dykes-Hoberg, M.; Pardo, C.A.; Kanai, Y.; Bristol, L.A.; Kuncl, R.W.; Welty, D.F.; Jin, L.; Hediger, M.A.; Wang, Y.; et al. Knockout of Glutamate Transporters Reveals a Major Role for Astroglial Transport in Excitotoxicity and Clearance of Glutamate. Neuron 1996, 16, 675–686. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, S.K.; Fehlings, M.G. Role of NMDA and Non-NMDA Ionotropic Glutamate Receptors in Traumatic Spinal Cord Axonal Injury. J. Neurosci. 1997, 17, 1055–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braughler, J.M.; Duncan, L.A.; Chase, R.L. Interaction of lipid peroxidation and calcium in the pathogenesis of neuronal injury. Cent. Nerv. Syst. Trauma 1985, 2, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Ruiz, A.; Rios, C.; Duarte, I.; Correa, D.; Guizar-Sahagun, G.; Grijalva, I.; Ibarra, A. Cyclosporin-A inhibits lipid peroxidation after spinal cord injury in rats. Neurosci. Lett. 1999, 266, 61–64. [Google Scholar] [CrossRef]
- Kaptanoglu, E.; Solaroglu, I.; Okutan, O.; Surucu, H.S.; Akbiyik, F.; Beskonakli, E. Erythropoietin exerts neuroprotection after acute spinal cord injury in rats: Effect on lipid peroxidation and early ultrastructural findings. Neurosurg. Rev. 2004, 27, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Winkler, E.A.; Sengillo, J.D.; Bell, R.D.; Wang, J.; Zlokovic, B.V. Blood-spinal cord barrier pericyte reductions contribute to increased capillary permeability. J. Cereb. Blood Flow Metab. 2012, 32, 1841–1852. [Google Scholar] [CrossRef] [PubMed]
- Whetstone, W.D.; Hsu, J.-Y.C.; Eisenberg, M.; Werb, Z.; Noble-Haeusslein, L.J. Blood-spinal cord barrier after spinal cord injury: Relation to revascularization and wound healing. J. Neurosci. Res. 2003, 74, 227–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noble, L.J.; Wrathall, J.R. Blood-spinal cord barrier disruption proximal to a spinal cord transection in the rat: Time course and pathways associated with protein leakage. Exp. Neurol. 1988, 99, 567–578. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Agrawal, S. Role of sodium in the pathophysiology of secondary spinal cord injury. Spine 1995, 20, 2187–2191. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.; Fehlings, M.G. Evaluation of the neuroprotective effects of sodium channel blockers after spinal cord injury: Improved behavioral and neuroanatomical recovery with riluzole. J. Neurosurg. Spine 2001, 94, 245–256. [Google Scholar] [CrossRef]
- Wilcox, J.T.; Satkunendrarajah, K.; Nasirzadeh, Y.; Laliberte, A.M.; Lip, A.; Cadotte, D.W.; Foltz, W.D.; Fehlings, M.G. Generating level-dependent models of cervical and thoracic spinal cord injury: Exploring the interplay of neuroanatomy, physiology, and function. Neurobiol. Dis. 2017, 105, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Ulndreaj, A.; Badner, A.; Fehlings, M.G. Promising neuroprotective strategies for traumatic spinal cord injury with a focus on the differential effects among anatomical levels of injury. F1000Res 2017, 6, 1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Guan, Z.; Reader, B.; Shawler, T.; Mandrekar-Colucci, S.; Huang, K.; Weil, Z.; Bratasz, A.; Wells, J.; Powell, N.D.; et al. Autonomic Dysreflexia Causes Chronic Immune Suppression after Spinal Cord Injury. J. Neurosci. 2013, 33, 12970–12981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucin, K.M.; Sanders, V.M.; Popovich, P.G. Stress hormones collaborate to induce lymphocyte apoptosis after high level spinal cord injury. J. Neurochem. 2009, 110, 1409–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Partida, E.; Mironets, E.; Hou, S.; Tom, V.J. Cardiovascular dysfunction following spinal cord injury. Neural Regen. Res. 2016, 11, 189–194. [Google Scholar] [PubMed]
- Brommer, B.; Engel, O.; Kopp, M.A.; Watzlawick, R.; Müller, S.; Prüss, H.; Chen, Y.; DeVivo, M.J.; Finkenstaedt, F.W.; Dirnagl, U.; et al. Spinal cord injury-induced immune deficiency syndrome enhances infection susceptibility dependent on lesion level. Brain 2016, 139, 692–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwab, J.M.; Zhang, Y.; Kopp, M.A.; Brommer, B.; Popovich, P.G. The paradox of chronic neuroinflammation, systemic immune suppression, autoimmunity after traumatic chronic spinal cord injury. Exp. Neurol. 2014, 258, 121–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waykole, Y.P.; Doiphode, S.S.; Rakhewar, P.S.; Mhaske, M. Anticytokine therapy for periodontal diseases: Where are we now? J. Indian Soc. Periodontol. 2009, 13, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Audet, M.-C.; Anisman, H. Interplay between pro-inflammatory cytokines and growth factors in depressive illnesses. Front. Cell. Neurosci. 2013, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Kofler, S.; Nickel, T.; Weis, M. Role of cytokines in cardiovascular diseases: A focus on endothelial responses to inflammation. Clin. Sci. 2005, 108, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Mukhamedshina, Y.O.; Akhmetzyanova, E.R.; Martynova, E.V.; Khaiboullina, S.F.; Galieva, L.R.; Rizvanov, A.A. Systemic and Local Cytokine Profile following Spinal Cord Injury in Rats: A Multiplex Analysis. Front. Neurol. 2017, 8, 581. [Google Scholar] [CrossRef] [PubMed]
- Bartholdi, D.; Schwab, M.E. Expression of pro-inflammatory cytokine and chemokine mRNA upon experimental spinal cord injury in mouse: An in situ hybridization study. Eur. J. Neurosci. 1997, 9, 1422–1438. [Google Scholar] [CrossRef] [PubMed]
- Klusman, I.; Schwab, M.E. Effects of pro-inflammatory cytokines in experimental spinal cord injury. Brain Res. 1997, 762, 173–184. [Google Scholar] [CrossRef]
- Schnell, L.; Fearn, S.; Schwab, M.E.; Perry, V.H.; Anthony, D.C. Cytokine-induced acute inflammation in the brain and spinal cord. J. Neuropathol. Exp. Neurol. 1999, 58, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Francos Quijorna, I.; Santos-Nogueira, E.; Gronert, K.; Sullivan, A.B.; Kopp, M.A.; Brommer, B.; David, S.; Schwab, J.M.; Lopez Vales, R. Maresin 1 promotes inflammatory resolution, neuroprotection and functional neurological recovery after spinal cord injury. J. Neurosci. 2017, 29, 11731–11743. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.J.; Robert, S.; Huang, W.; Young, W.; Cotman, C.W. Activation of complement pathways after contusion-induced spinal cord injury. J. Neurotrauma 2004, 21, 1831–1846. [Google Scholar] [CrossRef] [PubMed]
- Kasinathan, N.; Vanathi, M.B.; Subrahmanyam, V.M.; Rao, J.V. A review on response of immune system in spinal cord injury and therapeutic agents useful in treatment. Curr. Pharm. Biotechnol. 2015, 16, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Figley, S.A.; Liu, Y.; Karadimas, S.K.; Satkunendrarajah, K.; Fettes, P.; Spratt, S.K.; Lee, G.; Ando, D.; Surosky, R.; Giedlin, M.; et al. Delayed administration of a bio-engineered zinc-finger VEGF-A gene therapy is neuroprotective and attenuates allodynia following traumatic spinal cord injury. PLoS ONE 2014, 9, e96137. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Figley, S.; Spratt, S.K.; Lee, G.; Ando, D.; Surosky, R.; Fehlings, M.G. An engineered transcription factor which activates VEGF-A enhances recovery after spinal cord injury. Neurobiol. Dis. 2010, 37, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Y.; Li, D.; Liu, Z.; Zhao, Z.; Han, D.; Yuan, Y.; Bi, J.; Mei, X. VEGF inhibits the inflammation in spinal cord injury through activation of autophagy. Biochem. Biophys. Res. Commun. 2015, 464, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, X.; Zhang, H.; Yuan, J.; Ding, H.; Wei, Y. Elevated leptin expression in rat model of traumatic spinal cord injury and femoral fracture. J. Spinal Cord Med. 2011, 34, 501–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gezici, A.R.; Ergun, R.; Karakas, A.; Gunduz, B. Serum leptin levels following acute experimental spinal cord injury. J. Spinal Cord Med. 2009, 32, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Garshick, E.; Walia, P.; Goldstein, R.L.; Teylan, M.; Lazzari, A.A.; Tun, C.G.; Hart, J.E. Plasma Leptin and Reduced FEV1 and FVC in Chronic Spinal Cord Injury. PM R 2017, 10, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.Y.; Steadward, R.D.; Wheeler, G.D.; Bell, G.; McCargar, L.; Harber, V. Intact sympathetic nervous system is required for leptin effects on resting metabolic rate in people with spinal cord injury. J. Clin. Endocrinol. Metab. 2003, 88, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Martos, C.M.; González, P.; Rodriguez, F.J. Acute leptin treatment enhances functional recovery after spinal cord injury. PLoS ONE 2012, 7, e35594. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.K.; Streijger, F.; Fallah, N.; Noonan, V.K.; Bélanger, L.M.; Ritchie, L.; Paquette, S.J.; Ailon, T.; Boyd, M.C.; Street, J.; et al. Cerebrospinal Fluid Biomarkers To Stratify Injury Severity and Predict Outcome in Human Traumatic Spinal Cord Injury. J. Neurotrauma 2017, 34, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.L.; Shih, K.; Bao, P.; Ghirnikar, R.S.; Eng, L.F. Cytokine chemokine expression in contused rat spinal cord. Neurochem. Int. 2000, 36, 417–425. [Google Scholar] [CrossRef]
- Glaser, J.; Gonzalez, R.; Sadr, E.; Keirstead, H.S. Neutralization of the chemokine CXCL10 reduces apoptosis and increases axon sprouting after spinal cord injury. J. Neurosci. Res. 2006, 84, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Di Lernia, V. Targeting the IFN-γ/CXCL10 pathway in lichen planus. Med. Hypotheses 2016, 92, 60–61. [Google Scholar] [CrossRef] [PubMed]
- Glaser, J.; Gonzalez, R.; Perreau, V.M.; Cotman, C.W.; Keirstead, H.S. Neutralization of the chemokine CXCL10 enhances tissue sparing and angiogenesis following spinal cord injury. J. Neurosci. Res. 2004, 77, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.; Hickey, M.J.; Espinosa, J.M.; Nistor, G.; Lane, T.E.; Keirstead, H.S. Therapeutic neutralization of CXCL10 decreases secondary degeneration and functional deficit after spinal cord injury in mice. Regen. Med. 2007, 2, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Kim, J.H.; Choi, K.H.; Yoon, H.H.; Jeon, S.R. Is There Additive Therapeutic Effect When GCSF Combined with Adipose-Derived Stem Cell in a Rat Model of Acute Spinal Cord Injury? J. Korean Neurosurg. Soc. 2017, 60, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Nishio, Y.; Koda, M.; Kamada, T.; Someya, Y.; Kadota, R.; Mannoji, C.; Miyashita, T.; Okada, S.; Okawa, A.; Moriya, H.; et al. Granulocyte colony-stimulating factor attenuates neuronal death and promotes functional recovery after spinal cord injury in mice. J. Neuropathol. Exp. Neurol. 2007, 66, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Koda, M.; Takahashi, H.; Sakuma, T.; Inada, T.; Kamiya, K.; Ota, M.; Maki, S.; Okawa, A.; Takahashi, K.; et al. Granulocyte colony-stimulating factor attenuates spinal cord injury-induced mechanical allodynia in adult rats. J. Neurol. Sci. 2015, 355, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Dittgen, T.; Pitzer, C.; Plaas, C.; Kirsch, F.; Vogt, G.; Laage, R.; Schneider, A. Granulocyte-colony stimulating factor (G-CSF) improves motor recovery in the rat impactor model for spinal cord injury. PLoS ONE 2012, 7, e29880. [Google Scholar] [CrossRef] [PubMed]
- Kadota, R.; Koda, M.; Kawabe, J.; Hashimoto, M.; Nishio, Y.; Mannoji, C.; Miyashita, T.; Furuya, T.; Okawa, A.; Takahashi, K.; et al. Granulocyte colony-stimulating factor (G-CSF) protects oligodendrocyte and promotes hindlimb functional recovery after spinal cord injury in rats. PLoS ONE 2012, 7, e50391. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, S.; Wang, P.; Zhang, H.; Wang, F.; Bing, L.; Gao, J.; Yang, J.; Hao, A. Granulocyte colony-stimulating factor improves neuron survival in experimental spinal cord injury by regulating nucleophosmin-1 expression. J. Neurosci. Res. 2014, 92, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Koda, M.; Nishio, Y.; Kamada, T.; Someya, Y.; Okawa, A.; Mori, C.; Yoshinaga, K.; Okada, S.; Moriya, H.; Yamazaki, M. Granulocyte colony-stimulating factor (G-CSF) mobilizes bone marrow-derived cells into injured spinal cord and promotes functional recovery after compression-induced spinal cord injury in mice. Brain Res. 2007, 1149, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Yang, C.-C.; Kuo, Y.-M.; Sze, C.-I.; Hsu, J.-Y.C.; Huang, Y.-H.; Tzeng, S.-F.; Tsai, C.-L.; Chen, H.-H.; Jou, I.-M. Delayed granulocyte colony-stimulating factor treatment promotes functional recovery in rats with severe contusive spinal cord injury. Spine 2012, 37, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Yamazaki, M.; Okawa, A.; Sakuma, T.; Kato, K.; Hashimoto, M.; Hayashi, K.; Furuya, T.; Fujiyoshi, T.; Kawabe, J.; et al. Neuroprotective therapy using granulocyte colony-stimulating factor for acute spinal cord injury: A phase I/IIa clinical trial. Eur. Spine J. 2012, 21, 2580–2587. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Koda, M.; Furuya, T.; Kato, K.; Takahashi, H.; Sakuma, T.; Inada, T.; Ota, M.; Maki, S.; Okawa, A.; et al. Neuroprotective therapy with granulocyte colony-stimulating factor in acute spinal cord injury: A comparison with high-dose methylprednisolone as a historical control. Eur. Spine J. 2015, 24, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Shim, Y.S.; Park, Y.H.; Chung, J.K.; Nam, J.H.; Kim, M.O.; Park, H.C.; Park, S.R.; Min, B.-H.; Kim, E.Y.; et al. Complete Spinal Cord Injury Treatment Using Autologous Bone Marrow Cell Transplantation and Bone Marrow Stimulation with Granulocyte Macrophage-Colony Stimulating Factor: Phase I/II Clinical Trial. Stem Cells 2007, 25, 2066–2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poniatowski, Ł.A.; Wojdasiewicz, P.; Krawczyk, M.; Szukiewicz, D.; Gasik, R.; Kubaszewski, Ł.; Kurkowska-Jastrzębska, I. Analysis of the Role of CX3CL1 (Fractalkine) and Its Receptor CX3CR1 in Traumatic Brain and Spinal Cord Injury: Insight into Recent Advances in Actions of Neurochemokine Agents. Mol. Neurobiol. 2017, 54, 2167–2188. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, D.J.; Longbrake, E.E.; Shawler, T.M.; Kigerl, K.A.; Lai, W.; Tovar, C.A.; Ransohoff, R.M.; Popovich, P.G. Deficient CX3CR1 signaling promotes recovery after mouse spinal cord injury by limiting the recruitment and activation of Ly6Clo/iNOS+ macrophages. J. Neurosci. 2011, 31, 9910–9922. [Google Scholar] [CrossRef] [PubMed]
- Freria, C.M.; Hall, J.C.E.; Wei, P.; Guan, Z.; McTigue, D.M.; Popovich, P.G. Deletion of the Fractalkine Receptor, CX3CR1, Improves Endogenous Repair, Axon Sprouting, and Synaptogenesis after Spinal Cord Injury in Mice. J. Neurosci. 2017, 37, 3568–3587. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, J.T.; Fehlings, M.G. Acute Clip Compression Model of SCI. In Experimental Neurosurgery in Animal Models; Neuromethods; Springer: New York, NY, USA, 2016; Volume 116, pp. 111–117. [Google Scholar]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A Sensitive and Reliable Locomotor Rating Scale for Open Field Testing in Rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.; Chang, A.; Zavvarian, M.-M.; Wang, J.; Liu, Y.; Fehlings, M.G. Level-Specific Differences in Systemic Expression of Pro- and Anti-Inflammatory Cytokines and Chemokines after Spinal Cord Injury. Int. J. Mol. Sci. 2018, 19, 2167. https://doi.org/10.3390/ijms19082167
Hong J, Chang A, Zavvarian M-M, Wang J, Liu Y, Fehlings MG. Level-Specific Differences in Systemic Expression of Pro- and Anti-Inflammatory Cytokines and Chemokines after Spinal Cord Injury. International Journal of Molecular Sciences. 2018; 19(8):2167. https://doi.org/10.3390/ijms19082167
Chicago/Turabian StyleHong, James, Alex Chang, Mohammad-Masoud Zavvarian, Jian Wang, Yang Liu, and Michael G. Fehlings. 2018. "Level-Specific Differences in Systemic Expression of Pro- and Anti-Inflammatory Cytokines and Chemokines after Spinal Cord Injury" International Journal of Molecular Sciences 19, no. 8: 2167. https://doi.org/10.3390/ijms19082167
APA StyleHong, J., Chang, A., Zavvarian, M. -M., Wang, J., Liu, Y., & Fehlings, M. G. (2018). Level-Specific Differences in Systemic Expression of Pro- and Anti-Inflammatory Cytokines and Chemokines after Spinal Cord Injury. International Journal of Molecular Sciences, 19(8), 2167. https://doi.org/10.3390/ijms19082167