Stress-Induced Phosphorylation of Nuclear YB-1 Depends on Nuclear Trafficking of p90 Ribosomal S6 Kinase
Abstract
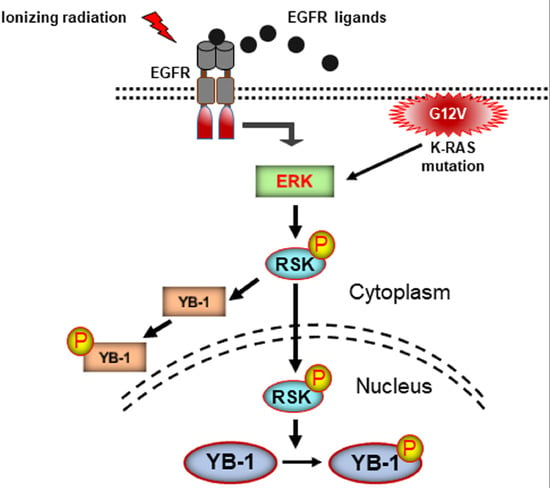
:1. Introduction
2. Results
2.1. YB-1 Is Not Translocated to the Nucleus after Irradiation
2.2. Phosphorylation of Nuclear YB-1 after Stimulation with EGF Is Associated with Nuclear Accumulation of RSK but Not of YB-1
2.3. Targeting RSK Inhibits Radiation-Induced YB-1 Phosphorylation in Nuclear Fractions without Affecting YB-1 Nuclear Expression
2.4. Conditional Expression of KRAS(G12V) Stimulates YB-1 Phosphorylation in Cytoplasmic and Nuclear Fractions without YB-1 Accumulation in the Nucleus
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Antibodies and Reagents
4.3. Irradiation, Ligand Stimulation, and Treatment with Inhibitors
4.4. Cellular Fractionation and Immunoblotting Analysis
4.5. Densitometry and Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kohno, K.; Izumi, H.; Uchiumi, T.; Ashizuka, M.; Kuwano, M. The pleiotropic functions of the Y-box-binding protein, YB-1. Bioessays 2003, 25, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Oyama, T.; Osaki, T.; Kohno, K.; Yasumoto, K. Expression of Y box-binding protein-1 correlates with DNA topoisomerase IIalpha and proliferating cell nuclear antigen expression in lung cancer. Anticancer Res. 2001, 21, 2357–2362. [Google Scholar] [PubMed]
- Eliseeva, I.A.; Kim, E.R.; Guryanov, S.G.; Ovchinnikov, L.P.; Lyabin, D.N. Y-box-binding protein 1 (YB-1) and its functions. Biochemistry 2011, 76, 1402–1433. [Google Scholar] [CrossRef] [PubMed]
- Lasham, A.; Print, C.G.; Woolley, A.G.; Dunn, S.E.; Braithwaite, A.W. YB-1: oncoprotein, prognostic marker and therapeutic target? Biochem. J. 2013, 449, 11–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosnopfel, C.; Sinnberg, T.; Schittek, B. Y-box binding protein 1—A prognostic marker and target in tumour therapy. Eur. J. Cell Biol. 2014, 93, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Wu, Y.; Wang, Y.; Zhu, X.; Yin, H.; He, Y.; Li, C.; Liu, Y.; Lu, X.; Chen, Y.; et al. Y-box-binding protein-1 (YB-1) promotes cell proliferation, adhesion and drug resistance in diffuse large B-cell lymphoma. Exp. Cell Res. 2016, 346, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Hua, L.; Wang, B.; Wei, H.; Prabhu, L.; Hartley, A.V.; Jiang, G.; Liu, Y.; Lu, T. Novel Serine 176 Phosphorylation of YBX1 Activates NF-kappaB in Colon Cancer. J. Biol. Chem. 2017, 292, 3433–3444. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, L.; Mundade, R.; Wang, B.; Wei, H.; Hartley, A.V.; Martin, M.; McElyea, K.; Temm, C.J.; Sandusky, G.; Liu, Y.; et al. Critical role of phosphorylation of serine 165 of YBX1 on the activation of NF-κB in colon cancer. Oncotarget 2015, 6, 29396–29412. [Google Scholar] [CrossRef] [PubMed]
- Jurchott, K.; Bergmann, S.; Stein, U.; Walther, W.; Janz, M.; Manni, I.; Piaggio, G.; Fietze, E.; Dietel, M.; Royer, H.D. YB-1 as a cell cycle-regulated transcription factor facilitating cyclin A and cyclin B1 gene expression. J. Biol. Chem. 2003, 278, 27988–27996. [Google Scholar] [CrossRef] [PubMed]
- Stein, U.; Jurchott, K.; Walther, W.; Bergmann, S.; Schlag, P.M.; Royer, H.D. Hyperthermia-induced nuclear translocation of transcription factor YB-1 leads to enhanced expression of multidrug resistance-related ABC transporters. J. Biol. Chem. 2001, 276, 28562–28569. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Ito, K.; Izumi, H.; Kimura, M.; Sano, M.; Nakagomi, H.; Maeno, K.; Hama, Y.; Shingu, K.; Tsuchiya, S.; et al. Increased nuclear localization of transcription factor Y-box binding protein 1 accompanied by up-regulation of P-glycoprotein in breast cancer pretreated with paclitaxel. Clin. Cancer Res. 2005, 11, 8837–8844. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Higashi, M.; Momose, S.; Morozumi, M.; Tamaru, J.I. Nuclear expression of Y box binding-1 is important for resistance to chemotherapy including gemcitabine in TP53-mutated bladder cancer. Int. J. Oncol. 2017, 51, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Shiraiwa, S.; Kinugasa, T.; Kawahara, A.; Mizobe, T.; Ohchi, T.; Yuge, K.; Fujino, S.; Katagiri, M.; Shimomura, S.; Tajiri, K.; et al. Nuclear Y-Box-binding Protein-1 Expression Predicts Poor Clinical Outcome in Stage III Colorectal Cancer. Anticancer Res. 2016, 36, 3781–3788. [Google Scholar] [PubMed]
- Heumann, A.; Kaya, O.; Burdelski, C.; Hube-Magg, C.; Kluth, M.; Lang, D.S.; Simon, R.; Beyer, B.; Thederan, I.; Sauter, G.; et al. Up regulation and nuclear translocation of Y-box binding protein 1 (YB-1) is linked to poor prognosis in ERG-negative prostate cancer. Sci. Rep. 2017, 7, 2056. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yuan, G.L.; Liang, Q.L.; Zhang, H.J.; Huang, J.; Cheng, S.A.; Peng, X.X. Positive expression of Y-box binding protein 1 and prognosis in non-small cell lung cancer: A meta-analysis. Oncotarget 2017, 8, 55613–55621. [Google Scholar] [CrossRef] [PubMed]
- Jeggo, P.A.; Lobrich, M. DNA non-homologous end-joining enters the resection arena. Oncotarget 2017, 8, 93317–93318. [Google Scholar] [CrossRef] [PubMed]
- Mladenov, E.; Magin, S.; Soni, A.; Iliakis, G. DNA double-strand-break repair in higher eukaryotes and its role in genomic instability and cancer: Cell cycle and proliferation-dependent regulation. Semin. Cancer Biol. 2016, 37–38, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Toulany, M.; Schickfluss, T.A.; Eicheler, W.; Kehlbach, R.; Schittek, B.; Rodemann, H.P. Impact of oncogenic K-RAS on YB-1 phosphorylation induced by ionizing radiation. Breast Cancer Res. 2011, 13, R28. [Google Scholar] [CrossRef] [PubMed]
- Alemasova, E.E.; Moor, N.A.; Naumenko, K.N.; Kutuzov, M.M.; Sukhanova, M.V.; Pestryakov, P.E.; Lavrik, O.I. Y-box-binding protein 1 as a non-canonical factor of base excision repair. Biochim. Biophys. Acta 2016, 1864, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.L.; Ren, E.C. Functional Aspects of PARP1 in DNA Repair and Transcription. Biomolecules 2012, 2, 524–548. [Google Scholar] [CrossRef] [PubMed]
- Stratford, A.L.; Fry, C.J.; Desilets, C.; Davies, A.H.; Cho, Y.Y.; Li, Y.; Dong, Z.; Berquin, I.M.; Roux, P.P.; Dunn, S.E. Y-box binding protein-1 serine 102 is a downstream target of p90 ribosomal S6 kinase in basal-like breast cancer cells. Breast Cancer Res. 2008, 10, R99. [Google Scholar] [CrossRef] [PubMed]
- Stratford, A.L.; Reipas, K.; Hu, K.; Fotovati, A.; Brough, R.; Frankum, J.; Takhar, M.; Watson, P.; Ashworth, A.; Lord, C.J.; et al. Targeting p90 ribosomal S6 kinase eliminates tumor-initiating cells by inactivating Y-box binding protein-1 in triple-negative breast cancers. Stem Cells 2012, 30, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Rossner, F.; Gieseler, C.; Morkel, M.; Royer, H.D.; Rivera, M.; Blaker, H.; Dietel, M.; Schafer, R.; Sers, C. Uncoupling of EGFR-RAS signaling and nuclear localization of YBX1 in colorectal cancer. Oncogenesis 2016, 5, e187. [Google Scholar] [CrossRef] [PubMed]
- Donaubauer, E.M.; Hunzicker-Dunn, M.E. Extracellular Signal-regulated Kinase (ERK)-dependent Phosphorylation of Y-Box-binding Protein 1 (YB-1) Enhances Gene Expression in Granulosa Cells in Response to Follicle-stimulating Hormone (FSH). J. Biol. Chem. 2016, 291, 12145–12160. [Google Scholar] [CrossRef] [PubMed]
- Izumi, H.; Imamura, T.; Nagatani, G.; Ise, T.; Murakami, T.; Uramoto, H.; Torigoe, T.; Ishiguchi, H.; Yoshida, Y.; Nomoto, M.; et al. Y box-binding protein-1 binds preferentially to single-stranded nucleic acids and exhibits 3′→5′ exonuclease activity. Nucleic Acids Res. 2001, 29, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Ohga, T.; Koike, K.; Ono, M.; Makino, Y.; Itagaki, Y.; Tanimoto, M.; Kuwano, M.; Kohno, K. Role of the human Y box-binding protein YB-1 in cellular sensitivity to the DNA-damaging agents cisplatin, mitomycin C, and ultraviolet light. Cancer Res. 1996, 56, 4224–4228. [Google Scholar] [PubMed]
- Jeggo, P.A.; Geuting, V.; Lobrich, M. The role of homologous recombination in radiation-induced double-strand break repair. Radiother. Oncol. 2011, 101, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, B.W.; Kucab, J.; Wu, J.; Lee, C.; Cheang, M.C.; Yorida, E.; Turbin, D.; Dedhar, S.; Nelson, C.; Pollak, M.; et al. Akt phosphorylates the Y-box binding protein 1 at Ser102 located in the cold shock domain and affects the anchorage-independent growth of breast cancer cells. Oncogene 2005, 24, 4281–4292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basaki, Y.; Hosoi, F.; Oda, Y.; Fotovati, A.; Maruyama, Y.; Oie, S.; Ono, M.; Izumi, H.; Kohno, K.; Sakai, K.; et al. Akt-dependent nuclear localization of Y-box-binding protein 1 in acquisition of malignant characteristics by human ovarian cancer cells. Oncogene 2007, 26, 2736–2746. [Google Scholar] [CrossRef] [PubMed]
- Mordovkina, D.A.; Kim, E.R.; Buldakov, I.A.; Sorokin, A.V.; Eliseeva, I.A.; Lyabin, D.N.; Ovchinnikov, L.P. Transportin-1-dependent YB-1 nuclear import. Biochem. Biophys. Res. Commun. 2016, 480, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Kose, S.; Kuwahara, I.; Yoshimura, M.; Imamoto, N.; Yoshida, M. Y-box protein-associated acidic protein (YBAP1/C1QBP) affects the localization and cytoplasmic functions of YB-1. Sci. Rep. 2018, 8, 6198. [Google Scholar] [CrossRef] [PubMed]
- Woolley, A.G.; Algie, M.; Samuel, W.; Harfoot, R.; Wiles, A.; Hung, N.A.; Tan, P.H.; Hains, P.; Valova, V.A.; Huschtscha, L.; et al. Prognostic association of YB-1 expression in breast cancers: A matter of antibody. PLoS ONE 2011, 6, e20603. [Google Scholar] [CrossRef] [PubMed]
- Houles, T.; Roux, P.P. Defining the role of the RSK isoforms in cancer. Semin. Cancer Biol. 2018, 48, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Minjgee, M.; Toulany, M.; Kehlbach, R.; Giehl, K.; Rodemann, H.P. K-RAS(V12) induces autocrine production of EGFR ligands and mediates radioresistance through EGFR-dependent Akt signaling and activation of DNA-PKcs. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Calvo, I.; Blazquez-Medela, A.M.; Santos, E.; Lopez-Novoa, J.M.; Martinez-Salgado, C. Analysis of k-ras nuclear expression in fibroblasts and mesangial cells. PLoS ONE 2010, 5, e8703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waters, A.M.; Ozkan-Dagliyan, I.; Vaseva, A.V.; Fer, N.; Strathern, L.A.; Hobbs, G.A.; Tessier-Cloutier, B.; Gillette, W.K.; Bagni, R.; Whiteley, G.R.; et al. Evaluation of the selectivity and sensitivity of isoform- and mutation-specific RAS antibodies. Sci Signal. 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, E.V. A cell line (HBL-100) established from human breast milk. Cell Tissue Res. 1982, 227, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Makino, K.; Xia, W.; Matin, A.; Wen, Y.; Kwong, K.Y.; Bourguignon, L.; Hung, M.C. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat. Cell Biol. 2001, 3, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Iida, M.; Brand, T.M.; Campbell, D.A.; Li, C.; Wheeler, D.L. Yes and Lyn play a role in nuclear translocation of the epidermal growth factor receptor. Oncogene 2013, 32, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Toulany, M.; Dittmann, K.; Baumann, M.; Rodemann, H.P. Radiosensitization of Ras-mutated human tumor cells in vitro by the specific EGF receptor antagonist BIBX1382BS. Radiother. Oncol. 2005, 74, 117–129. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwari, A.; Rebholz, S.; Maier, E.; Dehghan Harati, M.; Zips, D.; Sers, C.; Rodemann, H.P.; Toulany, M. Stress-Induced Phosphorylation of Nuclear YB-1 Depends on Nuclear Trafficking of p90 Ribosomal S6 Kinase. Int. J. Mol. Sci. 2018, 19, 2441. https://doi.org/10.3390/ijms19082441
Tiwari A, Rebholz S, Maier E, Dehghan Harati M, Zips D, Sers C, Rodemann HP, Toulany M. Stress-Induced Phosphorylation of Nuclear YB-1 Depends on Nuclear Trafficking of p90 Ribosomal S6 Kinase. International Journal of Molecular Sciences. 2018; 19(8):2441. https://doi.org/10.3390/ijms19082441
Chicago/Turabian StyleTiwari, Aadhya, Simone Rebholz, Eva Maier, Mozhgan Dehghan Harati, Daniel Zips, Christine Sers, H. Peter Rodemann, and Mahmoud Toulany. 2018. "Stress-Induced Phosphorylation of Nuclear YB-1 Depends on Nuclear Trafficking of p90 Ribosomal S6 Kinase" International Journal of Molecular Sciences 19, no. 8: 2441. https://doi.org/10.3390/ijms19082441
APA StyleTiwari, A., Rebholz, S., Maier, E., Dehghan Harati, M., Zips, D., Sers, C., Rodemann, H. P., & Toulany, M. (2018). Stress-Induced Phosphorylation of Nuclear YB-1 Depends on Nuclear Trafficking of p90 Ribosomal S6 Kinase. International Journal of Molecular Sciences, 19(8), 2441. https://doi.org/10.3390/ijms19082441