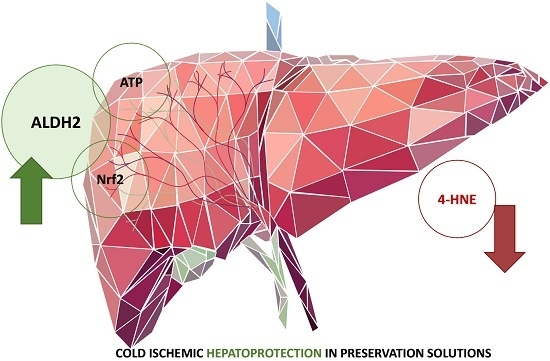
Aldehyde Dehydrogenase 2 (ALDH2) in Rat Fatty Liver Cold Ischemia Injury
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Groups
4.3. Biochemical Determinations
4.3.1. Transaminase Assay
4.3.2. Histology
4.3.3. ALDH2 Activity
4.3.4. 4-Hydroxynonenal
4.3.5. Malondialdehyde
4.3.6. Advanced Oxidation Protein Products
4.3.7. Caspase 3 Activity
4.3.8. ATP Measurements
4.3.9. Western-Blotting Analysis of ALDH2, 4-HNE, Caspase 3 and Caspase 3 Activity
4.3.10. TUNEL Assay
4.4. Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Belzer, F.O.; Southard, J.H. Principles of solid organ preservation by cold storage. Transplantation 1988, 45, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Southard, J.H.; Belzer, F.O. Organ Preservation. Annu. Rev. Med. 1995, 46, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Zaouali, M.A.; Abdennebi, H.B.; Padrissa-Altés, S.; Mahfoudh-Boussaid, A.; Roselló-Catafau, J. Pharmacological strategies against cold ischemia reperfusion injury. Expert Opin. Pharmacother. 2010, 11, 537–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casillas-Ramírez, A.; Mosbah, I.B.; Ramalho, F.; Roselló-Catafau, J.; Peralta, C. Past and future approaches to ischemia-reperfusion lesion associated with liver transplantation. Life Sci. 2006, 79, 1881–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serracino-Inglott, F.; Habib, N.A.; Mathie, R.T. Hepatic ischemia-reperfusion injury. Am. J. Surg. 2001, 181, 160–166. [Google Scholar] [CrossRef]
- Tashiro, H.; Kuroda, S.; Mikuriya, Y.; Ohdan, H. Ischemia–reperfusion injury in patients with fatty liver and the clinical impact of steatotic liver on hepatic surgery. Surg. Today 2014, 44, 1611–1625. [Google Scholar] [CrossRef] [PubMed]
- Selzner, M.; Clavien, P.A. Fatty liver in liver transplantation and surgery. Semin. Liver Dis. 2001, 21, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Delvart, V.; Karam, V.; Ducerf, C.; Navarro, F.; Letoublon, C.; Belghiti, J.; Pezet, D.; Castaign, D.; LeTreut, Y.; et al. The European liver, intestine trasplant association ELITA. Am. J. Trasplant. 2015, 15, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Guibert, E.E.; Petrenko, A.Y.; Balaban, C.L.; Somov, A.Y.; Rodriguez, J.V.; Fuller, B.J. Organ preservation: Current concepts and new strategies for the next decade. Transfus. Med. Hemother. 2011, 38, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Milkovic, L.; Cipak Gasparovic, A.; Zarkovic, N. Overview on major lipid peroxidation bioactive factor 4-hydoxynonenal as pluripotent growth-regulating factor. Free Radic. Res. 2015, 49, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Porter, N.A.; Brash, A.R. Routes to 4-hydroxynonenal: Fundamental issue in mechanisms of lipid peroxidation. J. Biol. Chem. 2008, 283, 15539–15543. [Google Scholar] [CrossRef] [PubMed]
- Kristal, B.S.; Park, B.K.; Yu, B.P. 4-Hydroxynonenal is a potent inducer of mitochondrial permability transition. J. Biol. Chem. 1996, 271, 6033–6038. [Google Scholar] [CrossRef] [PubMed]
- Kruman, I.; Bruce-Keller, A.J.; Bredesen, D.; Waeg, G.; Matson, M.P. Evidence that 4-hydroxynonenal mediates oxidative-stress-induced neuronal apoptosis. J. Neurosci. 1997, 17, 5089–5100. [Google Scholar] [CrossRef] [PubMed]
- Baskol, M.; Baskol, G.; Koçer, D.; Ozbakir, O.; Yucesoy, M. Advanced oxidation protein products: A novel marker of oxidative stress in ulcerative colitis. J. Clin. Gastroenterol. 2008, 42, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhong, Z.M.; Pan, Y.; Zeng, J.H.; Zheng, S.; Zhu, S.Y.; Chen, J.T. Advanced Oxidation Protein Products as a Novel Marker of Oxidative Stress in Postmenopausal Osteoporosis. Med. Sci. Monit. 2015, 21, 2428–2432. [Google Scholar] [PubMed] [Green Version]
- Dinkova-Kostova, A.T.; Abramov, A.V. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 2015, 88, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Chambel, S.S.; Santos-Gonçalves, A.; Duarte, T.L. The dual role of Nrf2 in nonalcoholic fatty liver disease: Regulation of antioxidant defenses and hepatic lipid metabolism. BioMed Res. Int. 2015, 2015, 597134. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Ferreira, J.C.; Gross, E.R.; Mochly-Rosen, D. Targeting aldehyde dehydrogenase 2: New theraprutic opportunities. Physiol. Rev. 2014, 94, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Paniselló-Roselló, A.; Lopez, A.; Folch-Puy, E.; Carbonell, T.; Rolo, A.; Palmeira, C.; Adam, R.; Net, M.; Roselló-Catafau, J. Role of aldehyde dehydrogenase 2 in ischemia reperfusion injury: An update. World J. Gastroenterol. 2018, in press. [Google Scholar]
- Chen, C.H.; Budas, G.R.; Churchill, E.N.; Disatnik, M.H.; Hurley, T.D.; Mochly-Rosen, D. Activation of aldehyde dehydrogenase 2 reduces ischemic damage to heart. Science 2008, 321, 1493–1495. [Google Scholar] [CrossRef] [PubMed]
- Budas, G.R.; Disatnik, M.H.; Mochly-Rosen, D. Aldehydedehydrogenase 2 in cardiac protection: A new therapeutic target? Trend Cardiovasc. Med. 2009, 19, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.H.; Zhang, H.F.; Yang, Z.B.; Li, T.B.; Liu, B.; Lou, Z.; Ma, Q.L.; Luo, X.J.; Peng, J. Alda 1 reduces cerebral ischemia-reperfusion injury in rat through clearance of reactive aldehydes. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 385, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; He, G.; Wang, J.; Wang, Y.; Chen, W. Pretreatment with the ALDH2 agonist Alda-1 reduces intestinal injury induced by ischemia-reperfusion in mice. Clin. Sci. 2017, 131, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhao, Q.; Ye, F.; Huang, C.Y.; Chen, W.M.; Huang, W.Q. Alda-1, an ALDH2 activator, protects against hepatic/ischemia reperfusion in rats injury via inhibition of oxidative stress. Free Radic. Res. 2018, 52, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Yamamoto, M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzyme Regul. 2006, 46, 113–140. [Google Scholar] [CrossRef] [PubMed]
- Kudoh, K.; Uchinami, H.; Yoshioka, M.; Seki, E.; Yamamoto, Y. Nrf2 activation protects the liver from ischemia/reperfusion injury in mice. Ann. Surg. 2014, 260, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, M.; Pantazi, E.; Folch-Puy, E.; Baptista, P.M.; Garcia-Gil, A.; Adam, R.; Roselló-Catafau, J. Emerging concepts in liver graft preservation. World J. Gastroenterol. 2015, 21, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Muller, X.; Dutkowski, P. Hypothermic machine preservation of the liver: State of the art. Curr. Transplant. Rep. 2018, 5, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Panisello-Roselló, A.; Lopez, A.; Castro-Benítez, C.; Balloji, S.; Folch-Puy, E.; Rolo, A.; Palmeira, C.; Adam, R.; Roselló-Catafau, J. Involvement of ALDH2 in fatty liver graft protection mechanisms against cold ischemic injury. In Proceedings of the American Transplantation Congress, Seattle, WA, USA, 2–6 June 2018. [Google Scholar]
- Ma, H.; Guo, R.; Lu, L.; Zhang, Y.; Ren, J. Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischemia reperfusion injury: Role of autophagy and toxic aldehyde paradox. Eur. Heart J. 2011, 32, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Peralta, C.; Roselló-Catafau, J. The future of fatty livers. J. Hepatol. 2004, 41, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Lemasters, J.J.; Thurman, R.G. Reperfusion injury after liver preservation for transplantation. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Montalvo-Jave, E.E.; Escalante-Tattersfield, T.; Ortega-salgado, J.A.; Pina, E.; Geller, D.A. Factors in the pathophysiology of liver ischemia-reperfusion injury. J. Surg. Res. 2008, 147, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Negre-Salvayre, A.; Coatrieux, C.; Ingueneau, C.; Salvayre, R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br. J. Pharmacol. 2008, 153, 6–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aberle, N.S.; Picklo, M.J.; Amarnath, V.; Ren, J. Inhibition of cardiac myocyte contraction by 4-hydroxy-trans-2-nonenal. Cardiovasc. Toxicol. 2004, 4, 21–28. [Google Scholar] [CrossRef]
- Perluigi, M.; Coccia, R.; Butterfield, D.A. 4-Hydroxy-2-nonenal, a reactive product of lipid peroxidation, and neurodegenerative diseases: A toxic combination illuminated by redox proteomics studies. Antioxid. Redox Signal. 2012, 17, 1590–1609. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.M.; Liu, A.J.; Zang, P.; Dong, W.Z.; Ying, L.; Wang, W.; Xu, P.; Song, X.R.; Cai, J.; Zhang, S.Q.; et al. ALDH2 protects against stroke by clearing 4-HNE. Cell Res. 2013, 23, 915–930. [Google Scholar] [CrossRef] [PubMed]
- Hermes-Lima, M.; Moreira, D.C.; Rivera-Ingraham, G.A.; Giraud-Billoud, M.; Genaro-Mattos, T.C.; Campos, É.G. Preparation for oxidative stress under hypoxia and metabolic depression: Revisiting the proposal two decades later. Free Radic. Biol. Med. 2015, 89, 1122–1143. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Luo, Q.; Zhu, H.; Liu, X.; Dong, Z.K.; Wu, J.; Ge, J.; Sun, A. Aldehyde dehydrogenase 2 activation ameliorates CCl4-induced chronic liver fibrosis in mice by up-regulating Nrf2/HO-1 antioxidant pathway. J. Cell. Mol. Med. 2018, 22. [Google Scholar] [CrossRef] [PubMed]
- Zaouali, M.A.; Abdennebi, H.B.; Padrissa-Altés, S.; Alfany-Fernández, I.; Rimola, A.; Roselló-Catafau, J. How Institut Georges Lopez preservation solution protects steatotic and non steatotic liver against ischemia-reperfsuion injury. Transplant. Proc. 2011, 43, 77–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaouali, M.A.; Bejaoui, M.; Calvo, M.; Folch-Puy, E.; Pantazi, E.; Pasut, G.; Rimola, A.; Abdennebi, H.B.; Adam, R.; Roselló-Catafau, J. Polyethylene glycol rinse solution: An effective way to prevent ischemia-reperfusion injury. World J. Gastroenterol. 2014, 20, 16203–16214. [Google Scholar] [CrossRef] [PubMed]
- Pasut, G.; Panisello, A.; Folch-Puy, E.; Lopez, A.; Castro-Benítez, C.; Calvo, M.; Carbonell, T.; García-Gil, A.; Adam, R.; Roselló-Catafau, J. Polyethylene glycols: An effective strategy for limiting liver ischemia reperfusion injury. World J. Gastroenterol. 2016, 22, 6501–6508. [Google Scholar] [CrossRef] [PubMed]
- Panisello-Roselló, A.; Verde, E.; Lopez, A.; Flores, M.; Folch-Puy, E.; Rolo, A.; Palmeira, C.; Hotter, G.; Carbonell, T.; Adam, R.; et al. Cytoprotective Mechanisms in Fatty Liver Preservation against Cold Ischemia Injury: A Comparison between IGL-1 and HTK. Int. J. Mol. Sci. 2018, 19, 348. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Panisello-Rosello, A.; Castro-Benitez, C.; Adam, R. Glycocalix preservation and NO production in fatty livers-The protective role of high molecular polyethylene glycol in cold ischemia injury. Int. J. Mol. Sci. 2018, 19, 2375. [Google Scholar] [CrossRef] [PubMed]






| Component | IGL-1 | UW | HTK | |
|---|---|---|---|---|
| Colloids (mmol/L) | PEG35 | 0.03 | ||
| HES | 0.25 | |||
| Antioxidants (mmol/L) | Glutathione | 3 | 3 | |
| Precursors (mmol/L) | Adenosine | 5 | 5 | |
| Ketoglutarate | 1 | |||
| Buffers (mmol/L) | Diphosphate | 25 | 25 | |
| Histidine | 198 | |||
| Histidine-HCl | 18 | |||
| Tryptophan | 2 | |||
| Electrolytes (mmol/L) | K+ | 25 | 125 | 10 |
| Na+ | 120 | 27 | 15 | |
| Mg2+ | 5 | 4 | ||
| Cl− | 50 | |||
| SO42− | 5 | 4 | ||
| Ca2+ | 0.5 | 0.015 | ||
| Impermeants (mmol/L) | Raffinose | 30 | 30 | |
| Lactobionic acid | 100 | 105 | ||
| Mannitol | 30 | |||
| Osmolarity (mOs mol/l) | 290 | 320 | 310 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panisello-Roselló, A.; Alva, N.; Flores, M.; Lopez, A.; Castro Benítez, C.; Folch-Puy, E.; Rolo, A.; Palmeira, C.; Adam, R.; Carbonell, T.; et al. Aldehyde Dehydrogenase 2 (ALDH2) in Rat Fatty Liver Cold Ischemia Injury. Int. J. Mol. Sci. 2018, 19, 2479. https://doi.org/10.3390/ijms19092479
Panisello-Roselló A, Alva N, Flores M, Lopez A, Castro Benítez C, Folch-Puy E, Rolo A, Palmeira C, Adam R, Carbonell T, et al. Aldehyde Dehydrogenase 2 (ALDH2) in Rat Fatty Liver Cold Ischemia Injury. International Journal of Molecular Sciences. 2018; 19(9):2479. https://doi.org/10.3390/ijms19092479
Chicago/Turabian StylePanisello-Roselló, Arnau, Norma Alva, Marta Flores, Alexandre Lopez, Carlos Castro Benítez, Emma Folch-Puy, Anabela Rolo, Carlos Palmeira, René Adam, Teresa Carbonell, and et al. 2018. "Aldehyde Dehydrogenase 2 (ALDH2) in Rat Fatty Liver Cold Ischemia Injury" International Journal of Molecular Sciences 19, no. 9: 2479. https://doi.org/10.3390/ijms19092479
APA StylePanisello-Roselló, A., Alva, N., Flores, M., Lopez, A., Castro Benítez, C., Folch-Puy, E., Rolo, A., Palmeira, C., Adam, R., Carbonell, T., & Roselló-Catafau, J. (2018). Aldehyde Dehydrogenase 2 (ALDH2) in Rat Fatty Liver Cold Ischemia Injury. International Journal of Molecular Sciences, 19(9), 2479. https://doi.org/10.3390/ijms19092479