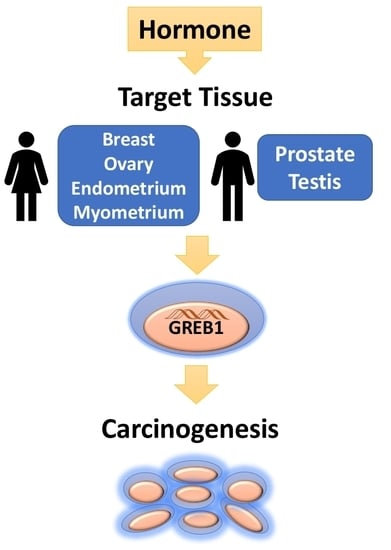
Role for Growth Regulation by Estrogen in Breast Cancer 1 (GREB1) in Hormone-Dependent Cancers
Abstract
:1. Hormone-Dependent Cancers
2. Nuclear Receptor Coactivators in Hormone-Dependent Cancers
3. GREB1 Structure and Function
4. Hormone Regulation of GREB1
5. Role of GREB1 in Breast Cancer
6. Role of GREB1 in Ovarian Cancer
7. Role of GREB1 in Prostate Cancer
8. The Emerging Role for GREB1 in Other Hormone-Dependent Cancers
9. Translational Relevance
10. Conclusions and Open Questions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Activation Domain |
| AR | Androgen Receptors |
| ARE | Androgen Response Element |
| b-HLH-PAS | Basic Helix-Loop-Helix-Per/ARNT/Sim |
| CCLE | Cancer Cell Line Encyclopedia |
| ChIP | Chromatin Immunoprecipitation |
| ER | Estrogen Receptors |
| ERE | Estrogen Response Element |
| GREB1 | Growth Regulation by Estrogen in Breast Cancer 1 |
| HR | Hormone Receptors |
| HRE | Hormone Response Element |
| LBD | Ligand Binding Domain |
| LNCaP cell | Lymph Node Carcinoma of the Prostate cell |
| LXXLL | Leu-X-X-Leu-Leu |
| MAS cell | Mouse Ovarian Cancer Ascites Cell |
| NCOA2 | Nuclear Receptor Coactivator 2 |
| PR | Progesterone Receptors |
| SERM | Selective Estrogen Receptor Modulator |
| SF II | Superfamily II |
| SRC | Steroid Receptor Coactivator |
| TAGT | TET/JBP-Associated Glycosyltransferase |
| TET/JBP | Ten-Eleven Translocation/J Binding Protein |
References
- Miller, W.L.; Bose, H.S. Early steps in steroidogenesis: Intracellular cholesterol trafficking. J. Lipid Res. 2011, 52, 2111–2135. [Google Scholar] [CrossRef] [PubMed]
- Marceau, K.; Ruttle, P.L.; Shirtcliff, E.A.; Essex, M.J.; Susman, E.J. Developmental and contextual considerations for adrenal and gonadal hormone functioning during adolescence: Implications for adolescent mental health. Dev. Psychobiol. 2015, 57, 742–768. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, J.C.; Srinivasan, S.; Zheng, Y.; Wang, S.; Min, J.; Dong, C.; Liao, Z.; Nowak, J.; Wright, N.J.; Houtman, R.; et al. Predictive features of ligand-specific signaling through the estrogen receptor. Mol. Syst. Biol. 2016, 12, 864. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.H.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E.L. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Eyster, K.M. Estrogen Receptor; Springer: Hertfordshire, UK, 2016. [Google Scholar]
- Chuffa, L.G.; Lupi-Junior, L.A.; Costa, A.B.; Amorim, J.P.; Seiva, F.R. The role of sex hormones and steroid receptors on female reproductive cancers. Steroids 2017, 118, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Johnston, R.; Campbell, D.C.; Nugent, S.; McDade, S.S.; Waugh, D.; Panov, K.I. Androgens and estrogens stimulate ribosome biogenesis in prostate and breast cancer cells in receptor dependent manner. Gene 2013, 526, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Dall, G.V.; Hawthorne, S.; Seyed-Razavi, Y.; Vieusseux, J.; Wu, W.; Gustafsson, J.A.; Byrne, D.; Murphy, L.; Risbridger, G.P.; Britt, K.L. Estrogen receptor subtypes dictate the proliferative nature of the mammary gland. J. Endocrinol. 2018, 237, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Strom, A.; Vega, V.B.; Kong, S.L.; Yeo, A.L.; Thomsen, J.S.; Chan, W.C.; Doray, B.; Bangarusamy, D.K.; Ramasamy, A.; et al. Discovery of estrogen receptor alpha target genes and response elements in breast tumor cells. Genome Biol. 2004, 5, R66. [Google Scholar] [CrossRef] [PubMed]
- Rae, J.M.; Johnson, M.D.; Scheys, J.O.; Cordero, K.E.; Larios, J.M.; Lippman, M.E. GREB 1 is a critical regulator of hormone dependent breast cancer growth. Breast Cancer Res. Treat. 2005, 92, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Bogush, T.A.; Polezhaev, B.B.; Mamichev, I.A.; Bogush, E.A.; Polotsky, B.E.; Tjulandin, S.A.; Ryabov, A.B. Tamoxifen Never Ceases to Amaze: New Findings on Non-Estrogen Receptor Molecular Targets and Mediated Effects. Cancer Investig. 2018, 36, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Singletary, S.E.; Allred, C.; Ashley, P.; Bassett, L.W.; Berry, D.; Bland, K.I.; Borgen, P.I.; Clark, G.; Edge, S.B.; Hayes, D.F.; et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J. Clin. Oncol. 2002, 20, 3628–3636. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, L.H.; Schnack, T.H.; Buchardi, K.; Hummelshoj, L.; Missmer, S.A.; Forman, A.; Blaakaer, J. Risk factors of epithelial ovarian carcinomas among women with endometriosis: A systematic review. Acta Obstet. Gynecol. Scand. 2017, 96, 761–778. [Google Scholar] [CrossRef] [PubMed]
- Webb, P.M.; Jordan, S.J. Epidemiology of epithelial ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, F.; Zhang, X.; Zhang, Y.; Ding, J.; Chen, Q. Hormone receptors expression in ovarian cancer taking into account menopausal status: A retrospective study in Chinese population. Oncotarget 2017, 8, 84019–84027. [Google Scholar] [CrossRef] [PubMed]
- Laviolette, L.A.; Hodgkinson, K.M.; Minhas, N.; Perez-Iratxeta, C.; Vanderhyden, B.C. 17β-Estradiol upregulates GREB1 and accelerates ovarian tumor progression in vivo. Int. J. Cancer Suppl. 2014, 135, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Spillman, M.A.; Manning, N.G.; Dye, W.W.; Sartorius, C.A.; Post, M.D.; Harrell, J.C.; Jacobsen, B.M.; Horwitz, K.B. Tissue-specific pathways for estrogen regulation of ovarian cancer growth and metastasis. Cancer Res. 2010, 70, 8927–8936. [Google Scholar] [CrossRef] [PubMed]
- Attard, G.; Parker, C.; Eeles, R.A.; Schroder, F.; Tomlins, S.A.; Tannock, I.; Drake, C.G.; de Bono, J.S. Prostate cancer. Lancet 2016, 387, 70–82. [Google Scholar] [CrossRef]
- Ojo, D.; Wei, F.; Liu, Y.; Wang, E.; Zhang, H.; Lin, X.; Wong, N.; Bane, A.; Tang, D. Factors Promoting Tamoxifen Resistance in Breast Cancer via Stimulating Breast Cancer Stem Cell Expansion. Curr. Med. Chem. 2015, 22, 2360–2374. [Google Scholar] [CrossRef] [PubMed]
- Deschenes, J.; Bourdeau, V.; White, J.H.; Mader, S. Regulation of GREB1 transcription by estrogen receptor alpha through a multipartite enhancer spread over 20 kb of upstream flanking sequences. J. Biol. Chem. 2007, 282, 17335–17339. [Google Scholar] [CrossRef] [PubMed]
- Onate, S.A.; Tsai, S.Y.; Tsai, M.J.; O’Malley, B.W. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 1995, 270, 1354–1357. [Google Scholar] [PubMed]
- Dasgupta, S.; Lonard, D.M.; O’Malley, B.W. Nuclear receptor coactivators: Master regulators of human health and disease. Annu. Rev. Med. 2014, 65, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Savkur, R.S.; Burris, T.P. The coactivator LXXLL nuclear receptor recognition motif. J. Pept. Res. 2004, 63, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.B.; O’Malley, B.W. Steroid receptor coactivators 1, 2, and 3: Critical regulators of nuclear receptor activity and steroid receptor modulator (SRM)-based cancer therapy. Mol. Cell. Endocrinol. 2012, 348, 430–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Duan, C.; Bian, C.; Xiong, Y.; Zhang, J. Steroid receptor coactivator-1: A versatile regulator and promising therapeutic target for breast cancer. J. Steroid Biochem. Mol. Biol. 2013, 138, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, C.; Zhao, M.; Chen, H.; Liu, X.; Chen, J.; Lonard, D.M.; Qin, L.; Xu, J.; Wang, X.; et al. Steroid Receptor Coactivator-3 (SRC-3/AIB1) as a Novel Therapeutic Target in Triple Negative Breast Cancer and Its Inhibition with a Phospho-Bufalin Prodrug. PLoS ONE 2015, 10, e0140011. [Google Scholar] [CrossRef] [PubMed]
- Fenne, I.S.; Helland, T.; Flageng, M.H.; Dankel, S.N.; Mellgren, G.; Sagen, J.V. Downregulation of steroid receptor coactivator-2 modulates estrogen-responsive genes and stimulates proliferation of MCF-7 breast cancer cells. PLoS ONE 2013, 8, e70096. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Wu, Y.L.; Toneff, M.J.; Li, D.; Liao, L.; Gao, X.; Bane, F.T.; Tien, J.C.; Xu, Y.; Feng, Z.; et al. NCOA1 Directly Targets M-CSF1 Expression to Promote Breast Cancer Metastasis. Cancer Res. 2014, 74, 3477–3488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lydon, J.P.; O’Malley, B.W. Minireview: Steroid receptor coactivator-3: A multifarious coregulator in mammary gland metastasis. Endocrinology 2011, 152, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Fleming, F.J.; Myers, E.; Kelly, G.; Crotty, T.B.; McDermott, E.W.; O’Higgins, N.J.; Hill, A.D.; Young, L.S. Expression of SRC-1, AIB1, and PEA3 in HER2 mediated endocrine resistant breast cancer; a predictive role for SRC-1. J. Clin. Pathol. 2004, 57, 1069–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, H.; Liu, J.; Samuel, S.; Cheng, W.; Rosen, D.; Naora, H. Steroid receptor coactivator-3, a homolog of Taiman that controls cell migration in the Drosophila ovary, regulates migration of human ovarian cancer cells. Mol. Cell. Endocrinol. 2005, 245, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, C.; Gojis, O.; Rudraraju, B.; Stamp-Vincent, C.; Wilson, D.; Langdon, S.; Gourley, C.; Faratian, D. Expression of steroid receptor coactivator 3 in ovarian epithelial cancer is a poor prognostic factor and a marker for platinum resistance. Br. J. Cancer 2013, 108, 2039–2044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakaguchi, H.; Fujimoto, J.; Sun, W.S.; Tamaya, T. Clinical implications of steroid receptor coactivator (SRC)-3 in uterine endometrial cancers. J. Steroid Biochem. Mol. Biol. 2007, 104, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Culig, Z.; Santer, F.R. Studies on Steroid Receptor Coactivators in Prostate Cancer. Methods Mol. Biol. 2018, 1786, 259–262. [Google Scholar] [PubMed]
- Dasgupta, S.; Putluri, N.; Long, W.; Zhang, B.; Wang, J.; Kaushik, A.K.; Arnold, J.M.; Bhowmik, S.K.; Stashi, E.; Brennan, C.A.; et al. Coactivator SRC-2-dependent metabolic reprogramming mediates prostate cancer survival and metastasis. J. Clin. Investg. 2015, 125, 1174–1188. [Google Scholar] [CrossRef] [PubMed]
- Agoulnik, I.U.; Vaid, A.; Bingman, W.E., 3rd; Erdeme, H.; Frolov, A.; Smith, C.L.; Ayala, G.; Ittmann, M.M.; Weigel, N.L. Role of SRC-1 in the promotion of prostate cancer cell growth and tumor progression. Cancer Res. 2005, 65, 7959–7967. [Google Scholar] [CrossRef] [PubMed]
- Tien, J.C.; Liu, Z.; Liao, L.; Wang, F.; Xu, Y.; Wu, Y.L.; Zhou, N.; Ittmann, M.; Xu, J. The steroid receptor coactivator-3 is required for the development of castration-resistant prostate cancer. Cancer Res. 2013, 73, 3997–4008. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Luo, X.; Nie, P.; Wu, B.; Zhang, T.; Wei, Y.; Wang, W.; Geng, G.; Jiang, J.; Mi, Y. Inhibition of SRC-3 enhances sensitivity of human cancer cells to histone deacetylase inhibitors. Biochem. Biophys. Res. Commun. 2016, 478, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, A.K.; Foulds, C.E.; Lanz, R.B.; Hamilton, R.A.; Yi, P.; Lonard, D.M.; Tsai, M.J.; Tsai, S.Y.; O’Malley, B.W. SRC-3 Coactivator Governs Dynamic Estrogen-Induced Chromatin Looping Interactions during Transcription. Mol. Cell 2018, 70, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Camden, A.J.; Szwarc, M.M.; Chadchan, S.B.; DeMayo, F.J.; O’Malley, B.W.; Lydon, J.P.; Kommagani, R. Growth regulation by estrogen in breast cancer 1 (GREB1) is a novel progesterone-responsive gene required for human endometrial stromal decidualization. Mol. Hum. Reprod. 2017, 23, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, M.; Panagopoulos, I.; Gorunova, L.; Davidson, B.; Heim, S.; Micci, F. RNA-sequencing identifies novel GREB1-NCOA2 fusion gene in a uterine sarcoma with the chromosomal translocation t(2;8)(p25;q13). Gene. Chromosome Cancer 2018, 57, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Nagase, T.; Ishikawa, K.; Miyajima, N.; Tanaka, A.; Kotani, H.; Nomura, N.; Ohara, O. Prediction of the coding sequences of unidentified human genes. IX. The complete sequences of 100 new cDNA clones from brain which can code for large proteins in vitro. DNA Res. 1998, 5, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.G.; Thompson, D.A.; Weigel, R.J. PDZK1 and GREB1 are estrogen-regulated genes expressed in hormone-responsive breast cancer. Cancer Res. 2000, 60, 6367–6375. [Google Scholar] [PubMed]
- Rae, J.M.; Johnson, M.D.; Cordero, K.E.; Scheys, J.O.; Larios, J.M.; Gottardis, M.M.; Pienta, K.J.; Lippman, M.E. GREB1 is a novel androgen-regulated gene required for prostate cancer growth. Prostate 2006, 66, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/ (accessed on 20 May 2018).
- Haines, C.N.; Braunreiter, K.M.; Mo, X.M.; Burd, C.J. GREB1 isoforms regulate proliferation independent of ERalpha co-regulator activities in breast cancer. Endocr. Relat. Cancer 2018, 25, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Iyer, L.M.; Zhang, D.; Burroughs, A.M.; Aravind, L. Computational identification of novel biochemical systems involved in oxidation, glycosylation and other complex modifications of bases in DNA. Nucleic Acids Res. 2013, 41, 7635–7655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drake, R.R. Glycosylation and cancer: Moving glycomics to the forefront. Adv. Cancer Res. 2015, 126, 1–10. [Google Scholar] [PubMed]
- Singh, C.; Shyanti, R.K.; Singh, V.; Kale, R.K.; Mishra, J.P.N.; Singh, R.P. Integrin expression and glycosylation patterns regulate cell-matrix adhesion and alter with breast cancer progression. Biochem. Biophys. Res. Commun. 2018, 499, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Milde-Langosch, K.; Karn, T.; Schmidt, M.; Zu Eulenburg, C.; Oliveira-Ferrer, L.; Wirtz, R.M.; Schumacher, U.; Witzel, I.; Schutze, D.; Muller, V. Prognostic relevance of glycosylation-associated genes in breast cancer. Breast Cancer Res. Treat. 2014, 145, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Ashkani, J.; Naidoo, K.J. Glycosyltransferase Gene Expression Profiles Classify Cancer Types and Propose Prognostic Subtypes. Sci. Rep. 2016, 6, 26451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plevin, M.J.; Mills, M.M.; Ikura, M. The LxxLL motif: A multifunctional binding sequence in transcriptional regulation. Trends Biochem. Sci. 2005, 30, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.; D’Santos, C.; Serandour, A.A.; Ali, H.R.; Brown, G.D.; Atkins, A.; Rueda, O.M.; Holmes, K.A.; Theodorou, V.; Robinson, J.L.; et al. Endogenous purification reveals GREB1 as a key estrogen receptor regulatory factor. Cell Rep. 2013, 3, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Nawaz, Z.; Slingerland, J.M. Long-range activation of GREB1 by estrogen receptor via three distal consensus estrogen-responsive elements in breast cancer cells. Mol. Endocrinol. 2007, 21, 2651–2662. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, K.; Forrest, L.A.; Vuong, N.; Garson, K.; Djordjevic, B.; Vanderhyden, B.C. GREB1 is an estrogen receptor-regulated tumour promoter that is frequently expressed in ovarian cancer. Oncogene 2018. [Google Scholar] [CrossRef] [PubMed]
- Stute, P.; Sielker, S.; Wood, C.E.; Register, T.C.; Lees, C.J.; Dewi, F.N.; Williams, J.K.; Wagner, J.D.; Stefenelli, U.; Cline, J.M. Life stage differences in mammary gland gene expression profile in non-human primates. Breast Cancer Res. Treat. 2012, 133, 617–634. [Google Scholar] [CrossRef] [PubMed]
- Haakensen, V.D.; Bjoro, T.; Luders, T.; Riis, M.; Bukholm, I.K.; Kristensen, V.N.; Troester, M.A.; Homen, M.M.; Ursin, G.; Borresen-Dale, A.L.; et al. Serum estradiol levels associated with specific gene expression patterns in normal breast tissue and in breast carcinomas. BMC Cancer 2011, 11, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourdeau, V.; Deschenes, J.; Metivier, R.; Nagai, Y.; Nguyen, D.; Bretschneider, N.; Gannon, F.; White, J.H.; Mader, S. Genome-wide identification of high-affinity estrogen response elements in human and mouse. Mol. Endocrinol. 2004, 18, 1411–1427. [Google Scholar] [CrossRef] [PubMed]
- Fung, J.N.; Holdsworth-Carson, S.J.; Sapkota, Y.; Zhao, Z.Z.; Jones, L.; Girling, J.E.; Paiva, P.; Healey, M.; Nyholt, D.R.; Rogers, P.A.; et al. Functional evaluation of genetic variants associated with endometriosis near GREB1. Hum. Rep. 2015, 30, 1263–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kariagina, A.; Xie, J.; Leipprandt, J.R.; Haslam, S.Z. Amphiregulin mediates estrogen, progesterone, and EGFR signaling in the normal rat mammary gland and in hormone-dependent rat mammary cancers. Horm. Cancer 2010, 1, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi, H.; Quesnel-Crooks, S.; Sherman, R.; Joseph, R.; Kohler, B.; Andall-Brereton, G.; Ivey, M.A.; Edwards, B.K.; Mery, L.; Gawryszewski, V.; et al. Leading Causes of Cancer Mortality—Caribbean Region, 2003–2013. Morb. Mortal. Wkly. Rep. 2016, 65, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Dunbier, A.K.; Anderson, H.; Ghazoui, Z.; Folkerd, E.J.; A’Hern, R.; Crowder, R.J.; Hoog, J.; Smith, I.E.; Osin, P.; Nerurkar, A.; et al. Relationship between plasma estradiol levels and estrogen-responsive gene expression in estrogen receptor-positive breast cancer in postmenopausal women. J. Clin. Oncol. 2010, 28, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Yau, C.; Fedele, V.; Roydasgupta, R.; Fridlyand, J.; Hubbard, A.; Gray, J.W.; Chew, K.; Dairkee, S.H.; Moore, D.H.; Schittulli, F.; et al. Aging impacts transcriptomes but not genomes of hormone-dependent breast cancers. Breast Cancer Res. 2007, 9, R59. [Google Scholar] [CrossRef] [PubMed]
- Hnatyszyn, H.J.; Liu, M.; Hilger, A.; Herbert, L.; Gomez-Fernandez, C.R.; Jorda, M.; Thomas, D.; Rae, J.M.; El-Ashry, D.; Lippman, M.E. Correlation of GREB1 mRNA with protein expression in breast cancer: Validation of a novel GREB1 monoclonal antibody. Breast Cancer Res. Treat. 2010, 122, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, Z.; Cenciarini, M.E.; Proietti, C.J.; Amasino, M.F.; Hong, T.; Yang, M.; Liao, Y.; Chiang, H.C.; Kaklamani, V.; et al. Tamoxifen resistance in breast cancer is regulated by the EZH2-ERalpha-GREB1 transcriptional axis. Cancer Res. 2017. [Google Scholar] [CrossRef]
- Peres, L.C.; Cushing-Haugen, K.L.; Kobel, M.; Harris, H.R.; Berchuck, A.; Rossing, M.A.; Schildkraut, J.M.; Doherty, J.A. Invasive Epithelial Ovarian Cancer Survival by Histotype and Disease Stage. J. Natl. Cancer Inst. 2018. [Google Scholar] [CrossRef] [PubMed]
- National Collaborating Centre for Cancer (UK). Ovarian Cancer: The Recognition and Initial Management of Ovarian Cancer; National Collaborating Centre for Cancer: Cardiff, UK, 2011. [Google Scholar]
- Committee on the State of the Science in Ovarian Cancer Research; Board on Health Care Services; Institute of Medicine; National Academies of Sciences and Medicine. Ovarian Cancers: Evolving Paradigms in Research and Care; National Academies Press (US): Washington, DC, USA, 2016. [Google Scholar]
- Langdon, S.P.; Hirst, G.L.; Miller, E.P.; Hawkins, R.A.; Tesdale, A.L.; Smyth, J.F.; Miller, W.R. The regulation of growth and protein expression by estrogen in vitro: A study of 8 human ovarian carcinoma cell lines. J. Steroid Biochem. Mol. Biol. 1994, 50, 131–135. [Google Scholar] [CrossRef]
- Brawley, O.W. Prostate cancer epidemiology in the United States. World J. Urol. 2012, 30, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Pernar, C.H.; Ebot, E.M.; Wilson, K.M.; Mucci, L.A. The Epidemiology of Prostate Cancer. Cold Spring Harb. Perspect. Med. 2003, 30, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Stephen Nussey, S.W. Endocrinology: An Integrated Approach; BIOS Scientific Publishers: Oxford, UK, 2001. [Google Scholar]
- Banerjee, P.P.; Banerjee, S.; Brown, T.R.; Zirkin, B.R. Androgen action in prostate function and disease. Am. J. Clin. Exp. Urol. 2018, 6, 62–77. [Google Scholar] [PubMed]
- Ferreira, L.B.; Palumbo, A.; de Mello, K.D.; Sternberg, C.; Caetano, M.S.; de Oliveira, F.L.; Neves, A.F.; Nasciutti, L.E.; Goulart, L.R.; Gimba, E.R. PCA3 noncoding RNA is involved in the control of prostate-cancer cell survival and modulates androgen receptor signaling. BMC Cancer 2012, 12, 507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, J.H.; Aguilera-Barrantes, I.; Shiau, C.W.; Sheng, X.; Wang, L.S.; Stoner, G.D.; Huang, Y.W. Urolithin A suppresses the proliferation of endometrial cancer cells by mediating estrogen receptor-alpha-dependent gene expression. Mol. Nutr. Food Res. 2016, 60, 2387–2395. [Google Scholar] [CrossRef] [PubMed]
- Painter, J.N.; O’Mara, T.A.; Morris, A.P.; Cheng, T.H.T.; Gorman, M.; Martin, L.; Hodson, S.; Jones, A.; Martin, N.G.; Gordon, S.; et al. Genetic overlap between endometriosis and endometrial cancer: Evidence from cross-disease genetic correlation and GWAS meta-analyses. Cancer Med. 2018, 7, 1978–1987. [Google Scholar] [CrossRef] [PubMed]
- Anglesio, M.S.; Papadopoulos, N.; Ayhan, A.; Nazeran, T.M.; Noe, M.; Horlings, H.M.; Lum, A.; Jones, S.; Senz, J.; Seckin, T.; et al. Cancer-Associated Mutations in Endometriosis without Cancer. N. Engl. J. Med. 2017, 376, 1835–1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrini, C.; Gori, I.; Achtari, C.; Hornung, D.; Chardonnens, E.; Wunder, D.; Fiche, M.; Canny, G.O. The expression of estrogen receptors as well as GREB1, c-MYC, and cyclin D1, estrogen-regulated genes implicated in proliferation, is increased in peritoneal endometriosis. Fertil. Steril. 2012, 98, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, Y.; Fassbender, A.; Bowdler, L.; Fung, J.N.; Peterse, D.; O, D.; Montgomery, G.W.; Nyholt, D.R.; D’Hooghe, T.M. Independent Replication and Meta-Analysis for Endometriosis Risk Loci. Twin Res. Hum. Genet. 2015, 18, 518–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapkota, Y.; Vivo, I.; Steinthorsdottir, V.; Fassbender, A.; Bowdler, L.; Buring, J.E.; Edwards, T.L.; Jones, S.; O, D.; Peterse, D.; et al. Analysis of potential protein-modifying variants in 9000 endometriosis patients and 150000 controls of European ancestry. Sci. Rep. 2017, 7, 11380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahmioglu, N.; Nyholt, D.R.; Morris, A.P.; Missmer, S.A.; Montgomery, G.W.; Zondervan, K.T. Genetic variants underlying risk of endometriosis: Insights from meta-analysis of eight genome-wide association and replication datasets. Hum. Reprod. Update 2014, 20, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, K.G.; Drummond, F.J.; Daly, M.; Shanahan, F.; Molloy, M.G. GREB1 genetic variants are associated with bone mineral density in Caucasians. J. Bone Miner. Metab. 2018, 36, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Cronin, K.A.; Lake, A.J.; Scott, S.; Sherman, R.L.; Noone, A.M.; Howlader, N.; Henley, S.J.; Anderson, R.N.; Firth, A.U.; Ma, J.; et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018. [Google Scholar] [CrossRef] [PubMed]
- Chern, J.Y.; Boyd, L.R.; Blank, S.V. Uterine Sarcomas: The Latest Approaches for These Rare but Potentially Deadly Tumors. Oncology 2017, 31, 229–236. [Google Scholar] [PubMed]
- Shores, M.M.; Matsumoto, A.M. Testosterone, aging and survival: Biomarker or deficiency. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhu, J.; Li, X.; Li, S.; Lan, Z.; Ko, J.; Lei, Z. Expression of genomic functional estrogen receptor 1 in mouse sertoli cells. Reprod. Sci. 2014, 21, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Lei, Z. Chromatin Immunoprecipitation with Estrogen Receptor 1 and the Promoter of Greb1 in TM4 Sertoli Cells. Methods Mol. Biol. 2016, 1366, 67–77. [Google Scholar] [PubMed]
- Sneddon, S.F.; Walther, N.; Saunders, P.T. Expression of androgen and estrogen receptors in sertoli cells: Studies using the mouse SK11 cell line. Endocrinology 2005, 146, 5304–5312. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.; Evans, J.; Brook, D.; Kenkre, J.; Jarvis, P.; Gower-Thomas, K. The Nottingham Prognostic Index: Five- and ten-year data for all-cause survival within a screened population. Ann. R. Coll. Surg. Engl. 2015, 97, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Bauerschlag, D.O.; Ammerpohl, O.; Brautigam, K.; Schem, C.; Lin, Q.; Weigel, M.T.; Hilpert, F.; Arnold, N.; Maass, N.; Meinhold-Heerlein, I.; et al. Progression-free survival in ovarian cancer is reflected in epigenetic DNA methylation profiles. Oncology 2011, 80, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Antunes, A.A.; Leite, K.R.; Reis, S.T.; Sousa-Canavez, J.M.; Camara-Lopes, L.H.; Dall’oglio, M.F.; Srougi, M. GREB1 tissue expression is associated with organ-confined prostate cancer. Urol. Oncol. 2012, 30, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.A.; Lange, C.A. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat. Res. 2008, 647, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Crowe, D.L. The histone methyltransferase EZH2 promotes mammary stem and luminal progenitor cell expansion, metastasis and inhibits estrogen receptor-positive cellular differentiation in a model of basal breast cancer. Oncol. Rep. 2015, 34, 455–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.A.; Yu, J. EZH2, an epigenetic driver of prostate cancer. Protein Cell 2013, 4, 331–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, B.A.; Varambally, S.; Arend, R.C. Histone Methyltransferase EZH2: A Therapeutic Target for Ovarian Cancer. Mol. Cancer Ther. 2018, 17, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Tsang, D.P.; Cheng, A.S. Epigenetic regulation of signaling pathways in cancer: Role of the histone methyltransferase EZH2. J. Gastroenterol. Hepatol. 2011, 26, 19–27. [Google Scholar] [CrossRef] [PubMed]


| Tissue Type and Expression | GREB1 | ER | AR | PR | ||||
|---|---|---|---|---|---|---|---|---|
| Normal | Cancer | Normal | Cancer | Normal | Cancer | Normal | Cancer | |
| Breast | + | + | ER-α + | ER-α + | + | + | + | + |
| ER-β − | ER-β + | |||||||
| Ovary | + | + | ER-α − | ER-α + | + | + | − | + |
| ER-β − | ER-β + | |||||||
| Endometrium | + | + | ER-α + | ER-α + | + | + | + | + |
| ER-β − | ER-β + | |||||||
| Myometrium of Uterus | + | ? | ER-α + | ER-α ? | + | ? | + | ? |
| ER-β − | ER-β ? | |||||||
| Prostate | + | + | ER-α − | ER-α + | − | + | − | + |
| ER-β − | ER-β + | |||||||
| Testis | + | + | ER-α − | ER-α + | + | + | − | + |
| ER-β + | ER-β + | |||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, M.; Michalski, S.; Kommagani, R. Role for Growth Regulation by Estrogen in Breast Cancer 1 (GREB1) in Hormone-Dependent Cancers. Int. J. Mol. Sci. 2018, 19, 2543. https://doi.org/10.3390/ijms19092543
Cheng M, Michalski S, Kommagani R. Role for Growth Regulation by Estrogen in Breast Cancer 1 (GREB1) in Hormone-Dependent Cancers. International Journal of Molecular Sciences. 2018; 19(9):2543. https://doi.org/10.3390/ijms19092543
Chicago/Turabian StyleCheng, Meng, Stephanie Michalski, and Ramakrishna Kommagani. 2018. "Role for Growth Regulation by Estrogen in Breast Cancer 1 (GREB1) in Hormone-Dependent Cancers" International Journal of Molecular Sciences 19, no. 9: 2543. https://doi.org/10.3390/ijms19092543
APA StyleCheng, M., Michalski, S., & Kommagani, R. (2018). Role for Growth Regulation by Estrogen in Breast Cancer 1 (GREB1) in Hormone-Dependent Cancers. International Journal of Molecular Sciences, 19(9), 2543. https://doi.org/10.3390/ijms19092543