Low Infection of Phelipanche aegyptiaca in Micro-Tom Mutants Deficient in CAROTENOID CLEAVAGE DIOXYGENASE 8
Abstract
:1. Introduction
2. Results
2.1. Screening for SlCCD8-Defective Micro-Tom Mutants
2.2. SL Levels Are Reduced in the slccd8 Mutants 5291 and 2757
2.3. Characterization of the Slccd8 Mutants 5291 and 2757
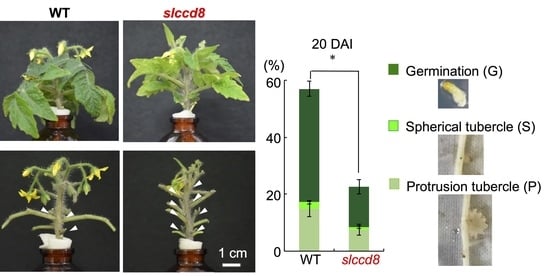
2.4. Phelipanche aegyptiaca Infection Is Decreased in Roots of Slccd8 Mutants
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Screening for slccd8 Mutants
4.3. CAPS Method
4.4. Analysis SlCCD8 Gene Expression
4.5. Chemicals
4.6. Quantitative Analysis of SLs by LC-MS/MS
4.7. Germination of Root-Parasitic Plant Seeds
4.8. Measurement of Membrane Ion Leakage and Chlorophyll Contents
4.9. Root Infection with Phelipanche aegyptiaca
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 4DO | 4-deoxyorobanchol |
| CAPS | cleaved amplified polymorphic sequence |
| CCD | carotenoid cleavage dioxygenase |
| CL | carlactone |
| DAI | day after innoculation |
| LC-MS/MS | liquid chromatography-tandem mass spectrometry |
| MS | Murashige and Skoog |
| N.D. | not detected |
| N.G. | no germination |
| n.s. | not significant |
| Pi | inorganic phosphate |
| S.E. | standard error |
| SL | strigolactone |
| TILLING | targeting-induced local lesions in genome |
| WT | wild-type |
References
- Steeves, T.A.; Sussex, I.M. Patterns in Plant Development, 2nd ed.; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- McSteen, P.; Leyser, O. Shoot branching. Annu. Rev. Plant Biol. 2005, 56, 353–374. [Google Scholar] [CrossRef] [PubMed]
- Ongaro, V.; Leyser, O. Hormonal control of shoot branching. J. Exp. Bot. 2008, 59, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pages, V.; Dun, E.A.; Pillot, J.P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.C.; et al. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.N.; Yoneyama, K.; Yoneyama, K. The strigolactone story. Annu. Rev. Phytopathol. 2010, 48, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Alder, A.; Jamil, M.; Marzorati, M.; Bruno, M.; Vermathen, M.; Bigler, P.; Ghisla, S.; Bouwmeester, H.; Beyer, P.; Al-Babili, S. The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 2012, 335, 1348–1351. [Google Scholar] [CrossRef] [PubMed]
- Seto, Y.; Sado, A.; Asami, K.; Hanada, A.; Umehara, M.; Akiyama, K.; Yamaguchi, S. Carlactone is an endogenous biosynthetic precursor for strigolactones. Proc. Natl. Acad. Sci. USA 2014, 111, 1640–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arite, T.; Iwata, H.; Ohshima, K.; Maekawa, M.; Nakajima, M.; Kojima, M.; Sakakibara, H.; Kyozuka, J. Dwarf10, an rms1/max4/dad1 ortholog, controls lateral bud outgrowth in rice. Plant J. 2007, 51, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Foo, E.; Bullier, E.; Goussot, M.; Foucher, F.; Rameau, C.; Beveridge, C.A. The branching gene RAMOSUS1 mediates interactions among two novel signals and auxin in pea. Plant Cell 2005, 17, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Sado, A.; Tanaka, K.; Kisugi, T.; Asami, K.; Ota, S.; Kim, H.I.; Yoneyama, K.; Xie, X.; Ohnishi, T.; et al. Carlactone is converted to carlactonoic acid by max1 in arabidopsis and its methyl ester can directly interact with atd14 in vitro. Proc. Natl. Acad. Sci. USA 2014, 111, 18084–18089. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; van Dijk, A.D.; Scaffidi, A.; Flematti, G.R.; Hofmann, M.; Charnikhova, T.; Verstappen, F.; Hepworth, J.; van der Krol, S.; Leyser, O.; et al. Rice cytochrome p450 max1 homologs catalyze distinct steps in strigolactone biosynthesis. Nat. Chem. Biol. 2014, 10, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Xie, X.; Kusumoto, D.; Sekimoto, H.; Sugimoto, Y.; Takeuchi, Y.; Yoneyama, K. Nitrogen deficiency as well as phosphorus deficiency in sorghum promotes the production and exudation of 5-deoxystrigol, the host recognition signal for arbuscular mycorrhizal fungi and root parasites. Planta 2007, 227, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Yoneyama, K.; Takeuchi, Y.; Sekimoto, H. Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasites. Planta 2007, 225, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Raez, J.A.; Charnikhova, T.; Gomez-Roldan, V.; Matusova, R.; Kohlen, W.; De Vos, R.; Verstappen, F.; Puech-Pages, V.; Becard, G.; Mulder, P.; et al. Tomato strigolactones are derived from carotenoids and their biosynthesis is promoted by phosphate starvation. New Phytol. 2008, 178, 863–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugimoto, Y.; Ueyama, T. Production of (+)-5-deoxystrigol by Lotus japonicus root culture. Phytochemistry 2008, 69, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Umehara, M.; Hanada, A.; Magome, H.; Takeda-Kamiya, N.; Yamaguchi, S. Contribution of strigolactones to the inhibition of tiller bud outgrowth under phosphate deficiency in rice. Plant Cell Physiol. 2010, 51, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Matsuzaki, K.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Kohlen, W.; Charnikhova, T.; Liu, Q.; Bours, R.; Domagalska, M.A.; Beguerie, S.; Verstappen, F.; Leyser, O.; Bouwmeester, H.; Ruyter-Spira, C. Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host arabidopsis. Plant Physiol. 2010, 155, 974–987. [Google Scholar] [CrossRef] [PubMed]
- Umehara, M. Strigolactone, a key regulator of nutrient allocation in plants. Plant Biotechnol. 2011, 28, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Tao, J.; Liu, S.; Huang, S.; Chen, S.; Xie, X.; Yoneyama, K.; Zhang, Y.; Xu, G. Strigolactones are involved in phosphate- and nitrate-deficiency-induced root development and auxin transport in rice. J. Exp. Bot. 2014, 65, 6735–6746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shindo, M.; Shimomura, K.; Yamaguchi, S.; Umehara, M. Upregulation of dwarf27 is associated with increased strigolactone levels under sulfur deficiency in rice. Plant Direct 2018, 2, e00050. [Google Scholar] [CrossRef]
- Consortium, T.G. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 2012, 485, 635–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohlen, W.; Charnikhova, T.; Bours, R.; Lopez-Raez, J.A.; Bouwmeester, H. Tomato strigolactones: A more detailed look. Plant Signal. Behav. 2013, 8, e22785. [Google Scholar] [CrossRef] [PubMed]
- Kohlen, W.; Charnikhova, T.; Lammers, M.; Pollina, T.; Toth, P.; Haider, I.; Pozo, M.J.; de Maagd, R.A.; Ruyter-Spira, C.; Bouwmeester, H.J.; et al. The tomato carotenoid cleavage dioxygenase8 (slccd8) regulates rhizosphere signaling, plant architecture and affects reproductive development through strigolactone biosynthesis. New Phytol. 2012, 196, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.T.; Walter, M.H.; Giavalisco, P.; Lytovchenko, A.; Kohlen, W.; Charnikhova, T.; Simkin, A.J.; Goulet, C.; Strack, D.; Bouwmeester, H.J.; et al. Slccd7 controls strigolactone biosynthesis, shoot branching and mycorrhiza-induced apocarotenoid formation in tomato. Plant J. 2010, 61, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, X.; Wang, Y.; Diez-Simon, C.; Flokova, K.; Bimbo, A.; Bouwmeester, H.J.; Ruyter-Spira, C. The tomato max1 homolog, slmax1, is involved in the biosynthesis of tomato strigolactones from carlactone. New Phytol. 2018, 219, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.E.; Whichard, L.P.; Turner, B.; Wall, M.E.; Egley, G.H. Germination of witchweed (striga lutea lour.): Isolation and properties of a potent stimulant. Science 1966, 154, 1189–1190. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.E.; Whichard, L.P.; Wall, M.E.; Egley, G.H.; Coggon, P.; Luhan, P.A.; McPhail, A.T. Germination stimulants. 2. The structure of strigol–a potent seed germination stmulant for witchweed (striga lutea lour.). J. Am. Chem. Soc. 1972, 94, 6198–6199. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.; Matusova, R.; Zhongkui, S.; Beale, M.H. Secondary metabolite signalling in host-parasitic plant interactions. Curr. Opin. Plant Biol. 2003, 6, 358–364. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.; Roux, C.; Lopez-Raez, J.A.; Becard, G. Rhizosphere communication of plants, parasitic plants and am fungi. Trends Plant Sci. 2007, 12, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Parker, C. Observations on the current status of orobanche and striga problems worldwide. Pest Manag. Sci. 2009, 65, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Mangnus, E.M.; Dommerholt, F.J.; Dejong, R.L.P.; Zwanenburg, B. Improved synthesis of strigol analog gr24 and evaluation of the biological-activity of its diastereomers. J. Agric. Food Chem. 1992, 40, 1230–1235. [Google Scholar] [CrossRef]
- Yamada, Y.; Furusawa, S.; Nagasaka, S.; Shimomura, K.; Yamaguchi, S.; Umehara, M. Strigolactone signaling regulates rice leaf senescence in response to a phosphate deficiency. Planta 2014, 240, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Kusaba, M. Strigolactone regulates leaf senescence in concert with ethylene in arabidopsis. Plant Physiol. 2015, 169, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, T.; Xu, B.; Jia, L.; Xiao, B.; Liu, H.; Liu, L.; Yan, H.; Xia, Q. Crispr/cas9-mediated mutagenesis of carotenoid cleavage dioxygenase 8 (ccd8) in tobacco affects shoot and root architecture. Int. J. Mol. Sci. 2018, 19, 1062. [Google Scholar] [CrossRef] [PubMed]
- Pasare, S.A.; Ducreux, L.J.; Morris, W.L.; Campbell, R.; Sharma, S.K.; Roumeliotis, E.; Kohlen, W.; van der Krol, S.; Bramley, P.M.; Roberts, A.G.; et al. The role of the potato (Solanum tuberosum) ccd8 gene in stolon and tuber development. New Phytol. 2013, 198, 1108–1120. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.L.; Zhao, L.J.; Challis, R.; Leyser, O. Strigolactone regulation of shoot branching in chrysanthemum (dendranthema grandiflorum). J. Exp. Bot. 2010, 61, 3069–3078. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, C.A.; Symons, G.M.; Murfet, I.C.; Ross, J.J.; Rameau, C. The rms1 mutant of pea has elevated indole-3-acetic acid levels and reduced root-sap zeatin riboside content but increased branching controlled by graft-transmissible signal(s). Plant Physiol. 1997, 115, 1251–1258. [Google Scholar] [CrossRef]
- Bainbridge, K.; Sorefan, K.; Ward, S.; Leyser, O. Hormonally controlled expression of the arabidopsis max4 shoot branching regulatory gene. Plant J. 2005, 44, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, T.; Kohlen, W.; Sasse, J.; Borghi, L.; Schlegel, M.; Bachelier, J.B.; Reinhardt, D.; Bours, R.; Bouwmeester, H.J.; Martinoia, E. A petunia abc protein controls strigolactone-dependent symbiotic signalling and branching. Nature 2012, 483, 341–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joel, D.M.; Hershenhorn, J.; Eizenberg, H.; Aly, R.; Ejeta, G.; Rich, P.J.; Ransom, J.K.; Sauerborn, J.; Rubiales, D. Biology and management of weedy root parasites. Hortic. Rev. 2007, 33, 267–349. [Google Scholar]
- Hershenhorn, J.; Eizenberg, H.; Dor, E.; Kapulnik, Y.; Goldwasser, Y. Phelipanche aegyptiaca management in tomato. Weed Res. 2009, 49, 34–47. [Google Scholar] [CrossRef]
- Dor, E.; Alperin, B.; Wininger, S.; Ben-Dor, B.; Somvanshi, V.S.; Koltai, H.; Kapulnik, Y.; Hershenhorn, J. Characterization of a novel tomato mutant resistant to the weedy parasites orobanche and phelipanche spp. Euphytica 2010, 171, 371–380. [Google Scholar] [CrossRef]
- Dor, E.; Yoneama, K.; Wininger, S.; Kapulnik, Y.; Koltai, H.; Xiaonan, X.; Hershenhorn, J. Strigolactone deficiency confers resistance in tomato line sl-ort1 to the parasitic weeds phelipanche spp. and orobanche spp. Phytopathology 2011, 101, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Koltai, H.; LekKala, S.P.; Bhattacharya, C.; Mayzlish-Gati, E.; Resnick, N.; Wininger, S.; Dor, E.; Yoneyama, K.; Hershenhorn, J.; Joel, D.M.; et al. A tomato strigolactone-impaired mutant displays aberrant shoot morphology and plant interactions. J. Exp. Bot. 2010, 61, 1739–1749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, X.; Flokova, K.; Bouwmeester, H.; Ruyter-Spira, C. The role of endogenous strigolactones and their interaction with aba during the infection process of the parasitic weed phelipanche ramosa in tomato plants. Front. Plant Sci. 2017, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Torres-Vera, R.; Garcia, J.M.; Pozo, M.J.; Lopez-Raez, J.A. Do strigolactones contribute to plant defence? Mol. Plant Pathol. 2014, 15, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Joel, D.M.; Chaudhuri, S.K.; Plakhine, D.; Ziadna, H.; Steffens, J.C. Dehydrocostus lactone is exuded from sunflower roots and stimulates germination of the root parasite orobanche cumana. Phytochemistry 2011, 72, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Auger, B.; Pouvreau, J.-B.; Pouponneau, K.; Yoneyama, K.; Montiel, G.; Le Bizec, B.; Yoneyama, K.; Delavault, P.; Delourme, R.; Simier, P. Germination stimulants of phelipanche ramosa in the rhizosphere of brassica napus are derived from the glucosinolate pathway. Mol. Plant Microbe Interact. 2012, 25, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Okabe, Y.; Asamizu, E.; Saito, T.; Matsukura, C.; Ariizumi, T.; Bres, C.; Rothan, C.; Mizoguchi, T.; Ezura, H. Tomato tilling technology: Development of a reverse genetics tool for the efficient isolation of mutants from micro-tom mutant libraries. Plant Cell Physiol. 2011, 52, 1994–2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, T.; Ariizumi, T.; Okabe, Y.; Asamizu, E.; Hiwasa-Tanase, K.; Fukuda, N.; Mizoguchi, T.; Yamazaki, Y.; Aoki, K.; Ezura, H. Tomatoma: A novel tomato mutant database distributing micro-tom mutant collections. Plant Cell Physiol. 2011, 52, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Triques, K.; Sturbois, B.; Gallais, S.; Dalmais, M.; Chauvin, S.; Clepet, C.; Aubourg, S.; Rameau, C.; Caboche, M.; Bendahmane, A. Characterization of arabidopsis thaliana mismatch specific endonucleases: Application to mutation discovery by tilling in pea. Plant J. 2007, 51, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Till, B.J.; Zerr, T.; Comai, L.; Henikoff, S. A protocol for tilling and ecotilling in plants and animals. Nat. Protoc. 2006, 1, 2465–2477. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, L.; Mauriat, M.; Guenin, S.; Pelloux, J.; Lefebvre, J.F.; Louvet, R.; Rusterucci, C.; Moritz, T.; Guerineau, F.; Bellini, C.; et al. The lack of a systematic validation of reference genes: A serious pitfall undervalued in reverse transcription-polymerase chain reaction (rt-pcr) analysis in plants. Plant Biotechnol. J. 2008, 6, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Zhang, J.; Li, H.; Yang, C.; Zhang, C.; Zhang, X.; Khurram, Z.; Zhang, Y.; Wang, T.; Fei, Z.; et al. Transcriptional profiles of drought-responsive genes in modulating transcription signal transduction, and biochemical pathways in tomato. J. Exp. Bot. 2010, 61, 3563–3575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumagai, H.; Fujiwara, M.; Kuse, M.; Takikawa, H. A concise synthesis of optically active solanacol, the germination stimulant for seeds of root parasitic weeds. Biosci. Biotechnol. Biochem. 2015, 79, 1240–1245. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, K.; Mori, K. Synthesis of (+)-strigol and (+)-orobanchol, the germination stimulants, and their stereoisomers by employing lipase-catalyzed asymmetric acetylationas the kkey step. Eur. J. Org. Chem. 1999, 1999, 2211–2217. [Google Scholar] [CrossRef]
- Yoneyama, K.; Xie, X.; Kim, H.I.; Kisugi, T.; Nomura, T.; Sekimoto, H.; Yokota, T.; Yoneyama, K. How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation? Planta 2012, 235, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthtic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Ekawa, M.; Aoki, K. Phloem-conducting cells in haustoria of the root-parasitic plant phelipanche aegyptiaca retain nuclei and are not mature sieve elements. Plants 2017, 6, 60. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasegawa, S.; Tsutsumi, T.; Fukushima, S.; Okabe, Y.; Saito, J.; Katayama, M.; Shindo, M.; Yamada, Y.; Shimomura, K.; Yoneyama, K.; et al. Low Infection of Phelipanche aegyptiaca in Micro-Tom Mutants Deficient in CAROTENOID CLEAVAGE DIOXYGENASE 8. Int. J. Mol. Sci. 2018, 19, 2645. https://doi.org/10.3390/ijms19092645
Hasegawa S, Tsutsumi T, Fukushima S, Okabe Y, Saito J, Katayama M, Shindo M, Yamada Y, Shimomura K, Yoneyama K, et al. Low Infection of Phelipanche aegyptiaca in Micro-Tom Mutants Deficient in CAROTENOID CLEAVAGE DIOXYGENASE 8. International Journal of Molecular Sciences. 2018; 19(9):2645. https://doi.org/10.3390/ijms19092645
Chicago/Turabian StyleHasegawa, Shoko, Takuya Tsutsumi, Shunsuke Fukushima, Yoshihiro Okabe, Junna Saito, Mina Katayama, Masato Shindo, Yusuke Yamada, Koichiro Shimomura, Kaori Yoneyama, and et al. 2018. "Low Infection of Phelipanche aegyptiaca in Micro-Tom Mutants Deficient in CAROTENOID CLEAVAGE DIOXYGENASE 8" International Journal of Molecular Sciences 19, no. 9: 2645. https://doi.org/10.3390/ijms19092645
APA StyleHasegawa, S., Tsutsumi, T., Fukushima, S., Okabe, Y., Saito, J., Katayama, M., Shindo, M., Yamada, Y., Shimomura, K., Yoneyama, K., Akiyama, K., Aoki, K., Ariizumi, T., Ezura, H., Yamaguchi, S., & Umehara, M. (2018). Low Infection of Phelipanche aegyptiaca in Micro-Tom Mutants Deficient in CAROTENOID CLEAVAGE DIOXYGENASE 8. International Journal of Molecular Sciences, 19(9), 2645. https://doi.org/10.3390/ijms19092645