Ex Vivo and in Vivo Study of Sucrosomial® Iron Intestinal Absorption and Bioavailability
Abstract
:1. Introduction
2. Results and Discussion
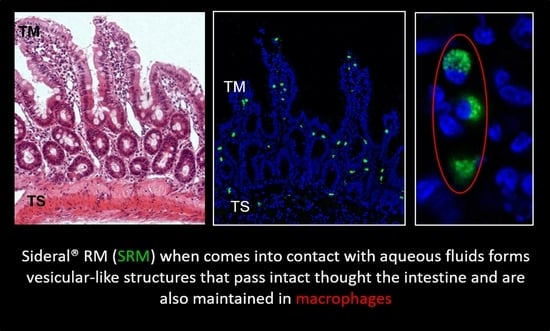
2.1. Study of Fluorescein Isothiocyanate (FITC)-Labeled SRM Internalization in and across Intestinal Barrier
2.2. Bioavailability Studies
3. Materials and Methods
3.1. Materials
3.2. Fluorescein Isothiocyanate Labeling of Sucrester
3.3. Biopharmaceutical Procedures
3.3.1. Study of FITC-Labeled SRM Internalization in and across Intestinal Barrier
3.3.2. Bioavailability Studies
3.4. Statistical Data Treatment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.D. Diagnosis and management of iron-deficiency anaemia. Best Pract. Res. Clin. Haematol. 2005, 18, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Kortman, G.A.; Raffatellu, M.; Swinkels, D.W.; Tjalsma, H. Nutritional iron turned inside out: Intestinal stress from a gut microbial perspective. FEMS Microbiol. Rev. 2014, 38, 1202–1234. [Google Scholar] [CrossRef] [PubMed]
- Gera, T.; Sachdev, H.P. Effect of iron supplementation on incidence of infectious illness in children: Systematic review. BMJ 2002, 325, 1142. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, H.P.; Gera, T.; Nestel, P. Effect of iron supplementation on physical growth in children: Systematic review of randomized controlled trials. Public Health Nutr. 2006, 9, 904–920. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Chassard, C.; Rohner, F.; N’Goran, E.K.; Nindjin, C.; Dostal, A.; Utzinger, J.; Ghattas, H.; Lacroix, C.; Hurrell, R.F. The effects of iron fortification on the gut microbiota in African children: A randomized controlled trial in Cote d’Ivoire. Am. J. Clin. Nutr. 2010, 92, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Jaeggi, T.; Kortman, G.A.; Moretti, D.; Chassard, C.; Holding, P.; Dostal, A.; Boekhorst, J.; Timmerman, H.M.; Swinkels, D.W.; Tjalsma, H.; et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 2014, 64, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, S.; Bhandari, S. Oral iron: Properties and current place in the treatment of anaemia. Prescriber 2012, 23, 12–18. [Google Scholar] [CrossRef]
- Prentice, A.M.; Mendoza, Y.A.; Pereira, D.; Cerami, C.; Wegmuller, R.; Constable, A.; Spieldenner, J. Dietary strategies for improving iron status: Balancing safety and efficacy. Nutr. Rev. 2017, 75, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Lund, E.K.; Wharf, S.G.; Fairweather-Tait, S.J.; Johnson, I.T. Oral ferrous sulfate supplements increase the free radical-generating capacity of feces from healthy volunteers. Am. J. Clin. Nutr. 1999, 69, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Ferruzza, S.; Scarino, M.L.; Gambling, L.; Natella, F.; Sambuy, Y. Biphasic effect of iron on human intestinal Caco-2 cells: Early effect on tight junction permeability with delayed onset of oxidative cytotoxic damage. Cell Mol. Biol. 2003, 49, 89–99. [Google Scholar] [PubMed]
- Natoli, M.; Felsani, A.; Ferruzza, S.; Sambuy, Y.; Canali, R.; Scarino, M.L. Mechanisms of defence from Fe(II) toxicityin human intestinal Caco-2 cells. Toxicol. In Vitro 2009, 23, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.I.A.; Mergler, B.I.; Faria, N.; Bruggraber, S.F.A.; Aslam, M.F.; Poots, L.K.; Prassmayer, L.; Lonnerndal, B.; Brown, A.P.; Powell, J.J. Caco-2 Cell Acquisition of Dietary Iron(III) Invokes a Nanoparticulate Endocytic Pathway. PLoS ONE 2013, 8, e81250. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, A.; Brilli, E.; Fogli, S.; Beconcini, D.; Carpi, S.; Tarantino, G.; Zambito, Y. Sucrosomial® iron absorption studied by in vitro and ex-vivo models. Eur. J. Pharm. Sci. 2018, 111, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Dev, S.; Babitt, J.L. Overview of iron metabolism in health and disease. Hemodial. Int. 2017, 21, S6–S20. [Google Scholar] [CrossRef] [PubMed]
- Rishi, G.; Subramaniam, V.N. The liver in regulation of iron homeostasis. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G157–G165. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Systemic iron homeostasis. Physiol. Rev. 2013, 93, 1721–1741. [Google Scholar] [CrossRef] [PubMed]
- Pantopoulos, K.; Porwal, S.K.; Tartakoff, A.; Devireddy, L. Mechanisms of mammalian iron homeostasis. Biochemistry 2012, 51, 5705–5724. [Google Scholar] [CrossRef] [PubMed]
- Di Colo, G.; Zambito, Y.; Zaino, C.; Sansò, M. Selected polysaccharides at comparison for their mucoadesiveness and effect on precorneal residence of different drugs in the rabbit model. Drug Dev. Ind. Pharm. 2009, 35, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, A.; Mattii, L.; Braca, A.; Felice, F.; Di Stefano, R.; Zambito, Y. Nanoparticles based on quaternary ammonium-chitosan conjugate: A vehicle for oral administration of antioxidants contained in red grapes. J. Drug Deliv. Technol. 2016, 32, 291–297. [Google Scholar] [CrossRef]
- Glahn, R.P.; Lee, O.A.; Yeung, A.; Goldman, M.I.; Miller, D.D. Caco-2 Cell Ferritin Formation Predicts Nonradiolabeled Food Iron Availability in an In Vitro Digestion/Caco-2 Cell Culture Model. J. Nutr. 1998, 128, 1555–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shyla, B.; Bhaskar, C.V.; Nagendrappa, G. Iron(III) oxidized nucleophilic coupling of catechol with o-tolidine/p-toluidine followed by 1,10-phenanthroline as new and sensitivity improved spectrophotometric methods for iron present in chemicals, pharmaceutical, edible green leaves, nuts and lake water samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 86, 152–158. [Google Scholar] [PubMed]
- Tozaki, H.; Odoriba, T.; Okada, N.; Fujita, T.; Terabe, A.; Suzuki, T.; Okabe, S.; Muranishi, S.; Yamamoto, A. Chitosan capsules for colon-specific drug delivery: Enhanced localization of 5-aminosalicylic acid in the large intestine accelerates healing of TNBS-induced colitis in rats. J. Control. Release 2002, 82, 51–61. [Google Scholar] [CrossRef]







| Item | Cmax (μg/mL) | tmax, h | AUC0–5 (μg h/mL) | AUCrel |
|---|---|---|---|---|
| FP | 352.7 ± 32.1 | 3 | 388.3 ± 151.6 | - |
| SRM | 463.5 * ± 31.8 | 3 | 694.5 * ± 57.2 | 1.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabiano, A.; Brilli, E.; Mattii, L.; Testai, L.; Moscato, S.; Citi, V.; Tarantino, G.; Zambito, Y. Ex Vivo and in Vivo Study of Sucrosomial® Iron Intestinal Absorption and Bioavailability. Int. J. Mol. Sci. 2018, 19, 2722. https://doi.org/10.3390/ijms19092722
Fabiano A, Brilli E, Mattii L, Testai L, Moscato S, Citi V, Tarantino G, Zambito Y. Ex Vivo and in Vivo Study of Sucrosomial® Iron Intestinal Absorption and Bioavailability. International Journal of Molecular Sciences. 2018; 19(9):2722. https://doi.org/10.3390/ijms19092722
Chicago/Turabian StyleFabiano, Angela, Elisa Brilli, Letizia Mattii, Lara Testai, Stefania Moscato, Valentina Citi, Germano Tarantino, and Ylenia Zambito. 2018. "Ex Vivo and in Vivo Study of Sucrosomial® Iron Intestinal Absorption and Bioavailability" International Journal of Molecular Sciences 19, no. 9: 2722. https://doi.org/10.3390/ijms19092722
APA StyleFabiano, A., Brilli, E., Mattii, L., Testai, L., Moscato, S., Citi, V., Tarantino, G., & Zambito, Y. (2018). Ex Vivo and in Vivo Study of Sucrosomial® Iron Intestinal Absorption and Bioavailability. International Journal of Molecular Sciences, 19(9), 2722. https://doi.org/10.3390/ijms19092722