Protein Phosphatase (PP2C9) Induces Protein Expression Differentially to Mediate Nitrogen Utilization Efficiency in Rice under Nitrogen-Deficient Condition
Abstract
:1. Introduction
2. Results
2.1. Physiological Performance of PP2C9TL and WT
2.2. NUE and Yield Performance of PP2C9TL and WT
2.3. Leaf Proteome Analysis of the Two Isogenic Lines under N Deficient Conditions
2.4. Functional Characterization of the Identified Proteins
2.5. Subcellular Characterization of the Identified Proteins
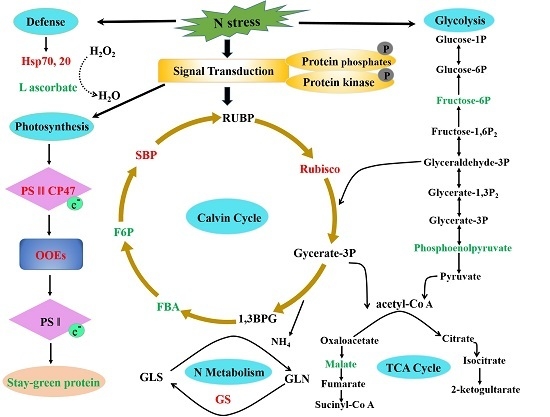
2.6. Potential Molecular Pathway Based on Differentially Expressed Proteins
2.7. Western Blotting of the Important Differentially Expressed Protein
3. Discussion
3.1. Regulatory Role of PP2C9 for Higher NUE in PP2C9TL under N Deficient Conditions
3.2. The Physiological Basis for NUE and Grain Yield
3.3. Proteins Expression Involved in Energy of PP2C9TL and WT Genotypes under N Deficient Conditions
3.4. Proteins Expression Involved in Photosynthesis of PP2C9TL and WT Genotypes under N Deficient Conditions
3.5. Proteins Expression Involved in Nitrogen Metabolism of PP2C9TL and WT Genotypes under N Deficient Conditions
3.6. Proteins Expression Involved in Defense and Protein Folding of PP2C9TL and WT Genotypes under N Deficient Conditions
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Transgenic Line Generation
4.3. Physiological Measurement
4.4. Protein Sample Preparation
4.5. Two-Dimensional Gel Electrophoresis
4.6. In-Gel Protein Digestion
4.7. LC-ESI-MS/MS Analysis and Protein Identification
4.8. Confirmation of Important Protein 14-3-3 by Western Blotting Analysis
4.9. Co-Immunoprecipitation (Co-IP) Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Amaral, L.R.; Molin, J.P.; Schepers, J.S. Algorithm for variable-rate nitrogen application in sugarcane based on active crop canopy sensor. Agron. J. 2015, 107, 1513–1523. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Shen, J.; Yu, W.; Yuan, F.; Cheng, S.; Huang, S.; Wang, H.; Yang, W.; Liu, F. Improving in-season estimation of rice yield potential and responsiveness to topdressing nitrogen application with crop circle active crop canopy sensor. Precis. Agric. 2016, 17, 136–154. [Google Scholar] [CrossRef]
- Khush, G.S. What it will take to feed 5.0 billion rice consumers in 2030. Plant Mol. Biol. 2005, 59, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Good, A.G.; Beatty, P.H. Fertilizing nature: A tragedy of excess in the commons. PLoS Biol. 2011, 9, e1001124. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Clark, M. Food, agriculture & the environment: Can we feed the world & save the earth? Daedalus 2015, 144, 8–23. [Google Scholar]
- Tilman, D.; Fargione, J.; Wolff, B.; D’antonio, C.; Dobson, A.; Howarth, R.; Schindler, D.; Schlesinger, W.H.; Simberloff, D.; Swackhamer, D. Forecasting agriculturally driven global environmental change. Science 2001, 292, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Prinsi, B.; Negri, A.S.; Pesaresi, P.; Cocucci, M.; Espen, L. Evaluation of protein pattern changes in roots and leaves of Zea mays plants in response to nitrate availability by two-dimensional gel electrophoresis analysis. BMC Plant Biol. 2009, 9, 113–129. [Google Scholar] [CrossRef] [PubMed]
- FAO. World Fertilizer Trends and Outlook to 2018; FAO: Rome, Italy, 2018; p. 66. [Google Scholar]
- Hakeem, K.R.; Ahmad, A.; Iqbal, M.; Gucel, S.; Ozturk, M. Nitrogen-efficient rice cultivars can reduce nitrate pollution. Environ. Sci. Pollut. Res. 2011, 18, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Kant, S.; Bi, Y.-M.; Rothstein, S.J. Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. J. Exp. Bot. 2010, 62, 1499–1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; Zhao, C.; Zhang, Y.; Wang, C. Nitrogen use efficiency in rice. In Nitrogen in Agriculture-Updates; InTech: Christchurch, New Zealand, 2018. [Google Scholar]
- Pathak, R.R.; Ahmad, A.; Lochab, S.; Raghuram, N. Molecular physiology of plant nitrogen use efficiency and biotechnological options for its enhancement. Curr. Sci. 2008, 1394–1403. [Google Scholar]
- Waqas, M.; Faheem, M.; Khan, A.S.; Shehzad, M.; Ansari, M.A.A. Estimation of heritability and genetic advance for some yield traits in eight f2 populations of wheat (Triticum aestivum L.). Sci. Lett. 2014, 2, 43–47. [Google Scholar]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Konishi, M.; Yanagisawa, S. The regulatory region controlling the nitrate-responsive expression of a nitrate reductase gene, nia1, in arabidopsis. Plant Cell Physiol. 2011, 52, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Bloom, A.J.; Burger, M.; Asensio, J.S.R.; Cousins, A.B. Carbon dioxide enrichment inhibits nitrate assimilation in wheat and arabidopsis. Science 2010, 328, 899–903. [Google Scholar] [CrossRef] [PubMed]
- McAllister, C.H.; Beatty, P.H.; Good, A.G. Engineering nitrogen use efficient crop plants: The current status. Plant Biotechnol. J. 2012, 10, 1011–1025. [Google Scholar] [CrossRef] [PubMed]
- Fraisier, V.; Gojon, A.; Tillard, P.; Daniel-Vedele, F. Constitutive expression of a putative high-affinity nitrate transporter in Nicotiana plumbaginifolia: Evidence for post-transcriptional regulation by a reduced nitrogen source. Plant. J. 2000, 23, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Laugier, E.; Bouguyon, E.; Mauriès, A.; Tillard, P.; Gojon, A.; Lejay, L. Regulation of high-affinity nitrate uptake in roots of arabidopsis depends predominantly on posttranscriptional control of the nrt2. 1/nar2. 1 transport system. Plant Physiol. 2012, 158, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Okamoto, M.; Beatty, P.H.; Rothstein, S.J.; Good, A.G. The genetics of nitrogen use efficiency in crop plants. Annu. Rev. Genet. 2015, 49, 269–289. [Google Scholar] [CrossRef] [PubMed]
- Hoshida, H.; Tanaka, Y.; Hibino, T.; Hayashi, Y.; Tanaka, A.; Takabe, T.; Takabe, T. Enhanced tolerance to salt stress in transgenic rice that overexpresses chloroplast glutamine synthetase. Plant Mol. Biol. 2000, 43, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Habash, D.; Massiah, A.; Rong, H.; Wallsgrove, R.; Leigh, R. The role of cytosolic glutamine synthetase in wheat. Ann. Appl. Biol. 2001, 138, 83–89. [Google Scholar] [CrossRef]
- Konishi, N.; Saito, M.; Imagawa, F.; Kanno, K.; Yamaya, T.; Kojima, S. Cytosolic glutamine synthetase isozymes play redundant roles in ammonium assimilation under low-ammonium conditions in roots of Arabidopsis thaliana. Plant Cell Physiol. 2018, 59, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Moose, S.; Below, F.E. Biotechnology approaches to improving maize nitrogen use efficiency. In Molecular Genetic Approaches to Maize Improvement; Springer: New York, NY, USA, 2009; pp. 65–77. [Google Scholar]
- Moorhead, G.B.G.; Trinkle-Mulcahy, L.; Ulke-Lemée, A. Emerging roles of nuclear protein phosphatases. Nat. Rev. Mol. Cell Biol. 2007, 8, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Pandey, A.; Srivastava, A.K.; Tran, L.-S.P.; Pandey, G.K. Plant protein phosphatases 2c: From genomic diversity to functional multiplicity and importance in stress management. Crit. Rev. Biotechnol. 2016, 36, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- de Torres-Zabala, M.; Truman, W.; Bennett, M.H.; Lafforgue, G.; Mansfield, J.W.; Egea, P.R.; Bögre, L.; Grant, M. Pseudomonas syringae pv. Tomato hijacks the arabidopsis abscisic acid signalling pathway to cause disease. EMBO J. 2007, 26, 1434–1443. [Google Scholar] [PubMed]
- Cai, Z.; Liu, J.; Wang, H.; Yang, C.; Chen, Y.; Li, Y.; Pan, S.; Dong, R.; Tang, G.; de Dios Barajas-Lopez, J. Gsk3-like kinases positively modulate abscisic acid signaling through phosphorylating subgroup iii snrk2s in arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 9651–9656. [Google Scholar] [CrossRef] [PubMed]
- Castillo, M.-C.; Lozano-Juste, J.; González-Guzmán, M.; Rodriguez, L.; Rodriguez, P.L.; León, J. Inactivation of pyr/pyl/rcar aba receptors by tyrosine nitration may enable rapid inhibition of aba signaling by nitric oxide in plants. Sci. Signal. 2015, 8, ra89. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; He, J.; Zhao, Y.; Wu, T.; Zhou, X.; Ding, Y.; Kong, L.; Wang, X.; Wang, Y.; Li, J. Ear1 negatively regulates aba signaling by enhancing 2c protein phosphatase activity. Plant Cell 2018, 30, 815–834. [Google Scholar] [CrossRef] [PubMed]
- Krzywińska, E.; Bucholc, M.; Kulik, A.; Ciesielski, A.; Lichocka, M.; Dębski, J.; Ludwików, A.; Dadlez, M.; Rodriguez, P.L.; Dobrowolska, G. Phosphatase abi1 and okadaic acid-sensitive phosphoprotein phosphatases inhibit salt stress-activated snrk2. 4 kinase. BMC Plant Biol. 2016, 16, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-H.; Lin, S.-H.; Hu, H.-C.; Tsay, Y.-F. Chl1 functions as a nitrate sensor in plants. Cell 2009, 138, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Léran, S.; Edel, K.H.; Pervent, M.; Hashimoto, K.; Corratgé-Faillie, C.; Offenborn, J.N.; Tillard, P.; Gojon, A.; Kudla, J.; Lacombe, B. Nitrate sensing and uptake in arabidopsis are enhanced by abi2, a phosphatase inactivated by the stress hormone abscisic acid. Sci. Signal. 2015, 8, ra43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, J.; Lin, S.; Li, Z.; Cheng, R.; Fang, C.; Chen, H.; Lin, W. Proteomic and phosphoproteomic determination of aba’s effects on grain-filling of Oryza sativa L. Inferior spikelets. Plant. Sci. 2012, 185, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, H.; Tang, J.; Li, Z.; Li, Z.; Chen, D.; Lin, W. A proteomic study on molecular mechanism of poor grain-filling of rice (Oryza sativa L.) inferior spikelets. PLoS ONE 2014, 9, e89140. [Google Scholar] [CrossRef] [PubMed]
- West, P.C.; Gerber, J.S.; Engstrom, P.M.; Mueller, N.D.; Brauman, K.A.; Carlson, K.M.; Cassidy, E.S.; Johnston, M.; MacDonald, G.K.; Ray, D.K. Leverage points for improving global food security and the environment. Science 2014, 345, 325–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, X.-T.; Xing, G.-X.; Chen, X.-P.; Zhang, S.-L.; Zhang, L.-J.; Liu, X.-J.; Cui, Z.-L.; Yin, B.; Christie, P.; Zhu, Z.-L. Reducing environmental risk by improving n management in intensive chinese agricultural systems. Proc. Natl. Acad. Sci. USA 2009, 106, 3041–3046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Cui, Z.; Fan, M.; Vitousek, P.; Zhao, M.; Ma, W.; Wang, Z.; Zhang, W.; Yan, X.; Yang, J. Producing more grain with lower environmental costs. Nature 2014, 514, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Buresh, R.J.; Huang, J.; Yang, J.; Zou, Y.; Zhong, X.; Wang, G.; Zhang, F. Strategies for overcoming low agronomic nitrogen use efficiency in irrigated rice systems in china. Field Crops Res. 2006, 96, 37–47. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Y.; Liu, X.; Li, Z.; Lin, W. The use of comparative quantitative proteomics analysis in rice grain-filling in determining response to moderate soil drying stress. Plant Growth Regul. 2017, 82, 219–232. [Google Scholar] [CrossRef]
- Shin, R.; Alvarez, S.; Burch, A.Y.; Jez, J.M.; Schachtman, D.P. Phosphoproteomic identification of targets of the arabidopsis sucrose nonfermenting-like kinase snrk2. 8 reveals a connection to metabolic processes. Proc. Natl. Acad. Sci. USA 2007, 104, 6460–6465. [Google Scholar] [CrossRef] [PubMed]
- Comparot, S.; Lingiah, G.; Martin, T. Function and specificity of 14-3-3 proteins in the regulation of carbohydrate and nitrogen metabolism. J. Exp. Bot. 2003, 54, 595–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz, C.; Kusano, M.; Sulpice, R.; Araki, M.; Redestig, H.; Saito, K.; Stitt, M.; Shin, R. Determining novel functions of arabidopsis 14-3-3 proteins in central metabolic processes. BMC Syst. Biol. 2011, 5, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Robertlee, J.; Kobayashi, K.; Suzuki, M.; Muranaka, T. Akin10, a representative arabidopsis snf1-related protein kinase 1 (snrk1), phosphorylates and downregulates plant hmg-coa reductase. FEBS Lett. 2017, 591, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ren, G.; Li, F.; Wang, B.; Yang, Y.; Ma, X.; Niu, Y.; Ye, Y.; Chen, X.; Fan, S. Overexpression of gmsnrk1, a soybean sucrose non-fermenting-1 related protein kinase 1 gene, results in directional alteration of carbohydrate metabolism in transgenic arabidopsis. Biotechnol. Biotechnol. Equip. 2018, 32, 1–11. [Google Scholar] [CrossRef]
- Ross, F.A.; MacKintosh, C.; Hardie, D.G. Amp-activated protein kinase: A cellular energy sensor that comes in 12 flavours. FEBS J. 2016, 283, 2987–3001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Zhao, X.; Guo, C.; Chen, L.; Li, K. Spinach 14-3-3 protein interacts with the plasma membrane h+-atpase and nitrate reductase in response to excess nitrate stress. Plant Physiol. Biochem. 2016, 106, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.; Huber, J.L.; Athwal, G.S.; Wu, K.; Ferl, R.J.; Huber, S.C. 14-3-3 proteins associate with the regulatory phosphorylation site of spinach leaf nitrate reductase in an isoform-specific manner and reduce dephosphorylation of ser-543 by endogenous protein phosphatases. FEBS Lett. 1996, 398, 26–30. [Google Scholar] [CrossRef]
- Moll, R.H.; Kamprath, E.J.; Jackson, W.A. Analysis and interpretation of factors which contribute to efficiency of nitrogen utilization. Agron. J. 1982, 74, 562–564. [Google Scholar] [CrossRef]
- Gallais, A.; Hirel, B. An approach to the genetics of nitrogen use efficiency in maize. J. Exp. Bot. 2004, 55, 295–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Zhang, H.; Zhao, P.; Zhang, Z.; Liang, W.; Tian, Z.; Zheng, Y. Mining of candidate maize genes for nitrogen use efficiency by integrating gene expression and QTL data. Plant Mol. Biol. Rep. 2012, 30, 297–308. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. F1000Res 2016, 5, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, M.J.; Hawkesford, M.J.; Barraclough, P.B.; Holdsworth, M.J.; Kerr, S.; Kightley, S.; Shewry, P.R. Identifying traits to improve the nitrogen economy of wheat: Recent advances and future prospects. Field Crops Res. 2009, 114, 329–342. [Google Scholar] [CrossRef]
- Rennenberg, H.; Wildhagen, H.; Ehlting, B. Nitrogen nutrition of poplar trees. Plant. Biol. 2010, 12, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ju, X.; Zhang, F.; Pan, J.; Christie, P. Nitrogen dynamics and budgets in a winter wheat–maize cropping system in the north china plain. Field Crops Res. 2003, 83, 111–124. [Google Scholar] [CrossRef]
- Wang, J.-G.; Liu, H.; Sun, H.; Hua, W.; Wang, H.; Liu, X.; Wei, B. One-pot synthesis of nitrogen-doped ordered mesoporous carbon spheres for high-rate and long-cycle life supercapacitors. Carbon 2018, 127, 85–92. [Google Scholar] [CrossRef]
- Hakeem, K.R.; Chandna, R.; Ahmad, A.; Qureshi, M.I.; Iqbal, M. Proteomic analysis for low and high nitrogen-responsive proteins in the leaves of rice genotypes grown at three nitrogen levels. Appl. Biochem. Biotechnol. 2012, 168, 834–850. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bian, Y.; Cheng, K.; Zou, H.; Sun, S.S.-M.; He, J.-X. A comprehensive differential proteomic study of nitrate deprivation in arabidopsis reveals complex regulatory networks of plant nitrogen responses. J. Proteome Res. 2012, 11, 2301–2315. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Suzuki, Y.; Mae, T.; Makino, A. Changes in the synthesis of RuBisCO in rice leaves in relation to senescence and n influx. Ann. Bot. 2008, 101, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Koyama, K.; Kang, S.-S.; Huang, W.; Yanagawa, Y.; Takahashi, Y.; Nagano, M. Aging-related changes in in vitro-matured bovine oocytes: Oxidative stress, mitochondrial activity and atp content after nuclear maturation. J. Reprod. Dev. 2014, 60, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Khueychai, S.; Jangpromma, N.; Daduang, S.; Jaisil, P.; Lomthaisong, K.; Dhiravisit, A.; Klaynongsruang, S. Comparative proteomic analysis of leaves, leaf sheaths, and roots of drought-contrasting sugarcane cultivars in response to drought stress. Acta Physiol. Plant. 2015, 37, 88–103. [Google Scholar] [CrossRef]
- Nazir, M.; Pandey, R.; Siddiqi, T.O.; Ibrahim, M.M.; Qureshi, M.I.; Abraham, G.; Vengavasi, K.; Ahmad, A. Nitrogen-deficiency stress induces protein expression differentially in low-n tolerant and low-n sensitive maize genotypes. Front. Plant Sci. 2016, 7, 298. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ratajczak, R.; Zhang, J. Activity, amount and subunit composition of vacuolar-type h+-atpase and h+-ppase in wheat roots under severe nacl stress. J. Plant Physiol. 2000, 157, 109–116. [Google Scholar] [CrossRef]
- Asaoka, M.; Segami, S.; Ferjani, A.; Maeshima, M. Contribution of ppi-hydrolyzing function of vacuolar h+-pyrophosphatase in vegetative growth of arabidopsis: Evidenced by expression of uncoupling mutated enzymes. Front. Plant Sci. 2016, 7, 415. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Murai, N.; Kasaoka, K.; Hiyoshi, T.; Imaseki, H.; Burnell, J.N.; Arai, M. Carbon metabolism in transgenic rice plants that express phosphoenolpyruvate carboxylase and/or phosphoenolpyruvate carboxykinase. Plant. Sci. 2006, 170, 1010–1019. [Google Scholar] [CrossRef]
- Melzer, E.; O’Leary, M.H. Anapleurotic CO2 fixation by phosphoenolpyruvate carboxylase in c3 plants. Plant Physiol. 1987, 84, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yi, K.; Liu, Y.; Xie, L.; Zhou, Z.; Chen, Y.; Hu, Z.; Zheng, T.; Liu, R.; Chen, Y. Phosphoenolpyruvate carboxylase in arabidopsis leaves plays a crucial role in carbon and nitrogen metabolism. Plant Physiol. 2015, 167, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, S.S.; Ibrahim, R.; Damalas, C.A.; Noorhosseini, S.A. Effects of gamma stress and carbon dioxide on eight bioactive flavonoids and photosynthetic efficiency in Centella asiatica. J. Plant Growth Regul. 2017, 36, 957–969. [Google Scholar] [CrossRef]
- Yang, Z.; Yuan, C.; Han, W.; Li, Y.; Xiao, F. Effects of low irradiation on photosynthesis and antioxidant enzyme activities in cucumber during ripening stage. Photosynthetica 2016, 54, 251–258. [Google Scholar] [CrossRef]
- Campos, H.; Trejo, C.; Peña-Valdivia, C.B.; García-Nava, R.; Conde-Martínez, F.V.; Cruz-Ortega, M.R. Stomatal and non-stomatal limitations of bell pepper (Capsicum annuum L.) plants under water stress and re-watering: Delayed restoration of photosynthesis during recovery. Environ. Exp. Bot. 2014, 98, 56–64. [Google Scholar] [CrossRef]
- Medrano, H.; Escalona, J.M.; Bota, J.; Gulías, J.; Flexas, J. Regulation of photosynthesis of c3 plants in response to progressive drought: Stomatal conductance as a reference parameter. Ann. Bot. 2002, 89, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.-S.; Liang, X.-N.; Li, X.; Wang, S.-L.; Lv, D.-W.; Ma, C.-Y.; Li, X.-H.; Ma, W.-J.; Yan, Y.-M. Wheat drought-responsive grain proteome analysis by linear and nonlinear 2-de and maldi-tof mass spectrometry. Int. J. Mol. Sci. 2012, 13, 16065–16083. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Shimoda, Y.; Matsuda, K.; Tanaka, A.; Ito, H. Mg-dechelation of chlorophyll a by stay-green activates chlorophyll b degradation through expressing non-yellow coloring 1 in Arabidopsis thaliana. J. Plant Physiol. 2018, 222, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, M.; Liang, N.; Yan, H.; Wei, Y.; Xu, X.; Liu, J.; Xu, Z.; Chen, F.; Wu, G. Molecular cloning and function analysis of the stay green gene in rice. Plant. J. 2007, 52, 197–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosales, E.P.; Iannone, M.F.; Groppa, M.D.; Benavides, M.P. Nitric oxide inhibits nitrate reductase activity in wheat leaves. Plant Physiol. Biochem. 2011, 49, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, B.S.; Park, S.W.; Lee, H.Y.; Song, J.T.; Seo, H.S. Nitrate reductases are relocalized to the nucleus by atsiz1 and their levels are negatively regulated by cop1 and ammonium. Int. J. Mol. Sci. 2018, 19, 1202–1216. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Mertens, J.A.; Kanamaru, K.; Campbell, W.H.; Crawford, N.M. Analysis of wild-type and mutant plant nitrate reductase expressed in the methylotrophic yeast Pichia pastoris. Plant Physiol. 1997, 115, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Lea, P.J.; Miflin, B.J. Nitrogen assimilation and its relevance to crop improvement. Annu. Plant. Rev. 2018, 50, 1–40. [Google Scholar]
- Zhang, H.; Liang, C.; Aoki, N.; Kawai, K.; Takane, K.-I.; Ohsugi, R. Introduction of a fungal nadp (h)-dependent glutamate dehydrogenase (gdha) improves growth, grain weight and salt resistance by enhancing the nitrogen uptake efficiency in forage rice. Plant Prod. Sci. 2016, 19, 267–278. [Google Scholar] [CrossRef]
- Wallsgrove, R.M.; Turner, J.C.; Hall, N.P.; Kendall, A.C.; Bright, S.W.J. Barley mutants lacking chloroplast glutamine synthetase—Biochemical and genetic analysis. Plant Physiol. 1987, 83, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Gazanchian, A.; Hajheidari, M.; Sima, N.K.; Salekdeh, G.H. Proteome response of Elymus elongatum to severe water stress and recovery. J. Exp. Bot. 2007, 58, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, G.; Huang, W.; Bi, T.; Chen, G.; Tang, Z.; Su, W.; Sun, W. Proteomic study of Carissa spinarum in response to combined heat and drought stress. Proteomics 2010, 10, 3117–3129. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dinler, B.S.; Vignjevic, M.; Jacobsen, S.; Wollenweber, B. Physiological and proteome studies of responses to heat stress during grain filling in contrasting wheat cultivars. Plant Sci. 2015, 230, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Kosová, K.; Vítámvás, P.; Prášil, I.T. Proteomics of stress responses in wheat and barley—Search for potential protein markers of stress tolerance. Front. Plant Sci. 2014, 5, 711. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-K.; Ma, Y.-H.; Zheng, J.-W.; Yang, W.-D.; Liu, J.-S.; Li, H.-Y. Proteomics to reveal metabolic network shifts towards lipid accumulation following nitrogen deprivation in the diatom Phaeodactylum tricornutum. J. Appl. Phycol. 2014, 26, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.-J. Thiol-based peroxidases and ascorbate peroxidases: Why plants rely on multiple peroxidase systems in the photosynthesizing chloroplast? Mol. Cells 2016, 39, 20–25. [Google Scholar] [PubMed]
- Turner, D.D.; Lad, L.; Kwon, H.; Basran, J.; Sharp, K.H.; Moody, P.C.E.; Raven, E.L. The role of ala134 in controlling substrate binding and reactivity in ascorbate peroxidase. J. Inorg. Biochem. 2017, 180, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Motojima, F.; Fujii, K.; Yoshida, M. Chaperonin facilitates protein folding by avoiding polypeptide collapse. bioRxiv 2017. [Google Scholar] [CrossRef]
- Rao, T.; Lund, P.A. Differential expression of the multiple chaperonins of Mycobacterium smegmatis. FEMS Microbiol. Lett. 2010, 310, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, H.; Chaurasia, A.K.; Potnis, A.A. Multiple chaperonins in cyanobacteria: Why one is not enough! In Prokaryotic Chaperonins; Springer: New York, NY, USA, 2017; pp. 93–109. [Google Scholar]
- Hiei, Y.; Komari, T. Agrobacterium-mediated transformation of rice using immature embryos or calli induced from mature seed. Nat. Protoc. 2008, 3, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S. Routine procedure for growing rice plants in culture solution. Lab. Man. Physiol. Stud. Rice 1976, 61–66. [Google Scholar]
- Xiong, D.; Chen, J.; Yu, T.; Gao, W.; Ling, X.; Li, Y.; Peng, S.; Huang, J. Spad-based leaf nitrogen estimation is impacted by environmental factors and crop leaf characteristics. Sci. Rep. 2015, 5, 13389–13399. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Plaza, P.; Navas, M.J.; Wybraniec, S.; Michałowski, T.; Asuero, A.G. An overview of the kjeldahl method of nitrogen determination. Part ii. Sample preparation, working scale, instrumental finish, and quality control. Crit. Rev. Anal. Chem. 2013, 43, 224–272. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Muhammad, W.; Lin, M.; Azeem, S.; Zhao, H.; Lin, S.; Chen, T.; Fang, C.; Letuma, P. Proteomic analysis of positive influence of alternate wetting and moderate soil drying on the process of rice grain filling. Plant Growth Regul. 2018, 84, 533–548. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ye, J.; Fang, L.; Zheng, H.; Zhang, Y.; Chen, J.; Zhang, Z.; Wang, J.; Li, S.; Li, R.; Bolund, L. Wego: A web tool for plotting go annotations. Nucleic Acids Res. 2006, 34, W293–W297. [Google Scholar] [CrossRef] [PubMed]
- Deschenes, L.A.; Vanden Bout, D.A. Origin 6.0: Scientific data analysis and graphing software origin lab corporation (formerly Microcal software, Inc.). J. Am. Chem. Soc. 2000, 122, 9567–9568. [Google Scholar] [CrossRef]
- Barrett, K.C.; Morgan, G.A.; Leech, N.L.; Gloeckner, G.W. IBM SPSS for Introductory Statistics: Use and Interpretation; Routledge: Abingdon, UK, 2012. [Google Scholar]









| SN a | AN b | Protein Name | Functional Characterization | Subcellular Location | Score c | MW (kDa)/pI d | MP e | CT f |
|---|---|---|---|---|---|---|---|---|
| Energy Metabolism | ||||||||
| 3 | A6N1P5.1 | Ribulose-1,5-bisphosphate carboxylase/oxygenase | Energy (photosynthesis) | Chloroplast | 69 | 30.36/5.22 | 12 | UR |
| 10 | P93431-1 | RuBisCO | Energy (photosynthesis) | Chloroplast | 45 | 51.42/5.16 | 4 | UR |
| 20 | Q6ZG90 | ATP synthase | Energy | Mitochondria | 46 | 27.3/6.93 | 4 | UR |
| 7 | Q8S6Z1.1 | ATP synthase subunit alpha | ATP energy | Mitochondria | 136 | 29.30/5.33 | 9 | UR |
| 2 | A3C6G9.1 | Glycine cleavage system H protein | Energy | Mitochondria | 108 | 17.3/5.03 | 11 | UR |
| 24 | A1YQK1 | Malate dehydrogenase | TCA cycle | Cytoplasm | 155 | 36.5/6.33 | 6 | DR |
| Photosynthesis | ||||||||
| 26 | Q9FYX8 | Phosphoenolpyruvate carboxylase | Photosynthesis | Cytosol | 98 | 105.2/5.73 | 8 | DR |
| 30 | E9KIQ8 | Photosystem II CP47 | Photosynthesis | Chloroplast | 89 | 53.24/5.67 | 7 | UR |
| 22 | Q652K1 | Stay-green protein | Photosynthesis | Chloroplast | 234 | 121.32/4.66 | 13 | DR |
| Carbohydrate Metabolism | ||||||||
| 1 | Q6Z8F4.1 | Phosphoribulokinase | Calvin cycle | Chloroplast | 43 | 44.83/6.02 | 2 | DR |
| 5 | Q53P94.1 | Fructose-bisphosphate aldolase | Glycolysis | Chloroplast | 138 | 14.24/7.50 | 4 | DR |
| 9 | Q6EQ16.1 | ATP-dependent 6-phosphofructokinase | Glycolysis | Chloroplast | 121 | 52.7/7.65 | 10 | DR |
| 14 | Q0INM3.1 | Beta-galactosidase 15 | Carbohydrate metabolism | Cytosol | 269 | 100.8/5.95 | 16 | DR |
| 6 | Q84JG8 | Chloroplast sedoheptulose-1,7-bisphosphatase | Calvin cycle | Chloroplast | 75 | 42.21/5.84 | 6 | UR |
| Nitrogen Metabolism | ||||||||
| 23 | Q6ZHH7 | Nitrate reductase | Nitrogen metabolism | Chloroplast | 271 | 97.79/534 | 13 | UR |
| 19 | P14655 | Glutamine synthetase | Nitrogen metabolism | Chloroplast | 67 | 47.08/5.96 | 7 | UR |
| Cell Growth and Division | ||||||||
| 18 | Q10PE7 | Deoxymugineic acid synthase 1 | Growth | Cytosol | 129 | 33.6/7.5.41 | 13 | UR |
| 13 | Q7XE16 | Cell division cycle 48 | Cell division | Cell membrane | 134 | 88.8/5.23 | 15 | DR |
| Signaling | ||||||||
| 15 | Q06967 | 14-3-3 protein GF 14-B | Defense | Nucleus | 114 | 18.27/4.46 | 21 | DR |
| 27 | Q18PR8 | Beta subunit 2 of SnRK1 | signaling | Membrane | 46 | 32.76/5.44 | 9 | DR |
| 25 | Q6K1U4 | Probable protein phosphatase 2C 16 | Phosphatase | Nucleus | 94 | 56.21/4.17 | 19 | UR |
| Transcription | ||||||||
| 11 | Q6IER3 | WRKY 8 | Transcription | Nucleus | 357 | 30.36/7.07 | 20 | DR |
| 8 | E5RQA1 | GHD 7 | Transcription | Nucleus | 112 | 26.83/7.23 | 14 | DR |
| 21 | Q84MM9 | Monoculm protein 1 | Transcription | Nucleus | 123 | 48.5/7.54 | 18 | UR |
| Storage and Structural Protein | ||||||||
| 29 | Q8H903 | Chaperonin 60 kDa protein | Protein synthesis, folding | Cytosol | 336 | 60.8/5.78 | 12 | DR |
| Defense | ||||||||
| 17 | Q943K7 | HSP 70 | Defense | Nucleus | 90 | 71.23/5.21 | 7 | UR |
| 28 | Q5QT28 | Remorin 1 protein/Hsp20 | Defense | Nucleus | 44 | 22.4/537 | 6 | UR |
| 16 | Q9FE01 | L-ascorbate peroxidase | Defense | Chloroplast | 75 | 28.49/5.36 | 14 | DR |
| 4 | Q6YUZ0 | Thaumatin-like protein | Defense | Cytoplasm | 97 | 18.81/4.88 | 10 | DR |
| AN a | Protein Name b | Functional Characterization | Subcellular Location | Score c | MW (kDa)/pI d |
|---|---|---|---|---|---|
| Energy Metabolism | |||||
| LOC_Os12g10580.1 | Ribulose bisphosphate carboxylase large chain | Energy (photosynthesis) | Chloroplast | 371.7 | 56/8.9 |
| LOC_Os10g21268.1 | Ribulose bisphosphate carboxylase large chain | Energy (photosynthesis) | Chloroplast | 683.23 | 53.7/7.03 |
| LOC_Os06g39740.1 | ATP synthase subunit beta | Energy | Mitochondria | 576.28 | 54.2/5.5 |
| LOC_Os06g45120.1 | ATP synthase | ATP energy | Mitochondria | 178.54 | 68.4/5.34 |
| LOC_Os10g17280.1 | ATP synthase gamma chain | Energy | Mitochondria | 16.83 | 35.2/7.03 |
| LOC_Os04g16740.1 | ATP synthase subunit alpha | ATP energy | Mitochondria | 279.38 | 55.6/6.25 |
| LOC_Os03g17070.1 | ATP synthase B chain chloroplast precursor | ATP energy | Mitochondria | 39.69 | 22.7/5.85 |
| LOC_Os10g37180.1 | Glycine cleavage system H protein | Energy | Mitochondria | 50.25 | 17.4/5.03 |
| LOC_Os03g56280.1 | Malate dehydrogenase | TCA cycle | Cytoplasm | 146.16 | 37/7.94 |
| Photosynthesis | |||||
| LOC_Os08g27840.1 | Phosphoenolpyruvate carboxylase | Photosynthesis | Cytosol | 260.03 | 110/5.8 |
| LOC_Os04g16874.1 | Photosystem II 44 kDa reaction center protein | Photosynthesis | Chloroplast | 94.42 | 44.7/6.6 |
| LOC_Os03g21560.1 | Photosystem II 11 kD protein | Photosynthesis | Chloroplast | 21.05 | 17.6/9.91 |
| LOC_Os07g05360.1 | Photosystem II 10 kDa polypeptide, chloroplast | Photosynthesis | Chloroplast | 22.11 | 14.2/9.81 |
| LOC_Os02g24634.1 | Photosystem II D2 protein | Photosynthesis | Chloroplast | 64.09 | 39.6/5.4 |
| LOC_Os06g39708.1 | Photosystem II P680 chlorophyll a Apoprotein | Photosynthesis | Chloroplast | 161.07 | 56.2/6.64 |
| Carbohydrate Metabolism | |||||
| LOC_Os02g47020.1 | Phosphoribulokinase | Calvin cycle | Chloroplast | 266.6 | 44.8/6.02 |
| LOC_Os04g16680.1 | Fructose-1,6-bisphosphatase | Glycolysis | Chloroplast | 256.8 | 42.2/6.09 |
| LOC_Os11g07020.1 | Fructose-bisphosphate aldolase isozyme | Glycolysis | Chloroplast | 403.24 | 42/6.8 |
| LOC_Os01g64660.2 | Fructose-1,6-bisphosphatase | Carbohydrate metabolism | Cytosol | 127.72 | 37/5.77 |
| LOC_Os08g03290.1 | Glyceraldehyde-3-phosphate dehydrogenase | Carbohydrate metabolism | Cytosol | 97.76 | 36.4/7.11 |
| Nitrogen Metabolism | |||||
| LOC_Os02g50240.1 | Glutamine synthetase | Nitrogen metabolism | Chloroplast | 134.89 | 39.2/5.73 |
| LOC_Os05g04220.1 | Nitrogen regulatory protein P-II | Nitrogen metabolism | Chloroplast | 5.02 | 22.7/9.91 |
| LOC_Os02g52730.1 | Ferredoxin-nitrite reductase | Nitrogen metabolism | Chloroplast | 3.35 | 72.4/8.29 |
| LOC_Os04g01590.1 | Arginase | Nitrogen metabolism | Mitochondria | 14.54 | 36.9/5.3 |
| LOC_Os09g28050.1 | Asparate aminotransferase | Nitrogen metabolism | Chloroplast | 19.62 | 50.6/6 |
| Cell Growth and Division | |||||
| LOC_Os10g30580.1 | Cell division | Growth | Cytosol | 42.5 | 89.8/5.21 |
| LOC_Os02g58790.1 | Cell division inhibitor | Growth | Cytosol | 6.35 | 38.8/9.16 |
| Signaling | |||||
| LOC_Os03g50290.1 | 14-3-3 protein | Defense | Nucleus | 112.53 | 29.2/4.88 |
| LOC_Os04g38870.3 | 14-3-3 protein | Signaling | Membrane | 88.34 | 29.8/4.81 |
| LOC_Os08g33370.2 | 14-3-3 protein | Phosphatase | Nucleus | 74.19 | 28.8/4.84 |
| LOC_Os05g11550.1 | Serine/threonine protein phosphatase 5 | Growth | Cytosol | 5.86 | 54.4/6.02 |
| LOC_Os07g32380.1 | Protein phosphatase 2C | Phosphatase | Nucleus | 4.9 | 20/8.18 |
| LOC_Os09g06230.1 | Serine/threonine-protein kinase 16 | Phosphatase | Nucleus | 3.21 | 25.5/4.7 |
| LOC_Os04g56450.1 | Protein phosphatase 2C | Signaling | Membrane | 1.89 | 30.6/5.15 |
| LOC_Os09g33790.1 | SnRK1-interacting protein 1 | Signaling | Membrane | 2.95 | 109/7.15 |
| LOC_Os02g38300.1 | SNF7 domain-containing protein | Signaling | Membrane | 2.69 | 25.5/4.7 |
| Transcription | |||||
| LOC_Os03g55164.1 | WRKY4 | Transcription | Nucleus | 2.47 | 14.8/7.28 |
| Storage and structural protein | |||||
| LOC_Os06g09679.2 | Chaperonin | Protein synthesis, folding | Cytosol | 81.66 | 26.3/8.02 |
| LOC_Os10g41710.1 | Chaperonin, putative expressed | Protein synthesis, folding | Cytosol | 5.18 | 21.1/8.92 |
| LOC_Os09g26730.1 | Chaperonin | Protein synthesis, folding | Cytosol | 70.68 | 69.1/6.37 |
| Defense | |||||
| LOC_Os10g07210.1 | Hsp20/alpha crystallin family protein | Defense | Nucleus | 2.26 | 18.6/8.2 |
| LOC_Os06g37150.1 | L-ascorbate oxidase precursor | Defense | Chloroplast | 2.9 | 27.4/7.65 |
| LOC_Os07g49400.2 | Cytosolic Ascorbate Peroxidase | Defense | Chloroplast | 68.37 | 25.2/5.71 |
| LOC_Os12g43440.1 | Thaumatin putative, | Defense | Cytoplasm | 9.64 | 22.4/5.5 |
| LOC_Os07g47510.1 | Stress-related protein, | Defense | Nucleus | 2.38 | 27.4/7.65 |
| LOC_Os09g29200.1 | Glutathione S-transferase, | Defense | Cytoplasm | 134.49 | 25.2/5.71 |
| LOC_Os04g45070.1 | Remorin | Defense | Nucleus | 45.89 | 22.4/5.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waqas, M.; Feng, S.; Amjad, H.; Letuma, P.; Zhan, W.; Li, Z.; Fang, C.; Arafat, Y.; Khan, M.U.; Tayyab, M.; et al. Protein Phosphatase (PP2C9) Induces Protein Expression Differentially to Mediate Nitrogen Utilization Efficiency in Rice under Nitrogen-Deficient Condition. Int. J. Mol. Sci. 2018, 19, 2827. https://doi.org/10.3390/ijms19092827
Waqas M, Feng S, Amjad H, Letuma P, Zhan W, Li Z, Fang C, Arafat Y, Khan MU, Tayyab M, et al. Protein Phosphatase (PP2C9) Induces Protein Expression Differentially to Mediate Nitrogen Utilization Efficiency in Rice under Nitrogen-Deficient Condition. International Journal of Molecular Sciences. 2018; 19(9):2827. https://doi.org/10.3390/ijms19092827
Chicago/Turabian StyleWaqas, Muhammad, Shizhong Feng, Hira Amjad, Puleng Letuma, Wenshan Zhan, Zhong Li, Changxun Fang, Yasir Arafat, Muhammad Umar Khan, Muhammad Tayyab, and et al. 2018. "Protein Phosphatase (PP2C9) Induces Protein Expression Differentially to Mediate Nitrogen Utilization Efficiency in Rice under Nitrogen-Deficient Condition" International Journal of Molecular Sciences 19, no. 9: 2827. https://doi.org/10.3390/ijms19092827
APA StyleWaqas, M., Feng, S., Amjad, H., Letuma, P., Zhan, W., Li, Z., Fang, C., Arafat, Y., Khan, M. U., Tayyab, M., & Lin, W. (2018). Protein Phosphatase (PP2C9) Induces Protein Expression Differentially to Mediate Nitrogen Utilization Efficiency in Rice under Nitrogen-Deficient Condition. International Journal of Molecular Sciences, 19(9), 2827. https://doi.org/10.3390/ijms19092827