Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) Reduces Oxidative and Mechanical Stress-Evoked Matrix Degradation in Chondrifying Cell Cultures
Abstract
:1. Introduction
2. Results
2.1. Hyaluronidase Expression in Chondrifying Cells
2.2. Identification of MMPs in Chondrifying Cell Cultures
2.3. Aggrecanase Activity in Chondrogenesis
2.4. Effects of PACAP and Various Stress on Metachromatic Cartilage Formation
2.5. Various Cellular Stress Increase Hyluronidase Activity
2.6. Expression of MMPs Affected by PACAP Treatment
2.7. Aggrecanase Function during Oxidative and Mechanical Stress
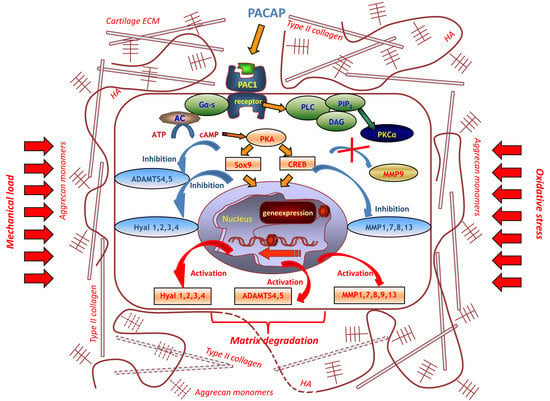
3. Discussion
4. Materials and Methods
4.1. Cell Culturing
4.2. Application of PACAP, H2O2, and Mechanical Stress
4.3. Light Microscopical Analysis
4.4. RT-PCR Reactions
4.5. Western Blot
4.6. Hyaluronidase Assay
4.7. Zymography
4.8. Aggrecanase Activity Assay
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| ADAMTS | a disintegrin and metalloprotease with thrombospondin motifs |
| BMP | bone morphogenetic protein |
| cAMP | cyclic adenosine monophosphate |
| CREB | cAMP response element-binding protein |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| dNTP | deoxynucleotide triphosphate |
| ECM | extracellular matrix |
| EDTA | ethylene diamine tetra-acetic acid |
| EGTA | ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetra acetic acid |
| FBS | foetal bovine serum |
| FGF | fibroblast growth factor |
| HH | hedgehog |
| HEPES | 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid |
| Hyal | hyaluronidase |
| IHH | Indian Hedgehog |
| MAPK | mitogen-activated protein kinase |
| MMP | matrix metalloproteinase |
| MS | mechanical stress |
| PAC1 | pituitary adenylate cyclase-activating polypeptide type I receptor |
| PACAP | pituitary adenylate cyclase activating polypeptide |
| PBS | phosphate buffered saline |
| PBST | phosphate buffered saline supplemented with 1% Tween-20 |
| PKA | protein kinase A |
| PKC | protein kinase C |
| RT-PCR | reverse transcription followed by polymerase chain reaction |
| Runx2 | Runt-related transcription factor 2 |
| SHH | Sonic Hedgehog |
| Sox9 | SRY (sex determining region Y)-box 9 |
| TGFβ | transforming growth factor-β |
| VIP | vasoactive intestinal polypeptide |
| VPAC | vasoactive intestinal polypeptide receptor |
| WNT | wingless int1 |
References
- Carballo, C.B.; Nakagawa, Y.; Sekiya, I.; Rodeo, S.A. Basic science of articular cartilage. Clin. Sports Med. 2017, 36, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Bastow, E.R.; Byers, S.; Golub, S.B.; Clarkin, C.E.; Pitsillides, A.A.; Fosang, A.J. Hyaluronan synthesis and degradationin cartilage and bone. Cell. Mol. Life Sci. 2008, 65, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Zakany, R.; Szucs, K.; Bako, E.; Felszeghy, S.; Czifra, G.; Biro, T.; Modis, L.; Gergely, P. Protein phosphatase 2A is involved in the regulation of protein kinase A signaling pathway during in vitro chondrogenesis. Exp. Cell Res. 2002, 275, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Matta, C.; Juhasz, T.; Szijgyarto, Z.; Kolozsvari, B.; Somogyi, C.; Nagy, G.; Gergely, P.; Zakany, R. PKCdelta is a positive regulator of chondrogenesis in chicken high density micromass cell cultures. Biochimie 2011, 93, 149–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakany, R.; Szijgyarto, Z.; Matta, C.; Juhasz, T.; Csortos, C.; Szucs, K.; Czifra, G.; Biro, T.; Modis, L.; Gergely, P. Hydrogen peroxide inhibits formation of cartilage in chicken micromass cultures and decreases the activity of calcineurin: Implication of ERK1/2 and Sox9 pathways. Exp. Cell Res. 2005, 305, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Zakany, R.; Bako, E.; Felszeghy, S.; Hollo, K.; Balazs, M.; Bardos, H.; Gergely, P.; Modis, L. Okadaic acid-induced inhibition of protein phosphatase 2A enhances chondrogenesis in chicken limb bud micromass cell cultures. Anat. Embryol. 2001, 203, 23–34. [Google Scholar]
- Juhasz, T.; Matta, C.; Katona, E.; Somogyi, C.; Takacs, R.; Gergely, P.; Csernoch, L.; Panyi, G.; Toth, G.; Reglodi, D.; et al. Pituitary adenylate cyclase activating polypeptide (PACAP) signalling exerts chondrogenesis promoting and protecting effects: Implication of calcineurin as a downstream target. PLoS ONE 2014, 9, e91541. [Google Scholar] [CrossRef]
- Huang, W.; Zhou, X.; Lefebvre, V.; de Crombrugghe, B. Phosphorylation of SOX9 by cyclic AMP-dependent protein kinase A enhances SOX9’ sability to transactivate a Col2a1 chondrocyte-specific enhancer. Mol. Cell. Biol. 2000, 20, 4149–4158. [Google Scholar] [CrossRef]
- Zakany, R.; Bakondi, E.; Juhasz, T.; Matta, C.; Szijgyarto, Z.; Erdelyi, K.; Szabo, E.; Modis, L.; Virag, L.; Gergely, P. Oxidative stress-induced poly(ADP-ribosyl)ation in chick limb bud-derived chondrocytes. Int. J. Mol. Med. 2007, 19, 597–605. [Google Scholar] [Green Version]
- Juhasz, T.; Szentleleky, E.; Somogyi, C.S.; Takacs, R.; Dobrosi, N.; Engler, M.; Tamas, A.; Reglodi, D.; Zakany, R. Pituitary adenylate cyclase activating polypeptide (PACAP) pathway is induced by mechanical load and reduces the activity of hedgehog signaling in chondrogenic micromass cell cultures. Int. J. Mol. Sci. 2015, 16, 17344–17367. [Google Scholar] [CrossRef]
- Fusco, M.; Skaper, S.D.; Coaccioli, S.; Varrassi, G.; Paladini, A. Degenerative joint diseases and neuroinflammation. Pain Pract. 2017, 17, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, H.; Kimura, T.; Dalal, S.; Okada, Y.; D’Armiento, J. Joint diseases and matrix metalloproteinases: A role for MMP-13. Curr. Pharm. Biotechnol. 2008, 9, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Snoek-van Beurden, P.A.M.; Vonden Hoff, J.W. Zymographic techniques for the analysis of matrix metalloproteinases and their inhibitors. Biotechniques 2005, 38, 73–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiraishi, A.; Mochizuki, S.; Miyakoshi, A.; Kojoh, K.; Okada, Y. Development of human neutralizing antibody to ADAMTS4 (aggrecanase-1) and ADAMTS5 (aggrecanase-2). Biochem. Biophys. Res. Commun. 2016, 469, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Knudson, W.; Ishizuka, S.; Terabe, K.; Askew, E.B.; Knudson, C.B. The pericellular hyaluronan of articular chondrocytes. Matrix Biol. 2018, 30437. [Google Scholar] [CrossRef]
- Miyata, A.; Arimura, A.; Dahl, R.R.; Minamino, N.; Uehara, A.; Jiang, L.; Culler, M.D.; Coy, D.H. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem. Biophys. Res. Commun. 1989, 164, 567–574. [Google Scholar] [CrossRef]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.; Hashimoto, H.; Galas, L.; et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef]
- Reglodi, D.; Cseh, S.; Somoskoi, B.; Fulop, B.D.; Szentleleky, E.; Szegeczki, V.; Kovacs, A.; Varga, A.; Kiss, P.; Hashimoto, H.; et al. Disturbed spermatogenic signaling in pituitary adenylate cyclase activating polypeptide-deficient mice. Reproduction 2018, 155, 129–139. [Google Scholar] [CrossRef]
- Sandor, B.; Fintor, K.; Felszeghy, S.; Juhasz, T.; Reglodi, D.; Mark, L.; Kiss, P.; Jungling, A.; Fulop, B.D.; Nagy, A.D.; et al. Structural and morphometric comparison of the molar teeth in pre-eruptive developmental stage of PACAP-deficient and wild-type mice. J. Mol. Neurosci. 2014, 54, 331–341. [Google Scholar] [CrossRef]
- Juhasz, T.; Matta, C.; Katona, E.; Somogyi, C.; Takacs, R.; Hajdu, T.; Helgadottir, S.L.; Fodor, J.; Csernoch, L.; Toth, G.; et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) signalling enhances osteogenesis in UMR-106 cell line. J. Mol. Neurosci. 2014, 54, 555–573. [Google Scholar] [CrossRef]
- Juhasz, T.; Helgadottir, S.L.; Tamas, A.; Reglodi, D.; Zakany, R. PACAP and VIP signaling in chondrogenesis and osteogenesis. Peptides 2015, 66, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banki, E.; Kovacs, K.; Nagy, D.; Juhasz, T.; Degrell, P.; Csanaky, K.; Kiss, P.; Jancso, G.; Toth, G.; Tamas, A.; et al. Molecular mechanisms underlying the nephroprotective effects of PACAP in diabetes. J. Mol. Neurosci. 2014, 54, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Laszlo, E.; Varga, A.; Kovacs, K.; Jancso, G.; Kiss, P.; Tamas, A.; Szakaly, P.; Fulop, B.; Reglodi, D. Ischemia/reperfusion-induced kidney injury in heterozygous PACAP-deficient mice. Transp. Proc. 2015, 47, 2210–2215. [Google Scholar] [CrossRef] [PubMed]
- Mester, L.; Kovacs, K.; Racz, B.; Solti, I.; Atlasz, T.; Szabadfi, K.; Tamas, A.; Reglodi, D. Pituitary adenylate cyclase-activating polypeptide is protective against oxidative stress in human retinal pigment epithelial cells. J. Mol. Neurosci. 2011, 43, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Gourlet, P.; Vandermeers, A.; Vertongen, P.; Rathe, J.; DeNeef, P.; Cnudde, J.; Waelbroeck, M.; Robberecht, P. Development of high affinity selective VIP1 receptor agonists. Peptides 1997, 18, 1539–1545. [Google Scholar] [CrossRef]
- Giunta, S.; Castorina, A.; Marzagalli, R.; Szychlinska, M.A.; Pichler, K.; Mobasheri, A.; Musumeci, G. Ameliorative effects of PACAP against cartilage degeneration. Morphological, immunohistochemical and biochemical evidence from in vivo and in vitro models of rat osteoarthritis. Int. J. Mol. Sci. 2015, 16, 5922–5944. [Google Scholar] [CrossRef] [PubMed]
- Sasano, Y.; Zhu, J.X.; Tsubota, M.; Takahashi, I.; Onodera, K.; Mizoguchi, I.; Kagayama, M. Gene Expression of MMP8 and MMP13 during embryonic development of bone and cartilage in the rat mandible and hind limb. J. Histochem. Cytochem. 2002, 50, 325–332. [Google Scholar] [CrossRef]
- Assis-Ribas, T.; Forni, M.F.; Winnischofer, S.M.B.; Sogayar, M.C.; Trombetta-Lima, M. Extracellular matrix dynamics during mesenchymal stem cells differentiation. Dev. Biol. 2018, 437, 63–74. [Google Scholar] [CrossRef]
- Kelwick, R.; Desanlis, I.; Wheeler, G.N.; Edwards, D.R. The ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) family. Genome Biol. 2015, 16, 113. [Google Scholar] [CrossRef]
- Higuchi, Y.; Nishida, Y.; Kozawa, E.; Zhuo, L.; Arai, E.; Hamada, S.; Morita, D.; Ikuta, K.; Kimata, K.; Ushida, T.; et al. Conditional knockdown of hyaluronidase 2 in articular cartilage stimulates osteoarthritic progression in a mice model. Sci. Rep. 2017, 7, 7028. [Google Scholar] [CrossRef]
- Nicoll, S.B.; Barak, O.; Csoka, A.B.; Bhatnagar, R.S.; Stern, R. Hyaluronidases and CD44 undergo differential modulation during chondrogenesis. Biochem. Biophys. Res. Commun. 2002, 292, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Kaneiwa, T.; Miyazaki, A.; Kogawa, R.; Mizumoto, S.; Sugahara, K.; Yamada, S. Identification of amino acid residues required for the substrate specificity of human and mouse chondroitinsulfate hydrolase (conventional hyaluronidase-4). J. Biol. Chem. 2012, 287, 42119–42128. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yue, S.X.; Zhou, G.; Greenfield, E.M.; Murakami, S. ERK1 and ERK2 regulate chondrocyte terminal differentiation during endochondral bone formation. J. Bone Miner. Res. 2015, 30, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.T.; Gao, Y.J.; Duan, C.C.; Li, D.D.; Tian, X.C.; Zhang, Q.L.; Guo, B.; Yue, Z.P. Effects of PTHrP on expression of MMP9 and MMP13 in sika deer antler chondrocytes. Cell Biol. Int. 2013, 37, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Parmar, P.A.; Chow, L.W.; St-Pierre, J.P.; Horejs, C.M.; Peng, Y.Y.; Werkmeister, J.A.; Ramshaw, J.A.; Stevens, M.M. Collagen-mimetic peptide-modifiable hydrogels for articular cartilage regeneration. Biomaterials 2015, 54, 213–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thirunavukkarasu, K.; Pei, Y.; Moore, T.L.; Wang, H.; Yu, X.P.; Geiser, A.G.; Chandrasekhar, S. Regulation of the human ADAMTS-4 promoter by transcription factors and cytokines. Biochem. Biophys. Res. Commun. 2006, 345, 197–204. [Google Scholar] [CrossRef]
- El Zein, N.; Badran, B.; Sariban, E. VIP differentially activates beta2 integrins, CR1, and Matrixmetalloproteinase-9 in human monocytes through cAMP/PKA, EPAC, and PI-3K signaling pathways via VIP receptor type1 and FPRL1. J. Leukoc. Biol. 2008, 83, 972–981. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta 2016, 1862, 576–591. [Google Scholar] [CrossRef]
- Juhasz, T.; Matta, C.; Somogyi, C.; Katona, E.; Takacs, R.; Soha, R.F.; Szabo, I.A.; Cserhati, C.; Szody, R.; Karacsonyi, Z.; et al. Mechanical loading stimulates chondrogenesis via the PKA/CREB-Sox9 and PP2A pathways in chicken micromass cultures. Cell. Signal. 2014, 26, 468–482. [Google Scholar] [CrossRef] [Green Version]
- Tanimoto, K.; Kitamura, R.; Tanne, Y.; Kamiya, T.; Kunimatsu, R.; Yoshioka, M.; Tanaka, N.; Tanaka, E.; Tanne, K. Modulation of hyaluronan catabolism in chondrocytes by mechanical stimuli. J. Biomed. Mater. Res. 2010, 93, 373–380. [Google Scholar] [CrossRef]
- Kitamura, R.; Tanimoto, K.; Tanne, Y.; Kamiya, T.; Huang, Y.C.; Tanaka, N.; Tanaka, E.; Tanne, K. Effects of mechanical load on the expression and activity of hyaluronidasein cultured synovial membrane cells. J. Biomed. Mater. Res. 2010, 92, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Albano, G.D.; Bonanno, A.; Cavalieri, L.; Ingrassia, E.; DiSano, C.; Siena, L.; Riccobono, L.; Gagliardo, R.; Profita, M. Effect of high, medium, and low molecular weight hyaluronan on inflammation and oxidative stress in an in vitro model of human nasal epithelial cells. Mediat. Inflamm. 2016, 2016, 8727289. [Google Scholar] [CrossRef] [PubMed]
- Katona, E.; Juhasz, T.; Somogyi, C.S.; Hajdu, T.; Szasz, C.; Racz, K.; Kokai, E.; Gergely, P.; Zakany, R. PP2B and ERK1/2 regulate hyaluronan synthesis of HT168 and WM35 human melanoma cell lines. Int. J. Oncol. 2016, 48, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.J.; Hardin, J.A.; Cobelli, N.J.; Sun, H.B. Mechanotransduction and cartilage integrity. Ann. N. Y. Acad. Sci. 2011, 1240, 32–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Tang, Y.; Xiao, L.; Liu, S.S.; Yu, H. Suture cartilage formation pattern varies with different expansive forces. Am. J. Orthod. Dentofac. Orthop. 2014, 146, 442–450. [Google Scholar] [CrossRef]
- Ibaragi, S.; Shimo, T.; Iwamoto, M.; Hassan, N.M.; Kodama, S.; Isowa, S.; Sasaki, A. Parathyroid hormone-related peptide regulates matrixmetalloproteinase-13 gene expression in bone metastatic breast cancer cells. Anticancer Res. 2010, 30, 5029–5036. [Google Scholar]
- Park, C.H.; Moon, Y.; Shin, C.M.; Chung, J.H. Cyclic AMP suppresses matrixmetalloproteinase-1 expression through inhibition of MAPK and GSK-3beta. J. Investig. Dermatol. 2010, 130, 2049–2056. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.; Forteza, J.; Lopez-Otin, C.; Gomez-Reino, J.J.; Gonzalez, A.; Conde, C. Matrixmetalloproteinase-8 deficiency increases joint inflammation and bone erosion in the K/BxN serum-transfer arthritis model. Arthritis Res. Ther. 2010, 12, R224. [Google Scholar] [CrossRef]
- Karsdal, M.A.; Sumer, E.U.; Wulf, H.; Madsen, S.H.; Christiansen, C.; Fosang, A.J.; Sondergaard, B.C. Induction of increased cAMP levels in articular chondrocytes blocks matrixmetalloproteinase-mediated cartilage degradation, but not aggrecanase-mediated cartilage degradation. Arthritis Rheum. 2007, 56, 1549–1558. [Google Scholar] [CrossRef]
- Harfi, I.; D’Hondt, S.; Corazza, F.; Sariban, E. Regulation of human polymorphonuclear leukocytes functions by the neuropeptide pituitary adenylate cyclase-activating polypeptide after activation of MAPKs. J. Immunol. 2004, 173, 4154–4163. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, W.; Huang, T.; Zhu, Y.; Huang, C. RUNX2 promotes hepatocellular carcinoma cell migration and invasion by upregulating MMP9 expression. Oncol. Rep. 2016, 36, 2777–2784. [Google Scholar] [CrossRef]
- Aisenbrey, E.A.; Bryant, S.J. Mechanical loading inhibits hypertrophy in chondrogenically differentiating hMSCs within a biomimetic hydrogel. J. Mater. Chem. B 2016, 4, 3562–3574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niewiadomski, P.; Zhujiang, A.; Youssef, M.; Waschek, J.A. Interaction of PACAP with sonic hedgehog reveals complex regulation of the hedgehog pathway by PKA. Cell. Signal. 2013, 25, 2222–2230. [Google Scholar] [CrossRef]
- Aisenbrey, E.A.; Bryant, S.J. A MMP7-sensitive photoclickable biomimetic hydrogel for MSC encapsulation towards engineering human cartilage. J. Biomed. Mater. Res. 2018, 106, 2344–2355. [Google Scholar] [CrossRef] [PubMed]
- Al-Alem, L.F.; McCord, L.A.; Southard, R.C.; Kilgore, M.W.; Curry, T.E., Jr. Activation of the PKC pathway stimulates ovarian cancer cell proliferation, migration, and expression of MMP7 and MMP10. Biol. Reprod. 2013, 89, 73. [Google Scholar] [CrossRef] [PubMed]
- Guan, P.P.; Yu, X.; Guo, J.J.; Wang, Y.; Wang, T.; Li, J.Y.; Konstantopoulos, K.; Wang, Z.Y.; Wang, P. By activating matrixmetalloproteinase-7, shearstress promotes chondrosarcoma cell motility, invasion and lung colonization. Oncotarget 2015, 6, 9140–9159. [Google Scholar] [CrossRef] [PubMed]
- LeMaitre, C.L.; Freemont, A.J.; Hoyland, J.A. Human discdegeneration is associated with increased MMP7 expression. Biotech. Histochem. 2006, 81, 125–131. [Google Scholar] [CrossRef]
- Verma, P.; Dalal, K.; Chopra, M. Pharmacophore development and screening for discovery of potential inhibitors of ADAMTS-4 for osteoarthritis therapy. J. Mol. Model. 2016, 22, 178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ji, Q.; Wang, X.; Kang, L.; Fu, Y.; Yin, Y.; Li, Z.; Liu, Y.; Xu, X.; Wang, Y. SOX9 is a regulator of ADAMTSs-induced cartilage degeneration at the early stage of human osteoarthritis. Osteoarthr. Cartil. 2015, 23, 2259–2268. [Google Scholar] [CrossRef] [Green Version]
- Latridis, J.C.; Godburn, K.; Wuertz, K.; Alini, M.; Roughley, P.J. Region-dependent aggrecan degradation patterns in the rat intervertebral disc are affected by mechanical loading in vivo. Spine 2011, 36, 203–209. [Google Scholar] [CrossRef]
- Shen, C.; Yan, J.; Erkocak, O.F.; Zheng, X.F.; Chen, X.D. Nitric oxide inhibits autophagy via suppression of JNK in meniscal cells. Rheumatology 2014, 53, 1022–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzgerald, J.B.; Jin, M.; Dean, D.; Wood, D.J.; Zheng, M.H.; Grodzinsky, A.J. Mechanical compression of cartilage explants induces multiple time-dependent gene expression patterns and involves intracellular calcium and cyclic AMP. J. Biol. Chem. 2004, 279, 19502–19511. [Google Scholar] [CrossRef]
- Perez-Garcia, S.; Carrion, M.; Gutierrez-Canas, I.; Gonzalez-Alvaro, I.; Gomariz, R.P.; Juarranz, Y. VIP and CRF reduce ADAMTS expression and function in osteoarthritis synovial fibroblasts. J. Cell. Mol. Med. 2016, 20, 678–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juhász, T.; Tamás, A.; Zákány, R.; Reglődi, D. Role of PACAP and VIP signalling in regulation of chondrogenesis and osteogenesis. In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Current Topics in Neurotoxicity; Springer: Cham, Switzerland, 2016; Volume 11, pp. 337–354. ISBN 978-3-319-35135-3. [Google Scholar]
- Matta, C.; Fodor, J.; Szijgyarto, Z.; Juhasz, T.; Gergely, P.; Csernoch, L.; Zakany, R. Cytosolic free Ca2+ concentration exhibits a characteristic temporal pattern during in vitro cartilage differentiation: A possible regulatory role of calcineurin in Ca-signalling of chondrogenic cells. Cell Calcium 2008, 44, 310–323. [Google Scholar] [CrossRef] [PubMed]







| Gene | Primer | Nucleotide Sequence (5’→3’) | GenBank ID | Annealing Temperature | Amplimer Size (bp) |
|---|---|---|---|---|---|
| Admats4 | sense | GTG GCA AGT ATT GTG AGG G(2079–2097) | NM_172845.3 | 54 °C | 124 |
| antisense | AGG TCG GTT CGG TGG TT(2186–2202) | ||||
| Hyal1 | sense | GGG GTC TTT GAT GTC GTG G(371–389) | XM_015292793.1 | 57 °C | 486 |
| antisense | CGG GTC GCT GAA GTT GTT(839–856) | ||||
| Hyal2 | sense | ACA ACC ACG ACT ACA GCA AGA A(784–805) | XM_414258.5 | 56 °C | 452 |
| antisense | CGC TGC CAT CGT CAC ATT(1218–1235) | ||||
| Hyal3 | sense | TAC GGC ATC GTG GAG AAC CG(265–284) | XM_003641994.3 | 59 °C | 450 |
| antisense | CCA GTC GTC GTT GAA GCA GTC G(693–714) | ||||
| Hyal4 | sense | CCA CCG TGC CTT GCT ATT(325–342) | XM_017011911.1 | 51 °C | 254 |
| antisense | GTT TGC TGC TGG TCC TTT(560–577) | ||||
| MMP1 | sense | TTT GTG ACC CTA ACT TGA(1021–1038) | XM_417176.4 | 47 °C | 473 |
| antisense | GAC ATA GCC ATC TTT CTG(1476–1493) | ||||
| MMP7 | sense | AAA AGA GTT ACC TCG GGA CA(483–502) | NM_007742.3 | 52 °C | 250 |
| antisense | CAC GGA CAT TTG AGT GGG(717–735) | ||||
| MMP8 | sense | TGT CAA GGG CTG AAG TGA(478–495) | NM_008611.4 | 51 °C | 382 |
| antisense | TGA GGT AGT GAA TAG GTG C(841–859) | ||||
| MMP9 | sense | TTC TGG ACT CTG GGA ACC G(625–643) | NM_204667.1 | 57 °C | 237 |
| antisense | GGG AGA CCC ATC GCT GTT(844–861) | ||||
| MMP13 | sense | CAT GCA GAA ACC ACG ATG(296–313) | NM_001293090.1 | 51 °C | 395 |
| antisense | GAG CAG CAA CAA GAA ACA AG(671–690) | ||||
| GAPDH | sense | GAG AAC GGG AAA CTT GTC AT(238–258) | NM_204305 | 54 °C | 556 |
| antisense | GGC AGG TCA GGT CAA CAA(775–793) |
| Antibody | Host Animal | Dilution | Distributor |
|---|---|---|---|
| Anti-Hyal1 | rabbit, polyclonal | 1:500 | Sigma-Aldrich, St. Louis, MO, USA |
| Anti-Hyal2 | rabbit, polyclonal | 1:500 | Sigma-Aldrich, St. Louis, MO, USA |
| Anti-Hyal3 | rabbit, polyclonal | 1:500 | Sigma-Aldrich, St. Louis, MO, USA |
| Anti-Hyal4 | rabbit, polyclonal | 1:500 | Sigma-Aldrich, St. Louis, MO, USA |
| Anti-MMP1 | rabbit, polyclonal | 1:500 | Sigma-Aldrich, St. Louis, MO, USA |
| Anti-MMP7 | rabbit, polyclonal | 1:500 | Sigma-Aldrich, St. Louis, MO, USA |
| Anti-MMP8 | rabbit, polyclonal | 1:500 | Sigma-Aldrich, St. Louis, MO, USA |
| Anti-MMP9 | rabbit, polyclonal | 1:500 | Sigma-Aldrich, St. Louis, MO, USA |
| Anti-MMP13 | rabbit, polyclonal | 1:500 | Sigma-Aldrich, St. Louis, MO, USA |
| Anti-Adamts4 | rabbit, polyclonal | 1:500 | Sigma-Aldrich, St. Louis, MO, USA |
| Anti-Actin | mouse, monoclonal | 1:10,000 | Sigma-Aldrich, St. Louis, MO, USA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szentléleky, E.; Szegeczki, V.; Karanyicz, E.; Hajdú, T.; Tamás, A.; Tóth, G.; Zákány, R.; Reglődi, D.; Juhász, T. Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) Reduces Oxidative and Mechanical Stress-Evoked Matrix Degradation in Chondrifying Cell Cultures. Int. J. Mol. Sci. 2019, 20, 168. https://doi.org/10.3390/ijms20010168
Szentléleky E, Szegeczki V, Karanyicz E, Hajdú T, Tamás A, Tóth G, Zákány R, Reglődi D, Juhász T. Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) Reduces Oxidative and Mechanical Stress-Evoked Matrix Degradation in Chondrifying Cell Cultures. International Journal of Molecular Sciences. 2019; 20(1):168. https://doi.org/10.3390/ijms20010168
Chicago/Turabian StyleSzentléleky, Eszter, Vince Szegeczki, Edina Karanyicz, Tibor Hajdú, Andrea Tamás, Gábor Tóth, Róza Zákány, Dóra Reglődi, and Tamás Juhász. 2019. "Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) Reduces Oxidative and Mechanical Stress-Evoked Matrix Degradation in Chondrifying Cell Cultures" International Journal of Molecular Sciences 20, no. 1: 168. https://doi.org/10.3390/ijms20010168
APA StyleSzentléleky, E., Szegeczki, V., Karanyicz, E., Hajdú, T., Tamás, A., Tóth, G., Zákány, R., Reglődi, D., & Juhász, T. (2019). Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) Reduces Oxidative and Mechanical Stress-Evoked Matrix Degradation in Chondrifying Cell Cultures. International Journal of Molecular Sciences, 20(1), 168. https://doi.org/10.3390/ijms20010168