Evaluation of the Biological Behavior of a Gold Nanocore-Encapsulated Human Serum Albumin Nanoparticle (Au@HSANP) in a CT-26 Tumor/Ascites Mouse Model after Intravenous/Intraperitoneal Administration
Abstract
:1. Introduction
2. Results
2.1. Characteristics of AuNP and Au@HSANP
2.2. Cell Viability after Incubation with Au@HSANPs
2.3. Preparation and the Serum Stability of 111In-Labeled Au@HSANP (111In-Au@HSANP)
2.4. Pharmacokinetic Studies
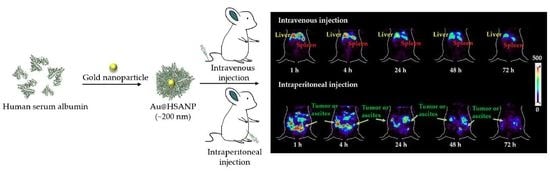
2.5. MicroSPECT Images
2.6. Biodistribution Studies
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Preparation of Gold Nanoparticles (AuNP)
4.3. Preparation of AuNP-Encapsulated Human Serum Albumin Nanoparticle (Au@HSANP)
4.4. Cytotoxicity of Au@HSANP in CT-26 Cell Culture
4.5. Preparation of 111In-Labeled Au@HSANP (111In-Au@HSANP)
4.6. Serum Stability Assay of 111In-Au@HSANP
4.7. Cell Culture and Tumor/Ascites Animal Model
4.8. Pharmacokinetic Study of the CT-26 Tumor/Ascites-Bearing Mouse Model after iv or ip Injections of 111In-Au@HSANP
4.9. MicroSPECT Imaging of the CT-26 Tumor/Ascites-Bearing Mouse Model after iv or ip Injection of 111In-Au@HSANP
4.10. Biodistribution of CT-26 Tumor/Ascites-Bearing Mouse Model after iv or ip Injections of 111In-Au@HSANP
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| AuNPs | Gold nanoparticle |
| Au@HSANP | Gold nanocore-encapsulated human serum albumin nanoparticles |
| CL | Clearance rate |
| CT | Computed tomography |
| EPR effect | Enhanced permeability and retention effect |
| HSA | Human serum albumin |
| HSANPs | Human serum albumin nanoparticle |
| ip | Intraperitoneal |
| IPC | Intraperitoneal chemotherapy |
| iv | Intravenous |
| MPS | mononuclear phagocyte system |
| NPs | Nanoparticles |
| PDI | Polydispersity index |
| p.i. | Post injection |
| SPECT | Single photon emission computed tomography |
| T/M ratio | Tumor-to-muscle ratio |
| TEM | Transmission electron microscopy |
| %ID/g | Percent of injected dose per gram |
References
- Haggar, F.A.; Boushey, R.P. Colorectal cancer epidemiology: Incidence, mortality, survival, and risk factors. Clin. Colon Rectal Surg. 2009, 22, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Sangisetty, S.L. Malignant ascites: A review of prognostic factors, pathophysiology and therapeutic measures. World J. Gastrointest. Surg. 2012, 4, 87. [Google Scholar] [CrossRef] [PubMed]
- Smolle, E.; Taucher, V.; Haybaeck, J. Malignant ascites in ovarian cancer and the role of targeted therapeutics. Anticancer. Res. 2014, 34, 1553–1561. [Google Scholar]
- Maeda, H.; Kobayashi, M.; Sakamoto, J. Evaluation and treatment of malignant ascites secondary to gastric cancer. World J. Gastroenterol. 2015, 21, 10936–10947. [Google Scholar] [CrossRef]
- Chakedis, J.; Schmidt, C.R. Surgical Treatment of Metastatic Colorectal Cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 377–399. [Google Scholar] [CrossRef]
- Franko, J.; Shi, Q.; Goldman, C.D.; Pockaj, B.A.; Nelson, G.D.; Goldberg, R.M.; Pitot, H.C.; Grothey, A.; Alberts, S.R.; Sargent, D.J. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: A pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J. Clin. Oncol. 2012, 30, 263–267. [Google Scholar] [CrossRef]
- Verwaal, V.J.; van Ruth, S.; de Bree, E.; van Sloothen, G.W.; van Tinteren, H.; Boot, H.; Zoetmulder, F.A. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J. Clin. Oncol. 2003, 21, 3737–3743. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [Green Version]
- Koo, H.; Huh, M.S.; Sun, I.C.; Yuk, S.H.; Choi, K.; Kim, K.; Kwon, I.C. In vivo targeted delivery of nanoparticles for theranosis. Acc. Chem. Res. 2011, 44, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amiens, C.; Ciuculescu-Pradines, D.; Philippot, K. Controlled metal nanostructures: Fertile ground for coordination chemists. Coord. Chem. Rev. 2016, 308, 409–432. [Google Scholar] [CrossRef]
- Khandelia, R.; Bhandari, S.; Pan, U.N.; Ghosh, S.S.; Chattopadhyay, A. Gold Nanocluster Embedded Albumin Nanoparticles for Two-Photon Imaging of Cancer Cells Accompanying Drug Delivery. Small 2015, 11, 4075–4081. [Google Scholar] [CrossRef]
- Xi, D.; Dong, S.; Meng, X.; Lu, Q.; Meng, L.; Ye, J. Gold nanoparticles as computerized tomography (CT) contrast agents. RSC Adv. 2012, 2, 12515–12524. [Google Scholar] [CrossRef]
- Cole, L.E.; Ross, R.D.; Tilley, J.M.; Vargo-Gogola, T.; Roeder, R.K. Gold nanoparticles as contrast agents in x-ray imaging and computed tomography. Nanomedicine 2015, 10, 321–341. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 49, N309–N315. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Rahme, K.; He, Y.; Li, L.L.; Holmes, J.D.; O’Driscoll, C.M. Gold nanoparticles enlighten the future of cancer theranostics. Int. J. Nanomed. 2017, 12, 6131–6152. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef]
- Loo, C.; Lowery, A.; Halas, N.; West, J.; Drezek, R. Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett. 2005, 5, 709–711. [Google Scholar] [CrossRef]
- Volk, L.D.; Flister, M.J.; Bivens, C.M.; Stutzman, A.; Desai, N.; Trieu, V.; Ran, S. Nab-paclitaxel Efficacy in the Orthotopic Model of Human Breast Cancer is Significantly Enhanced By Concurrent Anti–Vascular Endothelial Growth Factor A Therapy. Neoplasia 2008, 10, 613–623. [Google Scholar] [CrossRef]
- Qi, W.W.; Yu, H.Y.; Guo, H.; Lou, J.; Wang, Z.M.; Liu, P.; Sapin-Minet, A.; Maincent, P.; Hong, X.C.; Hu, X.M.; et al. Doxorubicin-loaded glycyrrhetinic acid modified recombinant human serum albumin nanoparticles for targeting liver tumor chemotherapy. Mol. Pharm. 2015, 12, 675–683. [Google Scholar] [CrossRef]
- Wan, X.; Zheng, X.; Pang, X.; Zhang, Z.; Jing, T.; Xu, W.; Zhang, Q. The potential use of lapatinib-loaded human serum albumin nanoparticles in the treatment of triple-negative breast cancer. Int. J. Pharm. 2015, 484, 16–28. [Google Scholar] [CrossRef]
- Hawkins, M.J.; Soon-Shiong, P.; Desai, N. Protein nanoparticles as drug carriers in clinical medicine. Adv. Drug Deliv. Rev. 2008, 60, 876–885. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef]
- Liang, J.F.; Wang, H.K.; Xiao, H.; Li, N.; Cheng, C.X.; Zhao, Y.Z.; Ma, Y.B.; Gao, J.Z.; Bai, R.B.; Zheng, H.X. Relationship and prognostic significance of SPARC and VEGF protein expression in colon cancer. J. Exp. Clin. Cancer Res. 2010, 29, 71. [Google Scholar] [CrossRef] [Green Version]
- Komiya, K.; Nakamura, T.; Nakashima, C.; Takahashi, K.; Umeguchi, H.; Watanabe, N.; Sato, A.; Takeda, Y.; Kimura, S.; Sueoka-Aragane, N. SPARC is a possible predictive marker for albumin-bound paclitaxel in non-small-cell lung cancer. OncoTargets Ther. 2016, 9, 6663–6668. [Google Scholar] [CrossRef] [Green Version]
- Esmaeili, F.; Dinarvand, R.; Ghahremani, M.H.; Amini, M.; Rouhani, H.; Sepehri, N.; Ostad, S.N.; Atyabi, F. Docetaxel-albumin conjugates: Preparation, in vitro evaluation and biodistribution studies. J. Pharm. Sci. 2009, 98, 2718–2730. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, X. Simple bioconjugate chemistry serves great clinical advances: Albumin as a versatile platform for diagnosis and precision therapy. Chem. Soc. Rev. 2016, 45, 1432–1456. [Google Scholar] [CrossRef]
- An, F.F.; Zhang, X.H. Strategies for Preparing Albumin-based Nanoparticles for Multifunctional Bioimaging and Drug Delivery. Theranostics 2017, 7, 3667–3689. [Google Scholar] [CrossRef] [Green Version]
- Elzoghby, A.O.; Hemasa, A.L.; Freag, M.S. Hybrid protein-inorganic nanoparticles: From tumor-targeted drug delivery to cancer imaging. J. Control. Release 2016, 243, 303–322. [Google Scholar] [CrossRef]
- Xie, J.; Chen, K.; Huang, J.; Lee, S.; Wang, J.; Gao, J.; Li, X.; Chen, X. PET/NIRF/MRI triple functional iron oxide nanoparticles. Biomaterials 2010, 31, 3016–3022. [Google Scholar] [CrossRef] [Green Version]
- Quan, Q.; Xie, J.; Gao, H.; Yang, M.; Zhang, F.; Liu, G.; Lin, X.; Wang, A.; Eden, H.S.; Lee, S.; et al. HSA coated iron oxide nanoparticles as drug delivery vehicles for cancer therapy. Mol. Pharm. 2011, 8, 1669–1676. [Google Scholar] [CrossRef]
- Peralta, D.V.; Heidari, Z.; Dash, S.; Tarr, M.A. Hybrid paclitaxel and gold nanorod-loaded human serum albumin nanoparticles for simultaneous chemotherapeutic and photothermal therapy on 4T1 breast cancer cells. ACS Appl. Mater. Interfaces 2015, 7, 7101–7111. [Google Scholar] [CrossRef]
- Al-Quteimat, O.M.; Al-Badaineh, M.A. Intraperitoneal chemotherapy: Rationale, applications, and limitations. J. Oncol. Pharm. Pract. 2014, 20, 369–380. [Google Scholar] [CrossRef]
- Williamson, S.K.; Johnson, G.A.; Maulhardt, H.A.; Moore, K.M.; McMeekin, D.S.; Schulz, T.K.; Reed, G.A.; Roby, K.F.; Mackay, C.B.; Smith, H.J.; et al. A phase I study of intraperitoneal nanoparticulate paclitaxel (Nanotax(R)) in patients with peritoneal malignancies. Cancer Chemother. Pharmacol. 2015, 75, 1075–1087. [Google Scholar] [CrossRef]
- Stewart, J.H.T.; Shen, P.; Levine, E.A. Intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: Current status and future directions. Ann. Surg. Oncol. 2005, 12, 765–777. [Google Scholar] [CrossRef]
- Vermorken, J.B. Intraperitoneal chemotherapy in advanced ovarian cancer: Recognition at last. Ann. Oncol. 2006, 17 (Suppl. 10), x241–x246. [Google Scholar] [CrossRef]
- Kinoshita, J.; Fushida, S.; Tsukada, T.; Oyama, K.; Watanabe, T.; Shoji, M.; Okamoto, K.; Nakanuma, S.; Sakai, S.; Makino, I.; et al. Comparative study of the antitumor activity of Nab-paclitaxel and intraperitoneal solvent-based paclitaxel regarding peritoneal metastasis in gastric cancer. Oncol. Rep. 2014, 32, 89–96. [Google Scholar] [CrossRef]
- Jones, A.; Harris, A.L. New developments in angiogenesis: A major mechanism for tumor growth and target for therapy. Cancer J. Sci. Am. 1998, 4, 209–217. [Google Scholar]
- Owens, D.E., 3rd; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Kunzmann, A.; Andersson, B.; Thurnherr, T.; Krug, H.; Scheynius, A.; Fadeel, B. Toxicology of engineered nanomaterials: Focus on biocompatibility, biodistribution and biodegradation. Biochim. Biophys. Acta 2011, 1810, 361–373. [Google Scholar] [CrossRef]
- Steichen, S.D.; Caldorera-Moore, M.; Peppas, N.A. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur. J. Pharm. Sci. 2013, 48, 416–427. [Google Scholar] [CrossRef] [Green Version]
- Venturoli, D.; Rippe, B. Ficoll and dextran vs. globular proteins as probes for testing glomerular permselectivity: Effects of molecular size, shape, charge, and deformability. Am. J. Physiol. Ren. Physiol. 2005, 288, F605–F613. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef]
- Kinoshita, R.; Ishima, Y.; Chuang, V.T.G.; Nakamura, H.; Fang, J.; Watanabe, H.; Shimizu, T.; Okuhira, K.; Ishida, T.; Maeda, H.; et al. Improved anticancer effects of albumin-bound paclitaxel nanoparticle via augmentation of EPR effect and albumin-protein interactions using S-nitrosated human serum albumin dimer. Biomaterials 2017, 140, 162–169. [Google Scholar] [CrossRef]
- Waser, P.G.; Müller, U.; Kreuter, J.; Berger, S.; Munz, K.; Kaiser, E.; Pfluger, B. Localization of colloidal particles (liposomes, hexylcyanoacrylate nanoparticles and albumin nanoparticles) by histology and autoradiography in mice. Int. J. Pharm. 1987, 39, 213–227. [Google Scholar] [CrossRef]
- John, T.A.; Vogel, S.M.; Tiruppathi, C.; Malik, A.B.; Minshall, R.D. Quantitative analysis of albumin uptake and transport in the rat microvessel endothelial monolayer. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 284, L187–L196. [Google Scholar] [CrossRef]
- Mirahmadi, N.; Babaei, M.H.; Vali, A.M.; Dadashzadeh, S. Effect of liposome size on peritoneal retention and organ distribution after intraperitoneal injection in mice. Int. J. Pharm. 2010, 383, 7–13. [Google Scholar] [CrossRef]
- Yan, T.D.; Cao, C.Q.; Munkholm-Larsen, S. A pharmacological review on intraperitoneal chemotherapy for peritoneal malignancy. World J. Gastrointest. Oncol. 2010, 2, 109–116. [Google Scholar] [CrossRef]
- Mohamed, F.; Marchettini, P.; Stuart, O.A.; Sugarbaker, P.H. Pharmacokinetics and tissue distribution of intraperitoneal paclitaxel with different carrier solutions. Cancer Chemother. Pharmacol. 2003, 52, 405–410. [Google Scholar] [CrossRef]
- Mohamed, F.; Stuart, O.A.; Sugarbaker, P.H. Pharmacokinetics and tissue distribution of intraperitoneal docetaxel with different carrier solutions. J. Surg. Res. 2003, 113, 114–120. [Google Scholar] [CrossRef]
- Tsai, M.; Lu, Z.; Wang, J.; Yeh, T.K.; Wientjes, M.G.; Au, J.L. Effects of carrier on disposition and antitumor activity of intraperitoneal Paclitaxel. Pharm. Res. 2007, 24, 1691–1701. [Google Scholar] [CrossRef]
- De Smet, L.; Ceelen, W.; Remon, J.P.; Vervaet, C. Optimization of drug delivery systems for intraperitoneal therapy to extend the residence time of the chemotherapeutic agent. Sci. World J. 2013, 2013, 720858. [Google Scholar] [CrossRef]
- Gelderblom, H.; Verweij, J.; van Zomeren, D.M.; Buijs, D.; Ouwens, L.; Nooter, K.; Stoter, G.; Sparreboom, A. Influence of Cremophor El on the bioavailability of intraperitoneal paclitaxel. Clin. Cancer Res. 2002, 8, 1237–1241. [Google Scholar]
- Weiss, R.B.; Donehower, R.C.; Wiernik, P.H.; Ohnuma, T.; Gralla, R.J.; Trump, D.L.; Baker, J.R., Jr.; Van Echo, D.A.; Von Hoff, D.D.; Leyland-Jones, B. Hypersensitivity reactions from taxol. J. Clin. Oncol. 1990, 8, 1263–1268. [Google Scholar] [CrossRef]
- Fujiyama, J.; Nakase, Y.; Osaki, K.; Sakakura, C.; Yamagishi, H.; Hagiwara, A. Cisplatin incorporated in microspheres: Development and fundamental studies for its clinical application. J. Control. Release 2003, 89, 397–408. [Google Scholar] [CrossRef]
- Lu, Z.; Tsai, M.; Lu, D.; Wang, J.; Wientjes, M.G.; Au, J.L. Tumor-penetrating microparticles for intraperitoneal therapy of ovarian cancer. J. Pharmacol. Exp. Ther. 2008, 327, 673–682. [Google Scholar] [CrossRef]
- Kohane, D.S.; Tse, J.Y.; Yeo, Y.; Padera, R.; Shubina, M.; Langer, R. Biodegradable polymeric microspheres and nanospheres for drug delivery in the peritoneum. J. Biomed. Mater. Res. Part A 2006, 77, 351–361. [Google Scholar] [CrossRef]
- Cheng, C.J.; Tietjen, G.T.; Saucier-Sawyer, J.K.; Saltzman, W.M. A holistic approach to targeting disease with polymeric nanoparticles. Nat. Rev. Drug Discov. 2015, 14, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Yang, F.; Cocco, E.; Song, E.; Zhang, J.; Cui, J.; Mohideen, M.; Bellone, S.; Santin, A.D.; Saltzman, W.M. Improved i.p. drug delivery with bioadhesive nanoparticles. Proc. Natl. Acad. Sci. USA 2016, 113, 11453–11458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghimi, S.M.; Szebeni, J. Stealth liposomes and long circulating nanoparticles: Critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog. Lipid Res. 2003, 42, 463–478. [Google Scholar] [CrossRef]
- Hirano, K.; Hunt, C.A. Lymphatic transport of liposome-encapsulated agents: Effects of liposome size following intraperitoneal administration. J. Pharm. Sci. 1985, 74, 915–921. [Google Scholar] [CrossRef]
- Dadashzadeh, S.; Mirahmadi, N.; Babaei, M.H.; Vali, A.M. Peritoneal retention of liposomes: Effects of lipid composition, PEG coating and liposome charge. J. Control. Release 2010, 148, 177–186. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Li, J.J.; Chang, C.H.; Lu, Y.C.; Hwang, J.J.; Tseng, Y.L.; Lin, W.J.; Ting, G.; Wang, H.E. Evaluation of pharmacokinetics of 111In-labeled VNB-PEGylated liposomes after intraperitoneal and intravenous administration in a tumor/ascites mouse model. Cancer Biother. Radiopharm. 2009, 24, 453–460. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV-vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Yedomon, B.; Fessi, H.; Charcosset, C. Preparation of Bovine Serum Albumin (BSA) nanoparticles by desolvation using a membrane contactor: A new tool for large scale production. Eur. J. Pharm. Biopharm. 2013, 85, 398–405. [Google Scholar] [CrossRef]
- Woods, A.; Patel, A.; Spina, D.; Riffo-Vasquez, Y.; Babin-Morgan, A.; de Rosales, R.T.; Sunassee, K.; Clark, S.; Collins, H.; Bruce, K.; et al. In vivo biocompatibility, clearance, and biodistribution of albumin vehicles for pulmonary drug delivery. J. Control. Release 2015, 210, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.Y.; Chang, C.H.; Li, J.J.; Stabin, M.G.; Chang, Y.J.; Chen, L.C.; Lin, M.H.; Tseng, Y.L.; Lin, W.J.; Lee, T.W.; et al. Pharmacokinetics and dosimetry of (111)In/(188)Re-labeled PEGylated liposomal drugs in two colon carcinoma-bearing mouse models. Cancer Biother. Radiopharm. 2011, 26, 373–380. [Google Scholar] [CrossRef]






| Particle | Particle Size | Polydispersity Index (PDI) | Zeta Potential (mV) |
|---|---|---|---|
| AuNP | 22.5 ± 5.9 | 0.16 ± 0.05 | −43 ± 6 |
| Au@HSANP | 213.3 ± 32.9 | 0.08 ± 0.04 | −46.9 ± 9 |
| Parameters | Unit | iv Injection * | ip Injection ** |
|---|---|---|---|
| Half-life | h | NA | 24.4 |
| T1/2α | h | 0.05 | NA |
| T1/2β | h | 19.7 | NA |
| AUC0→∞ | h·[%ID/mL] | 44.5 | 7.1 |
| CL | mL/h | 2.2 | 14.8 |
| Organs | Mice Received iv Administration of 111In-Au@HSANP | |||
|---|---|---|---|---|
| 1 h | 24 h | 48 h | 96 h | |
| Blood | 0.44 ± 0.08 | 0.12 ± 0.04 | 0.07 ± 0.01 | 0.09 ± 0.03 |
| Heart | 0.17 ± 0.05 | 0.10 ± 0.06 | 0.06 ± 0.05 | 0.08 ± 0.03 |
| Lung | 13.31 ± 6.49 | 1.68 ± 0.85 | 0.34 ± 0.25 | 0.49 ± 0.40 |
| Liver | 16.09 ± 3.27 | 19.22 ± 2.78 | 24.39 ± 2.03 | 19.06 ± 3.74 |
| Stomach | 0.17 ± 0.06 | 0.09 ± 0.02 | 0.08 ± 0.03 | 0.10 ± 0.02 |
| S.I. | 0.45 ± 0.18 | 0.23 ± 0.10 | 0.15 ± 0.08 | 0.25 ± 0.16 |
| L.I | 0.15 ± 0.04 | 0.19 ± 0.11 | 0.14 ± 0.07 | 0.22 ± 0.06 |
| Pancreas | 0.15 ± 0.04 | 0.11 ± 0.03 | 0.22 ± 0.16 | 0.16 ± 0.02 |
| Spleen | 40.47 ± 2.36 | 46.45 ± 7.73 | 45.08 ± 11.19 | 34.90 ± 8.50 |
| Kidneys | 3.55 ± 1.00 | 5.44 ± 0.88 | 4.28 ± 0.67 | 4.36 ± 0.79 |
| Muscle | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 |
| Urine | 23.68 ± 25.19 | 51.59 ± 18.76 | 22.11 ± 10.96 | 16.19 ± 7.41 |
| Feces | 0.03 ± 0.03 | 2.80 ± 1.71 | 1.80 ± 1.58 | 7.55 ± 2.38 |
| Bladder | 0.16 ± 0.06 | 0.22 ± 0.14 | 0.09 ± 0.02 | 0.19 ± 0.09 |
| Bone | 0.14 ± 0.05 | 0.29 ± 0.18 | 0.28 ± 0.11 | 0.21 ± 0.05 |
| CT-26 tumor | 0.29 ± 0.10 | 0.33 ± 0.07 | 0.19 ± 0.05 | 0.21 ± 0.03 |
| Ascites | 0.28 ± 0.07 | 0.22 ± 0.10 | 0.13 ± 0.02 | 0.22 ± 0.09 |
| T/M ratio | 9.5 | 11.1 | 9.3 | 7.0 |
| Organs | Mice Received ip Administration of 111In-Au@HSANP | |||
|---|---|---|---|---|
| 1 h | 24 h | 48 h | 96 h | |
| Blood | 0.17 ± 0.07 | 0.15 ± 0.08 | 0.10 ± 0.04 | 0.11 ± 0.05 |
| Heart | 0.06 ± 0.03 | 0.08 ± 0.01 | 0.06 ± 0.02 | 0.17 ± 0.07 |
| Lung | 0.29 ± 0.18 | 2.78 ± 1.59 | 2.62 ± 0.71 | 15.70 ± 7.48 |
| Liver | 0.42 ± 0.23 | 1.11 ± 0.44 | 1.25 ± 0.33 | 3.78 ± 1.59 |
| Stomach | 0.41 ± 0.14 | 0.31 ± 0.10 | 0.33 ± 0.06 | 0.20 ± 0.04 |
| S.I. | 0.38 ± 0.41 | 0.23 ± 0.08 | 0.21 ± 0.09 | 0.22 ± 0.11 |
| L.I | 0.29 ± 0.25 | 0.30 ± 0.21 | 0.24 ± 0.04 | 0.19 ± 0.07 |
| Pancreas | 0.70 ± 0.19 | 0.55 ± 0.21 | 0.42 ± 0.06 | 0.46 ± 0.16 |
| Spleen | 1.14 ± 0.36 | 2.33 ± 0.18 | 2.38 ± 0.35 | 8.76 ± 3.96 |
| Kidneys | 0.41 ± 0.06 | 3.20 ± 0.65 | 3.11 ± 0.45 | 2.44 ± 0.35 |
| Muscle | 0.09 ± 0.12 | 0.04 ± 0.02 | 0.03 ± 0.02 | 0.05 ± 0.03 |
| Urine | 9.53 ± 2.36 | 62.81 ± 21.07 | 25.47 ± 19.24 | 10.54 ± 2.42 |
| Feces | 0.21 ± 0.24 | 7.76 ± 5.84 | 1.13 ± 0.72 | 3.48 ± 4.49 |
| Bladder | 0.31 ± 0.23 | 0.22 ± 0.07 | 0.31 ± 0.18 | 0.18 ± 0.07 |
| Bone | 0.02 ± 0.01 | 0.12 ± 0.07 | 0.06 ± 0.03 | 0.23 ± 0.10 |
| CT-26 tumor | 7.77 ± 2.99 | 8.89 ± 2.53 | 3.40 ± 0.94 | 1.45 ± 0.48 |
| Ascites | 41.33 ± 12.64 | 22.36 ± 5.28 | 12.86 ± 1.53 | 9.64 ± 3.39 |
| T/M ratio | 89.4 | 217.4 | 128.8 | 28.3 |
| Organ | iv Injection | ip Injection |
|---|---|---|
| Blood * | 44.5 | 7.1 |
| Liver | 1987.9 | 167.3 |
| Spleen | 4057.8 | 365.0 |
| Kidney | 430.9 | 251.0 |
| Tumor | 23.1 | 463.3 |
| Ascites | 18.6 | 1736.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-C.; Li, J.-J.; Guo, N.-H.; Chang, D.-Y.; Wang, C.-Y.; Chen, J.-T.; Lin, W.-J.; Chi, K.-H.; Lee, Y.-J.; Liu, R.-S.; et al. Evaluation of the Biological Behavior of a Gold Nanocore-Encapsulated Human Serum Albumin Nanoparticle (Au@HSANP) in a CT-26 Tumor/Ascites Mouse Model after Intravenous/Intraperitoneal Administration. Int. J. Mol. Sci. 2019, 20, 217. https://doi.org/10.3390/ijms20010217
Chen C-C, Li J-J, Guo N-H, Chang D-Y, Wang C-Y, Chen J-T, Lin W-J, Chi K-H, Lee Y-J, Liu R-S, et al. Evaluation of the Biological Behavior of a Gold Nanocore-Encapsulated Human Serum Albumin Nanoparticle (Au@HSANP) in a CT-26 Tumor/Ascites Mouse Model after Intravenous/Intraperitoneal Administration. International Journal of Molecular Sciences. 2019; 20(1):217. https://doi.org/10.3390/ijms20010217
Chicago/Turabian StyleChen, Chao-Cheng, Jia-Je Li, Nai-Hua Guo, Deng-Yuan Chang, Chung-Yih Wang, Jenn-Tzong Chen, Wuu-Jyh Lin, Kwan-Hwa Chi, Yi-Jang Lee, Ren-Shyan Liu, and et al. 2019. "Evaluation of the Biological Behavior of a Gold Nanocore-Encapsulated Human Serum Albumin Nanoparticle (Au@HSANP) in a CT-26 Tumor/Ascites Mouse Model after Intravenous/Intraperitoneal Administration" International Journal of Molecular Sciences 20, no. 1: 217. https://doi.org/10.3390/ijms20010217
APA StyleChen, C. -C., Li, J. -J., Guo, N. -H., Chang, D. -Y., Wang, C. -Y., Chen, J. -T., Lin, W. -J., Chi, K. -H., Lee, Y. -J., Liu, R. -S., Chen, C. -L., & Wang, H. -E. (2019). Evaluation of the Biological Behavior of a Gold Nanocore-Encapsulated Human Serum Albumin Nanoparticle (Au@HSANP) in a CT-26 Tumor/Ascites Mouse Model after Intravenous/Intraperitoneal Administration. International Journal of Molecular Sciences, 20(1), 217. https://doi.org/10.3390/ijms20010217