Making Medicines Baby Size: The Challenges in Bridging the Formulation Gap in Neonatal Medicine
Abstract
:1. Introduction
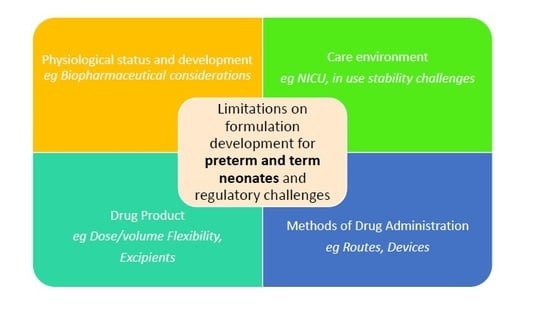
2. Formulation Considerations
2.1. Environment of Care
- Premature birth <37 weeks gestation
- Delayed birth >42 weeks gestation
- Birth weight <2500 g
- Concern about size, e.g., intrauterine growth restriction (IUGR)
- Medication or resuscitation required in the delivery room
- Birth defects, e.g., congenital heart defects, intraventricular haemorrhage, macrosomia, retinopathy of prematurity (ROP)
- Respiratory problems including RDS (respiratory distress syndrome) and BDP (bronchopulmonary dysplasia)
- Infection (including neonatal sepsis)
- Seizures
- Hypoglycemia
- Requiring additional support (extra oxygen or monitoring, body temperature control support, intravenous (IV) therapy, or medications) or specialized treatments (blood transfusion)
- Feeding issues
- Jaundice
2.2. Ability to Dose: Patient (Developmental Age)/Physiological/Administration Routes Factors to Consider
2.2.1. Parenteral Delivery
2.2.2. Oral Delivery
2.2.3. Rectal Delivery
2.2.4. Pulmonary Delivery
2.2.5. Nasal Delivery
- The ratio between the ideal volume per nostril and the concentration of solution/ suspension to be administered. In practice, the maximum volume for single administration into one nostril is 0.1 mL in neonates and 0.5 mL in older children [89]. There is no agreement about the volume that can be given to preterm neonates. Larger doses can be given by using these dose volumes (or half the total volume provided this does not exceed the safe total volume) in both nostrils.
- The need for a ‘baby size’ device able to dose accurately very low volumes of liquids without causing physical damage to the nasal mucosa.
- The potential irritancy of highly concentrated solutions, especially if these are hypertonic.
- The choice of excipients. For example, a penetration enhancer may be required to aid the absorption of polar drugs. Many of these could cause irritation of nasal epithelium of neonates, and for most common penetration enhancers, no safety data are available in the neonatal population.
2.2.6. Dermal and Transdermal Delivery
2.3. Ability to Administer: Product Factors to Consider
2.3.1. In use Stability Issues
2.3.2. Excipients
2.3.3. A Shift towards Solid Dosage Forms?
2.4. Ability to Administer: Device Factors to Consider
2.4.1. Accuracy of Small Volumes
2.4.2. Enteral Tubes Administration
2.4.3. Parenteral Catheters and Administration Sets
2.4.4. IV Polypharmacy
2.4.5. Inhalation Devices
2.5. Biopharmaceutical Considerations
2.6. Regulatory Challenges
3. Burden of Proof
4. Conclusions
Funding
Conflicts of Interest
References
- European Medicines Agency. Ich e11(r1) Guideline on Clinical Investigation of Medicinal Products in the Pediatric Population. Available online: https://www.ema.europa.eu/documents/scientific-guideline/ich-e11r1-guideline-clinical-investigation-medicinal-products-pediatric-population-revision-1_en.pdf (accessed on 19 March 2019).
- March of Dimes; PMNCH; Save the Children; WHO. Born too Soon: The Global Action Report on Preterm Birth. Available online: https://www.who.int/pmnch/media/news/2012/201204_borntoosoon-report.pdf (accessed on 19 March 2019).
- Allegaert, K.; van de Velde, M.; van den Anker, J. Neonatal clinical pharmacology. Paediatr. Anaesth. 2014, 24, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Allegaert, K.; Samardzic, J.; Bajcetic, M.; van den Anker, J.N. Developmental pharmacology and therapeutics in neonatal medicine. In Neonatology: A Practical Approach to Neonatal Diseases, 2nd ed.; Buonocore, G., Bracci, R., Weindling, M., Eds.; Springer: Cham, Switzerland, 2018; pp. 693–707. [Google Scholar]
- Allegaert, K.; Simons, S.; van den Anker, J. Research on medication use in the neonatal intensive care unit. Expert Rev. Clin. Pharmacol. 2019, 12, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Van den Anker, J.; Reed, M.D.; Allegaert, K.; Kearns, G.L. Developmental changes in pharmacokinetics and pharmacodynamics. J. Clin. Pharmacol. 2018, 58, S10–S25. [Google Scholar] [CrossRef] [PubMed]
- European Commission. State of Paediatric Medicines in the eu. 10 Years of the eu paediatric Regulation. Report from the Commission to the European Pparliament and the Council. Available online: https://ec.europa.eu/health/sites/health/files/files/paediatrics/docs/2017_childrensmedicines_report_en.pdf (accessed on 19 March 2019).
- Cuzzolin, L.; Atzei, A.; Fanos, V. Off-label and unlicensed prescribing for newborns and children in different settings: A review of the literature and a consideration about drug safety. Expert Opin. Drug Saf. 2006, 5, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Gore, R.; Chugh, P.K.; Tripathi, C.D.; Lhamo, Y.; Gautam, S. Pediatric off-label and unlicensed drug use and its implications. Curr. Clin. Pharmacol. 2017, 12, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, P.A.; Egger, G.F.; Pallidis, C.; Saint-Raymond, A. Enabling development of paediatric medicines in europe: 10 years of the eu paediatric regulation. Paediatr. Drugs 2017, 19, 505–513. [Google Scholar] [CrossRef]
- Yen, E.; Davis, J.M.; Milne, C.-P. Impact of regulatory incentive programs on the future of pediatric drug development. Ther. Innov. Regul. Sci. 2019. Published online 14 April 2019. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.M.; Benjamin, D.; Barrett, J.S.; Allegaert, K.; Portman, R.; Davis, J.M.; Turner, M.A. Safety, dosing, and pharmaceutical quality for studies that evaluate medicinal products (including biological products) in neonates. Pediatr. Res. 2017, 81, 692–711. [Google Scholar] [CrossRef] [PubMed]
- Linakis, M.W.; Roberts, J.K.; Lala, A.C.; Spigarelli, M.G.; Medlicott, N.J.; Reith, D.M.; Ward, R.M.; Sherwin, C.M.T. Challenges associated with route of administration in neonatal drug delivery. Clin. Pharmacokinet. 2016, 55, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Allegaert, K.; Cosaert, K.; van den Anker, J.N. Neonatal formulations: The need for a tailored, knowledge driven approach. Curr. Pharm. Des. 2015, 21, 5674–5679. [Google Scholar] [CrossRef] [PubMed]
- Valeur, K.S.; Holst, H.; Allegaert, K. Excipients in neonatal medicinal products: Never prescribed, commonly administered. Pharmaceut. Med. 2018, 32, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Stanford Children’s Health. The Neonatal Intensive Care Unit (nicu). Available online: https://www.stanfordchildrens.org/en/topic/default?id=the-neonatal-intensive-care-unit-nicu-90-P02389 (accessed on 21 March 2019).
- Rennie, J.M.; Roberton, N.R.C. Textbook of Neonatology, 3rd ed.; Churchill Livingstone: Edinburgh, UK, 1999. [Google Scholar]
- Zhou, D. Understanding physicochemical properties for pharmaceutical product development and manufacturing ii: Physical and chemical stability and excipient compatibility. J. Validation Tech. 2009, 15, 36–47. [Google Scholar]
- Allegaert, K.; van den Anker, J. Neonatal drug therapy: The first frontier of therapeutics for children. Clin. Pharmacol. Ther. 2015, 98, 288–297. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Guideline on Pharmaceutical Development of Medicines for Paediatric Use. Available online: https://www.ema.europa.eu/documents/scientific-guideline/guideline-pharmaceutical-development-medicines-paediatric-use_en.pdf (accessed on 19 March 2019).
- Concepcion, N.D.P.; Laya, B.F.; Lee, E.Y. Current updates in catheters, tubes and drains in the pediatric chest: A practical evaluation approach. Eur. J. Radiol. 2017, 95, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Farrelly, J.S.; Stitelman, D.H. Complications in pediatric enteral and vascular access. Semin. Pediatr. Surg. 2016, 25, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Goulet, O.; Hunt, J.; Krohn, K.; Shamir, R.; for the Parenteral Nutrition Guidelines Working Group. 1. Guidelines on paediatric parenteral nutrition of the european society of paediatric gastroenterology, hepatology and nutrition (espghan) and the european society for clinical nutrition and metabolism (espen), supported by the european society of paediatric research (espr). J. Pediatr. Gastroenterol. Nutr. 2005, 41, S1–S4. [Google Scholar] [PubMed]
- Dongara, A.R.; Patel, D.V.; Nimbalkar, S.M.; Potana, N.; Nimbalkar, A.S. Umbilical venous catheter versus peripherally inserted central catheter in neonates: A randomized controlled trial. J. Trop. Pediatr. 2017, 63, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Detaille, T.; Pirotte, T.; Veyckemans, F. Vascular access in the neonate. Best Pract. Res. Clin. Anaesthesiol. 2010, 24, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Pedreira, M.L. [obstruction of peripherally inserted central catheters in newborns: Prevention is the best intervention]. Revista paulista de pediatria: orgão oficial da Sociedade de Pediatria de São Paulo 2015, 33, 255–257. [Google Scholar] [CrossRef]
- Sherwin, C.M.; Medlicott, N.J.; Reith, D.M.; Broadbent, R.S. Intravenous drug delivery in neonates: Lessons learnt. Arch. Dis. Child. 2014, 99, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Kalikstad, B.; Skjerdal, A.; Hansen, T.W. Compatibility of drug infusions in the nicu. Arch. Dis. Child. 2010, 95, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Gaetani, M.; Frndova, H.; Seto, W.; Parshuram, C. Concurrent intravenous drug administration to critically ill children: Evaluation of frequency and compatibility. J. Crit. Care 2017, 41, 198–203. [Google Scholar] [CrossRef]
- Bradley, J.S.; Wassel, R.T.; Lee, L.; Nambiar, S. Intravenous ceftriaxone and calcium in the neonate: Assessing the risk for cardiopulmonary adverse events. Pediatrics 2009, 123, e609–e613. [Google Scholar] [CrossRef]
- Manrique-Rodriguez, S.; Sanchez-Galindo, A.; Mora-Garcia, T.; Fernandez-Llamazares, C.M.; Echarri-Martinez, L.; Lopez-Herce, J.; Rodriguez-Gomez, M.; Bellon-Cano, J.M.; Sanjuro-Saez, M. Development of a compatibility chart for intravenous y-site drug administration in a pediatric intensive care unit. J. Infus. Nurs. 2012, 35, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Décaudin, B.; Maiguy-Foinard, A.; Barthélémy, C.; Lebuffe, G.; Storme, L.; Odou, P. Dynamic image analysis to evaluate subvisible particles during continuous drug infusion in a neonatal intensive care unit. Sci. Rep. 2017, 7, 9404. [Google Scholar] [CrossRef] [PubMed]
- Rouse, C.; Mistry, P.; Rayner, O.; Nickless, J.; Wan, M.; Southern, K.W.; Batchelor, H.K. A mixed methods study of the administration of flucloxacillin oral liquid; identifying strategies to overcome administration issues of medicines with poor palatability. Int. J. Pharm. Pract. 2017, 25, 326–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheppard, J.J.; Mysak, E.D. Ontogeny of infantile oral reflexes and emerging chewing. Child Dev. 1984, 55, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Somani, A.A.; Thelen, K.; Zheng, S.; Trame, M.N.; Coboeken, K.; Meyer, M.; Schnizler, K.; Ince, I.; Willmann, S.; Schmidt, S. Evaluation of changes in oral drug absorption in preterm and term neonates for biopharmaceutics classification system (bcs) class i and ii compounds. Br. J. Clin. Pharmacol. 2016, 81, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.J.; van Lingen, R.A.; Hansen, T.G.; Lin, Y.C.; Holford, N.H.G. Acetaminophen developmental pharmacokinetics in premature neonates and infants—A pooled population analysis. Anesthesiology 2002, 96, 1336–1345. [Google Scholar] [CrossRef]
- European Medicines Agency. First Medicine to Treat Neonatal Diabetes. Press Release 23/02/2018. Available online: https://www.ema.europa.eu/en/news/first-medicine-treat-neonatal-diabetes (accessed on 19 March 2019).
- European Medicines Agency. Reflection Paper: Formulations of Choice for the Paediatric Population. Available online: https://www.ema.europa.eu/documents/scientific-guideline/reflection-paper-formulations-choice-paediatric-population_en.pdf (accessed on 19 March 2019).
- Ainscough, L.P.; Ford, J.L.; Morecroft, C.W.; Peak, M.; Turner, M.A.; Nunn, A.J.; Roberts, M. Accuracy of intravenous and enteral preparations involving small volumes for paediatric use: A review. Eur. J. Hosp. Pharm. 2018, 25, 66–71. [Google Scholar] [CrossRef]
- Gurung, K.; Arenas-Lopez, S.; Wei, L.; Tuleu, C. Accuracy of enteral syringes for liquid medicines prescribed in children. Arch. Dis. Child. 2014, 99, e3. [Google Scholar] [CrossRef]
- Brown, D.; Ford, J.L.; Nunn, A.J.; Rowe, P.H. An assessment of dose-uniformity of samples delivered from paediatric oral droppers. J. Clin. Pharm. Ther. 2004, 29, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Maier, T.; Scheuerle, R.L.; Markl, D.; Bruggraber, S.; Zeitler, A.; Fruk, L.; Slater, N.K.H. Zinc delivery from non-woven fibres within a therapeutic nipple shield. Int. J. Pharm. 2018, 537, 290–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, C.W.; Israel-Ballard, K.A.; Joanis, C.L.; Baniecki, M.L.; Thungu, F.; Gerrard, S.E.; Kneen, E.; Sokal, D.C. Acceptability of a nipple shield delivery system administering antiviral agents to prevent mother-to-child transmission of hiv through breastfeeding. J. Hum. Lact. 2015, 31, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Ducki, S.; Martin, C.; Bohn, L. Oral administration of medicines to infants: The dummy which relieves [2]. Arch. Pediatr. 2002, 9, 1298–1299. [Google Scholar]
- Hansen, K.; Yee, L.; Lee, J.; Horeczko, T.; Saidinejad, M.; Padlipsky, P.S.; Gausche-Hill, M.; Tanen, D.A. Parent and nurse satisfaction using pacidose® oral medication delivery device in the pediatric emergency department: A pilot study. J. Pediatr. Nurs. 2018, 42, 100–103. [Google Scholar] [CrossRef] [PubMed]
- WHO. Development of Paediatric Medicines: Points to Consider in Formulation. Who Technical Report Series, no. 970, 2012, annex 5. Available online: http://apps.who.int/medicinedocs/en/m/abstract/Js19833en/ (accessed on 19 March 2019).
- Paroche, M.M.; Caton, S.J.; Vereijken, C.; Weenen, H.; Houston-Price, C. How infants and young children learn about food: A systematic review. Front. Psychol. 2017, 8, 33. [Google Scholar] [CrossRef]
- Jannin, V.; Lemagnen, G.; Gueroult, P.; Larrouture, D.; Tuleu, C. Rectal route in the 21st century to treat children. Adv. Drug Deliv. Rev. 2014, 73, 34–49. [Google Scholar] [CrossRef]
- Kim, T.W.; Rognerud, C.L.; Ou, C.N. Accuracy in the alteration of acetaminophen suppositories. Anesth. Analg. 2005, 100, 1303–1305. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, M.; Yung, J.; Chen, J.; McNair, C.; Lee, K.S. Safety of rectal administration of acetaminophen in neonates. Can. J. Hosp. Pharm. 2018, 71, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Demir, N.; Peker, E.; Ece, I.; Balahoroglu, R.; Tuncer, O. Efficacy and safety of rectal ibuprofen for patent ductus arteriosus closure in very low birth weight preterm infants. J. Matern. Fetal Neonatal Med. 2017, 30, 2119–2125. [Google Scholar] [CrossRef] [PubMed]
- Surkov, D.; Obolonskiy, A.; Kapustina, O.; Volkov, D. Use of rectal ibuprofen for pda closure in preterm neonates. PACCJ 2014, 2, 11–16. [Google Scholar]
- Tozan, Y.; Klein, E.Y.; Darley, S.; Panicker, R.; Laxminarayan, R.; Breman, J.G. Prereferral rectal artesunate for treatment of severe childhood malaria: A cost-effectiveness analysis. Lancet 2010, 376, 1910–1915. [Google Scholar] [CrossRef]
- Kauss, T.; Langlois, M.H.; Guyonnet-Duperat, A.; Phoeung, T.; Xie, X.Y.; Cartwright, A.; White, N.; Gomes, M.; Gaudin, K. Development of rectodispersible tablets and granulate capsules for the treatment of serious neonatal sepsis in developing countries. J. Pharm. Sci. 2019. [Google Scholar] [CrossRef]
- Jobe, A.H. What is rds in 2012? Early Hum. Dev. 2012, 88, S42–S44. [Google Scholar] [CrossRef]
- Jobe, A. Metabolism of endogenous surfactant and exogenous surfactants for replacement therapy. Semin. Perinatol. 1988, 12, 231–244. [Google Scholar] [PubMed]
- Kandraju, H.; Murki, S.; Subramanian, S.; Gaddam, P.; Deorari, A.; Kumar, P. Early routine versus late selective surfactant in preterm neonates with respiratory distress syndrome on nasal continuous positive airway pressure: A randomized controlled trial. Neonatology 2013, 103, 148–154. [Google Scholar] [CrossRef]
- Verder, H.; Albertsen, P.; Ebbesen, F.; Greisen, G.; Robertson, B.; Bertelsen, A.; Agertoft, L.; Djernes, B.; Nathan, E.; Reinholdt, J. Nasal continuous positive airway pressure and early surfactant therapy for respiratory distress syndrome in newborns of less than 30 weeks’ gestation. Pediatrics 1999, 103, E24. [Google Scholar] [CrossRef]
- Kribs, A.; Roll, C.; Gopel, W.; Wieg, C.; Groneck, P.; Laux, R.; Teig, N.; Hoehn, T.; Bohm, W.; Welzing, L.; et al. Nonintubated surfactant application vs conventional therapy in extremely preterm infants: A randomized clinical trial. JAMA Pediatr. 2015, 169, 723–730. [Google Scholar] [CrossRef]
- Fabbri, L.; Klebermass-Schrehof, K.; Aguar, M.; Harrison, C.; Gulczynska, E.; Santoro, D.; Di Castri, M.; Rigo, V. Five-country manikin study found that neonatologists preferred using the lisacath rather than the angiocath for less invasive surfactant administration. Acta Paediatr. 2018, 107, 780–783. [Google Scholar] [CrossRef]
- Pillow, J.J.; Minocchieri, S. Innovation in surfactant therapy ii: Surfactant administration by aerosolization. Neonatology 2012, 101, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, G.; Iwatschenko, P.; Koch, W.; Windt, H.; Rast, M.; de Abreu, M.G.; Taut, F.J.; De Muynck, C. A novel continuous powder aerosolizer (cpa) for inhalative administration of highly concentrated recombinant surfactant protein-c (rsp-c) surfactant to preterm neonates. J. Aerosol Med. Pulm. Drug Deliv. 2013, 26, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Delara, M.; Chauhan, B.F.; Le, M.-L.; Abou-Setta, A.M.; Zarychanski, R.; ’tJong, G.W. Efficacy and safety of pulmonary application of corticosteroids in preterm infants with respiratory distress syndrome: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F137–F144. [Google Scholar] [CrossRef] [PubMed]
- MacLoughlin, R.; Telfer, C.; Clark, A.; Fink, J. Aerosol: A novel vehicle for pharmacotherapy in neonates. Curr. Pharm. Des. 2017, 23, 5928–5934. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Catozzi, C.; Ravanetti, F.; Murgia, X.; D’Alo, F.; Macchidani, N.; Sgarbi, E.; Di Lallo, V.; Saccani, F.; Pertile, M.; et al. In vitro and in vivo characterization of poractant alfa supplemented with budesonide for safe and effective intratracheal administration. Pediatr. Res. 2017, 82, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Barry, P.W.; O’Callaghan, C. An in vitro analysis of the output of budesonide from different nebulizers. J. Allergy Clin. Immunol. 1999, 104, 1168–1173. [Google Scholar] [CrossRef]
- Barry, P.W.; O’Callaghan, C. Drug output from nebulizers is dependent on the method of measurement. Eur. Respir. J. 1998, 12, 463–466. [Google Scholar] [CrossRef] [Green Version]
- Minocchieri, S.; Burren, J.M.; Bachmann, M.A.; Stern, G.; Wildhaber, J.; Buob, S.; Schindel, R.; Kraemer, R.; Frey, U.P.; Nelle, M. Development of the premature infant nose throat-model (print-model): An upper airway replica of a premature neonate for the study of aerosol delivery. Pediatr. Res. 2008, 64, 141–146. [Google Scholar] [CrossRef]
- Cates, C.J.; Crilly, J.A.; Rowe, B.H. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst. Rev. 2006, CD000052. [Google Scholar] [CrossRef]
- Uhlig, T.; Eber, E.; Devadason, S.G.; Pemberton, P.; Badawi, N.; Lesouef, P.N.; Wildhaber, J.H. Aerosol delivery to spontaneously breathing neonates: Spacer or nebulizer? Pediatr. Asthma Allergy Immunol. 1997, 11, 111–117. [Google Scholar] [CrossRef]
- NICE. Guidance on the Use of Inhaler Systems (Devices) in Children under the Age of 5 Years with Chronic Asthma. Technology Appraisal Guidance [ta10]. Available online: https://www.nice.org.uk/guidance/ta10 (accessed on 21 February 2019).
- Harlos, M.S.; Stenekes, S.; Lambert, D.; Hohl, C.; Chochinov, H.M. Intranasal fentanyl in the palliative care of newborns and infants. J. Pain Symptom Manage. 2013, 46, 265–274. [Google Scholar] [CrossRef]
- Milési, C.; Baleine, J.; Mura, T.; Benito-Castro, F.; Ferragu, F.; Thiriez, G.; Thévenot, P.; Combes, C.; Carbajal, R.; Cambonie, G. Nasal midazolam vs ketamine for neonatal intubation in the delivery room: A randomised trial. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F221–F226. [Google Scholar] [CrossRef]
- Hanson, L.R.; Frey, W.H., 2nd. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008, 9, S5. [Google Scholar] [CrossRef]
- Misra, A.; Kher, G. Drug delivery systems from nose to brain. Curr. Pharm. Biotechnol. 2012, 13, 2355–2379. [Google Scholar] [CrossRef]
- Kozlovskaya, L.; Abou-Kaoud, M.; Stepensky, D. Quantitative analysis of drug delivery to the brain via nasal route. J. Control. Release 2014, 189, 133–140. [Google Scholar] [CrossRef]
- Ainsworth, S.B. Neonatal Formulary 7: Drug Use in Pregnancy and the First Year of Life, 7th ed.; John Wiley & Sons Inc.: Chichester, UK, 2014. [Google Scholar]
- Demirjian, A.; Levy, O. Safety and efficacy of neonatal vaccination. Eur. J. Immunol. 2009, 39, 36–46. [Google Scholar] [CrossRef]
- Ghersi-Egea, J.-F.; Saudrais, E.; Strazielle, N.J.P.R. Barriers to drug distribution into the perinatal and postnatal brain. Pharm. Res. 2018, 35, 84. [Google Scholar] [CrossRef]
- Saunders, N.R.; Dziegielewska, K.M.; Møllgård, K.; Habgood, M.D. Physiology and molecular biology of barrier mechanisms in the fetal and neonatal brain. J. Physiol. (Lond.) 2018, 596, 5723–5756. [Google Scholar] [CrossRef] [Green Version]
- Webster, R. Presentation-Blood Brain Barrier Maturation: Implications for Drug Development. Available online: https://www.ema.europa.eu/documents/presentation/presentation-blood-brain-barrier-maturation-implications-drug-development_en.pdf (accessed on 13 March 2019).
- Vecsernyés, M.; Fenyvesi, F.; Bácskay, I.; Deli, M.A.; Szente, L.; Fenyvesi, É. Cyclodextrins, blood–brain barrier, and treatment of neurological diseases. Arch. Med. Res. 2014, 45, 711–729. [Google Scholar] [CrossRef]
- Merkus, F.W.; Verhoef, J.C.; Marttin, E.; Romeijn, S.G.; van der Kuy, P.H.; Hermens, W.A.; Schipper, N.G. Cyclodextrins in nasal drug delivery. Adv. Drug Deliv. Rev. 1999, 36, 41–57. [Google Scholar] [CrossRef]
- Rivers, J.R.; Maggo, S.D.; Ashton, J.C. Neuroprotective effect of hydroxypropyl-beta-cyclodextrin in hypoxia-ischemia. Neuroreport 2012, 23, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Millar, L.J.; Shi, L.; Hoerder-Suabedissen, A.; Molnár, Z. Neonatal hypoxia ischaemia: Mechanisms, models, and therapeutic challenges. Front. Cell. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Archambault, J.; Moreira, A.; McDaniel, D.; Winter, L.; Sun, L.; Hornsby, P. Therapeutic potential of mesenchymal stromal cells for hypoxic ischemic encephalopathy: A systematic review and meta-analysis of preclinical studies. PLoS One 2017, 12, e0189895. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.L.A.; Michael-Titus, A.T.; Shah, D.K. Hypoxic-ischaemic encephalopathy and the blood-brain barrier in neonates. Dev. Neurosci. 2017, 39, 49–58. [Google Scholar] [CrossRef] [PubMed]
- van Velthoven, C.T.; Kavelaars, A.; van Bel, F.; Heijnen, C.J. Nasal administration of stem cells: A promising novel route to treat neonatal ischemic brain damage. Pediatr. Res. 2010, 68, 419–422. [Google Scholar] [CrossRef]
- Wolfe, T.R.; Braude, D.A. Intranasal medication delivery for children: A brief review and update. Pediatrics 2010, 126, 532–537. [Google Scholar] [CrossRef]
- Kalia, Y.N.; Nonato, L.B.; Lund, C.H.; Guy, R.H. Development of skin barrier function in premature infants. J. Invest. Dermatol. 1998, 111, 320–326. [Google Scholar] [CrossRef]
- Delgado-Charro, M.B.; Guy, R.H. Effective use of transdermal drug delivery in children. Adv. Drug Deliv. Rev. 2014, 73, 63–82. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency. Sodium Laurilsulfate Used as an Excipient. Available online: www.ema.europa.eu/docs/en_GB/document_library/Report/2017/10/WC500235925.pdf (accessed on 17 May 2019).
- Oranges, T.; Dini, V.; Romanelli, M. Skin physiology of the neonate and infant: Clinical implications. Adv. Wound Care (New Rochelle) 2015, 4, 587–595. [Google Scholar] [CrossRef]
- Chiou, Y.B.; Blume-Peytavi, U. Stratum corneum maturation. A review of neonatal skin function. Skin Pharmacol. Physiol. 2004, 17, 57–66. [Google Scholar] [CrossRef]
- Cuzzolin, L. Off-label drug in the newborn. J. Pediatr. Neonat. Individual. Med. 2014, 3, e030224. [Google Scholar]
- Krzyzaniak, N.; Pawlowska, I.; Bajorek, B. Review of drug utilization patterns in nicus worldwide. J. Clin. Pharm. Ther. 2016, 41, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Lopez, S.; Stanley, I.M.; Tunstell, P.; Aguado-Lorenzo, V.; Philip, J.; Perkins, J.; Durward, A.; Calleja-Hernandez, M.A.; Tibby, S.M. Safe implementation of standard concentration infusions in paediatric intensive care. J. Pharm. Pharmacol. 2017, 69, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, R.; Bates, D.W.; Landrigan, C.; McKenna, K.J.; Clapp, M.D.; Federico, F.; Goldmann, D.A. Medication errors and adverse drug events in pediatric inpatients. JAMA 2001, 285, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Larsen, G.Y.; Parker, H.B.; Cash, J.; O’Connell, M.; Grant, M.C. Standard drug concentrations and smart-pump technology reduce continuous-medication-infusion errors in pediatric patients. Pediatrics 2005, 116, e21–e25. [Google Scholar] [CrossRef] [PubMed]
- The Joint Commission. Preventing Pediatric Medication Errors. Available online: https://www.jointcommission.org/sentinel_event_alert_issue_39_preventing_pediatric_medication_errors/ (accessed on 19 March 2019).
- Christie-Taylor, S.A.; Tait, P.A. Implementation of standard concentration medication infusions for preterm infants. Infant 2012, 8, 155–159. [Google Scholar]
- ISMP. High-Alert Medications in Acute Care Settings. Available online: https://www.ismp.org/recommendations/high-alert-medications-acute-list (accessed on 22 February 2019).
- Perkins, J.; Aguado-Lorenzo, V.; Arenas-Lopez, S. Standard concentration infusions in paediatric intensive care: The clinical approach. J. Pharm. Pharmacol. 2017, 69, 537–543. [Google Scholar] [CrossRef]
- Lala, A.C.; Broadbent, R.S.; Medlicott, N.J.; Sherwin, C.M.; Reith, D.M. Illustrative neonatal cases regarding drug delivery issues. J. Paediatr. Child Health 2015, 51, 478–481. [Google Scholar] [CrossRef]
- Medlicott, N.J.; Reith, D.M.; McCaffrey, F.; Krittaphol, W.; Broadbent, R.S. Delayed delivery of intravenous gentamicin in neonates: Impact of infusion variables. J. Pharm. Pharmacol. 2013, 65, 370–378. [Google Scholar] [CrossRef]
- Sherwin, C.M.; McCaffrey, F.; Broadbent, R.S.; Reith, D.M.; Medlicott, N.J. Discrepancies between predicted and observed rates of intravenous gentamicin delivery for neonates. J. Pharm. Pharmacol. 2009, 61, 465–471. [Google Scholar] [CrossRef]
- RCPCH; NPPG. Position Statement 18-01: Using Standardised Strengths of Unlicensed Liquid Medicines in Children. Available online: http://nppg.org.uk/wp-content/uploads/2019/03/NPPG-Position-Statement-18-01-Dec-2018.pdf (accessed on 19 March 2019).
- Allen, L.V., Jr.; Levinson, R.S.; Phisutsinthop, D. Compatibility of various admixtures with secondary additives at y-injection sites of intravenous administration sets. Am. J. Hosp. Pharm. 1977, 34, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Staven, V.; Iqbal, H.; Wang, S.; Gronlie, I.; Tho, I. Physical compatibility of total parenteral nutrition and drugs in y-site administration to children from neonates to adolescents. J. Pharm. Pharmacol. 2017, 69, 448–462. [Google Scholar] [CrossRef]
- Tonnesen, H.H. Formulation and stability testing of photolabile drugs. Int. J. Pharm. 2001, 225, 1–14. [Google Scholar] [CrossRef]
- Baertschi, S.W.; Clapham, D.; Foti, C.; Jansen, P.J.; Kristensen, S.; Reed, R.A.; Templeton, A.C.; Tonnesen, H.H. Implications of in-use photostability: Proposed guidance for photostability testing and labeling to support the administration of photosensitive pharmaceutical products, part 1: Drug products administered by injection. J. Pharm. Sci. 2013, 102, 3888–3899. [Google Scholar] [CrossRef]
- Baertschi, S.W.; Clapham, D.; Foti, C.; Kleinman, M.H.; Kristensen, S.; Reed, R.A.; Templeton, A.C.; Tonnesen, H.H. Implications of in-use photostability: Proposed guidance for photostability testing and labeling to support the administration of photosensitive pharmaceutical products, part 2: Topical drug product. J. Pharm. Sci. 2015, 104, 2688–2701. [Google Scholar] [CrossRef] [PubMed]
- Brustugun, J.; Tønnesen, H.H.; Edge, R.; Navaratnam, S. Formation and reactivity of free radicals in 5-hydroxymethyl-2-furaldehyde—The effect on isoprenaline photostability. J. Photochem. Photobiol. B Biol. 2005, 79, 109–119. [Google Scholar] [CrossRef]
- Kogermann, K.; Lass, J.; Nellis, G.; Metsvaht, T.; Lutsar, I. Age-appropriate formulations including pharmaceutical excipients in neonatal medicines. Curr. Pharm. Des. 2017, 23, 5779–5789. [Google Scholar] [CrossRef] [PubMed]
- Nellis, G.; Metsvaht, T.; Varendi, H.; Toompere, K.; Lass, J.; Mesek, I.; Nunn, A.J.; Turner, M.A.; Lutsar, I.; Esnee Consortium. Potentially harmful excipients in neonatal medicines: A pan-european observational study. Arch. Dis. Child. 2015, 100, 694–699. [Google Scholar] [CrossRef]
- Whittaker, A.; Currie, A.E.; Turner, M.A.; Field, D.J.; Mulla, H.; Pandya, H.C. Toxic additives in medication for preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2009, 94, F236–F240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, S.; Turner, M. European study of neonatal exposure to excipients (esnee). Infant 2011, 7, 196–199. [Google Scholar]
- Turner, M.A.; Duncan, J.C.; Shah, U.; Metsvaht, T.; Varendi, H.; Nellis, G.; Lutsar, I.; Yakkundi, S.; McElnay, J.C.; Pandya, H.; et al. Risk assessment of neonatal excipient exposure: Lessons from food safety and other areas. Adv. Drug Deliv. Rev. 2014, 73, 89–101. [Google Scholar] [CrossRef]
- EuPFI. The Step Database. Available online: http://www.eupfi.org/step-database-info/ (accessed on 22 February 2019).
- European Medicines Agency. Excipients Labelling. Available online: https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation/product-information/reference-guidelines/excipients-labelling (accessed on 22 February 2019).
- European Medicines Agency. Propylene Glycol and Esters. Available online: https://www.ema.europa.eu/en/propylene-glycol-esters (accessed on 22 February 2019).
- Tuleu, C.; Breitkreutz, J. Educational paper: Formulation-related issues in pediatric clinical pharmacology. Eur. J. Pediatr. 2013, 172, 717–720. [Google Scholar] [CrossRef]
- Walker, N. Trends in Excipient Demand. Available online: https://www.pharmamanufacturing.com/articles/2017/trends-in-excipient-demand (accessed on 17 May 2019).
- Reker, D.; Blum, S.M.; Steiger, C.; Anger, K.E.; Sommer, J.M.; Fanikos, J.; Traverso, G. “Inactive” ingredients in oral medications. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef]
- Richey, R.H.; Craig, J.V.; Shah, U.U.; Nunn, A.J.; Turner, M.A.; Barker, C.E.; Ford, J.L.; Peak, M. Modric—Manipulation of drugs in children. Int. J. Pharm. 2013, 457, 339–341. [Google Scholar] [CrossRef]
- Orubu, E.S.; Tuleu, C. Medicines for children: Flexible solid oral formulations. Bull. World Health Organ. 2017, 95, 238–240. [Google Scholar] [CrossRef]
- Kayitare, E.; Vervaet, C.; Ntawukulilyayo, J.D.; Seminega, B.; Bortel, V.; Remon, J.P. Development of fixed dose combination tablets containing zidovudine and lamivudine for paediatric applications. Int. J. Pharm. 2009, 370, 41–46. [Google Scholar] [CrossRef]
- Broadhurst, E.C.; Ford, J.L.; Nunn, A.J.; Rowe, P.H.; Roberts, M. Dose uniformity of samples prepared from dispersible aspirin tablets for paediatric use. Eur. J. Hosp. Pharm. Sci. 2008, 14, 27–31. [Google Scholar]
- JustMilk. Available online: http://www.justmilk.org/ (accessed on 22 February 2019).
- Orubu, S.E.; Hobson, N.J.; Basit, A.W.; Tuleu, C. The milky way: Paediatric milk-based dispersible tablets prepared by direct compression—A proof-of-concept study. J. Pharm. Pharmacol. 2017, 69, 417–431. [Google Scholar] [CrossRef]
- Binte Abu Bakar, S.Y.; Salim, M.; Clulow, A.J.; Hawley, A.; Boyd, B.J. Revisiting dispersible milk-drug tablets as a solid lipid formulation in the context of digestion. Int. J. Pharm. 2019, 554, 179–189. [Google Scholar] [CrossRef]
- Thabet, Y.; Klingmann, V.; Breitkreutz, J. Drug formulations: Standards and novel strategies for drug administration in pediatrics. J. Clin. Pharmacol. 2018, 58, S26–S35. [Google Scholar] [CrossRef]
- Thabet, Y.; Walsh, J.; Breitkreutz, J. Flexible and precise dosing of enalapril maleate for all paediatric age groups utilizing orodispersible minitablets. Int. J. Pharm. 2018, 541, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Orlu, M.; Ranmal, S.R.; Sheng, Y.; Tuleu, C.; Seddon, P. Acceptability of orodispersible films for delivery of medicines to infants and preschool children. Drug Deliv. 2017, 24, 1243–1248. [Google Scholar] [CrossRef] [Green Version]
- Parshuram, C.S.; To, T.; Seto, W.; Trope, A.; Koren, G.; Laupacis, A. Systematic evaluation of errors occurring during the preparation of intravenous medication. CMAJ 2008, 178, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Bhambhani, V.; Beri, R.S.; Puliyel, J.M. Inadvertent overdosing of neonates as a result of the dead space of the syringe hub and needle. Arch. Dis. Child. Fetal Neonatal Ed. 2005, 90, F444–F445. [Google Scholar] [CrossRef] [Green Version]
- Stucki, C.; Sautter, A.M.; Wolff, A.; Fleury-Souverain, S.; Bonnabry, P. Accuracy of preparation of i.V. Medication syringes for anesthesiology. Am. J. Health Syst. Pharm. 2013, 70, 137–142. [Google Scholar] [CrossRef]
- Aguado-Lorenzo, V.; Weeks, K.; Tunstell, P.; Turnock, K.; Watts, T.; Arenas-Lopez, S. Accuracy of the concentration of morphine infusions prepared for patients in a neonatal intensive care unit. Arch. Dis. Child. 2013, 98, 975–979. [Google Scholar] [CrossRef]
- Isaac, R.E.; Duncan, H.; Marriott, J.F.; Ng, A. The Effect of Different Manipulation Techniques on the Accuracy and Reproducibility of Small Dose Volume i.V. Measurements. In Proceedings of the 6th World Congress on Pediatric Critical Care: One World Sharing Knowledge, Sydney, Australia, 13–17 March 2011; p. A99.
- Howard, C.; Macken, W.L.; Connolly, A.; Keegan, M.; Coghlan, D.; Webb, D.W. Percutaneous endoscopic gastrostomy for refractory epilepsy and medication refusal. Arch. Dis. Child. 2019. Published Online First: 4 March 2019. [Google Scholar] [CrossRef]
- European Medicines Agency. Quality of Medicines Questions and Answers: Part 2: Administration of Oral Immediate Release Medicinal Products through Enteral Feeding Tubes New December 2018. Available online: https://www.ema.europa.eu/en/human-regulatory/research-development/scientific-guidelines/qa-quality/quality-medicines-questions-answers-part-2#administration-of-oral-immediate-release-medicinal-products-through-enteral-feeding-tubes-new-december-2018-section (accessed on 22 February 2019).
- Duesing, L.A.; Fawley, J.A.; Wagner, A.J. Central venous access in the pediatric population with emphasis on complications and prevention strategies. Nutr. Clin. Pract. 2016, 31, 490–501. [Google Scholar] [CrossRef]
- Zahid, N.; Taylor, K.M.; Gill, H.; Maguire, F.; Shulman, R. Adsorption of insulin onto infusion sets used in adult intensive care unit and neonatal care settings. Diabetes Res. Clin. Pract. 2008, 80, e11–e13. [Google Scholar] [CrossRef]
- Hooymans, P.M.; Janknegt, R.; Lohman, J.J. Comparison of clonazepam sorption to polyvinyl chloride-coated and polyethylene-coated tubings. Pharm. Weekbl. Sci. 1990, 12, 188–189. [Google Scholar] [CrossRef] [PubMed]
- Fuloria, M.; Friedberg, M.A.; DuRant, R.H.; Aschner, J.L. Effect of flow rate and insulin priming on the recovery of insulin from microbore infusion tubing. Pediatrics 1998, 102, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Simeon, P.S.; Geffner, M.E.; Levin, S.R.; Lindsey, A.M. Continuous insulin infusions in neonates: Pharmacologic availability of insulin in intravenous solutions. J. Pediatr. 1994, 124, 818–820. [Google Scholar] [CrossRef]
- Trissel, L.A. Handbook on Injectable Drugs, 17th ed.; American Society of Health-System Pharmacists: Bethesda, MD, USA, 2013. [Google Scholar]
- United States Pharmacopeial Convention (Ed.) USP 41 NF 36. [1664] assessment of drug product leachables associated with pharmaceutical pacakaging/delivery systems. In The United States Pharmacopoeia|the National Formulary, 41|36 ed.; United States Pharmacopeial Convention: Rockville, MD, USA, 2017; Volume 5, pp. 7924–7937. [Google Scholar]
- WHO; Children’s Health and the Environment. Children are not Little Adults. Available online: http://www.who.int/ceh/capacity/Children_are_not_little_adults.pdf (accessed on 3 April 2019).
- ISO. Iso 10993-17:2002 Biological Evaluation of Medical Devices-Part 17: Establishment of Allowable Limits for Leachable Substances; ISO: Geneva, Switzerland, 2016. [Google Scholar]
- Bagel-Boithias, S.; Sautou-Miranda, V.; Bourdeaux, D.; Tramier, V.; Boyer, A.; Chopineau, J. Leaching of diethylhexyl phthalate from multilayer tubing into etoposide infusion solutions. Am. J. Health Syst. Pharm. 2005, 62, 182–188. [Google Scholar] [CrossRef]
- Malarvannan, G.; Onghena, M.; Verstraete, S.; van Puffelen, E.; Jacobs, A.; Vanhorebeek, I.; Verbruggen, S.C.A.T.; Joosten, K.F.M.; Van den Berghe, G.; Jorens, P.G.; et al. Phthalate and alternative plasticizers in indwelling medical devices in pediatric intensive care units. J. Hazard. Mater. 2019, 363, 64–72. [Google Scholar] [CrossRef]
- Demirel, A.; et al. Hidden toxicity in neonatal intensive care units: Phthalate exposure in very low birth weight infants. J. Clin. Res. Pediatr. Endocrinol. 2016, 8, 298–304. [Google Scholar] [CrossRef]
- CERHR. Ntp-cerhr Monograph on the Potential Human Reproductive and Developmental Effects of di(2-ethylhexyl) Phthalate (dehp); NIH Publication No. 06-4476; National Toxicology Program: Bethesda, MD, USA, 2006. [Google Scholar]
- European Chemicals Bureau. European Union Risk Assessment Report: Bis(2-ethylhexyl)phthalate (dehp): Risk Assessment; Institute of Health and Consumer Protection (IHCP): Ispra, Italy, 2008. [Google Scholar]
- Wedekind, C.A.; Fidler, B.D. Compatibility of commonly used intravenous infusions in a pediatric intensive care unit. Crit. Care Nurse 2001, 21, 45–51. [Google Scholar]
- Staven, V.; Wang, S.; Gronlie, I.; Tho, I. Development and evaluation of a test program for y-site compatibility testing of total parenteral nutrition and intravenous drugs. Nutr. J. 2016, 15, 29. [Google Scholar] [CrossRef]
- Fink, J.B. Delivery of inhaled drugs for infants and small children: A commentary on present and future needs. Clin. Ther. 2012, 34, S36–S45. [Google Scholar] [CrossRef]
- Amirav, I.; Halamish, A.; Gorenberg, M.; Omar, H.; Newhouse, M.T. More realistic face model surface improves relevance of pediatric in-vitro aerosol studies. PLoS One 2015, 10, e0128538. [Google Scholar] [CrossRef]
- Amirav, I.; Newhouse, M.T.; Luder, A.; Halamish, A.; Omar, H.; Gorenberg, M. Feasibility of aerosol drug delivery to sleeping infants: A prospective observational study. BMJ Open 2014, 4, e004124. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, H.K.; Fotaki, N.; Klein, S. Paediatric oral biopharmaceutics:Key considerations and current challenges. Adv. Drug Deliv. Rev. 2014, 73, 102–126. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.; Aye Mya Thein, N.; Turner, P.; Nosten, F.; White, N.J. Rectal ph in well and unwell infants. J. Trop. Pediatr. 2012, 58, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Kamstrup, D.; Berthelsen, R.; Sassene, P.J.; Selen, A.; Müllertz, A. In vitro model simulating gastro-intestinal digestion in the pediatric population (neonates and young infants). AAPS PharmSciTech. 2017, 18, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.M.; Bouzom, F.; Hugues, C.; Ungell, A.L. Oral drug absorption in pediatrics: The intestinal wall, its developmental changes and current tools for predictions. Biopharm. Drug Dispos. 2017, 38, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Villiger, A.; Stillhart, C.; Parrott, N.; Kuentz, M. Using physiologically based pharmacokinetic (pbpk) modelling to gain insights into the effect of physiological factors on oral absorption in paediatric populations. AAPS J. 2016, 18, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, H.; Kaukonen, A.M.; Klein, S.; Davit, B.; Ju, R.; Ternik, R.; Heimbach, T.; Lin, W.; Wang, J.; Storey, D. Food effects in paediatric medicines development for products co-administered with food. Int. J. Pharm. 2018, 536, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Martir, J.; Flanagan, T.; Mann, J.; Fotaki, N. Recommended strategies for the oral administration of paediatric medicines with food and drinks in the context of their biopharmaceutical properties: A review. J. Pharm. Pharmacol. 2017, 69, 384–397. [Google Scholar] [CrossRef]
- Fernandez Polo, A.; Cabanas Poy, M.J.; Clemente Bautista, S.; Oliveras Arenas, M.; Castillo Salinas, F.; Hidalgo Albert, E. [osmolality of oral liquid dosage forms to be administered to newborns in a hospital]. Farm. Hosp. 2007, 31, 311–314. [Google Scholar]
- White, K.C.; Harkavy, K.L. Hypertonic formula resulting from added oral medications. Am. J. Dis. Child. 1982, 136, 931–933. [Google Scholar] [CrossRef]
- Ellis, Z.-M.; Tan, H.S.G.; Embleton, N.D.; Sangild, P.T.; van Elburg, R.M. Milk feed osmolality and adverse events in newborn infants and animals: A systematic review. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F333–F340. [Google Scholar] [CrossRef]
- Akram, G.; Mullen, A.B. Paediatric nurses’ knowledge and practice of mixing medication into foodstuff. Int. J. Pharm. Pract. 2012, 20, 191–198. [Google Scholar] [CrossRef]
- Wollmer, E.; Neal, G.; Whitaker, M.J.; Margetson, D.; Klein, S. Biorelevant in vitro assessment of dissolution and compatibility properties of a novel paediatric hydrocortisone drug product following exposure of the drug product to child-appropriate administration fluids. Eur. J. Pharm. Biopharm. 2018, 133, 277–284. [Google Scholar] [CrossRef]
- Karkossa, F.; Krueger, A.; Urbaniak, J.; Klein, S. Simulating different dosing scenarios for a child-appropriate valproate er formulation in a new pediatric two-stage dissolution model. AAPS PharmSciTech. 2017, 18, 309–316. [Google Scholar] [CrossRef]
- Boberg, M.; Vrana, M.; Mehrotra, A.; Pearce, R.E.; Gaedigk, A.; Bhatt, D.K.; Leeder, J.S.; Prasad, B. Age-dependent absolute abundance of hepatic carboxylesterases (ces1 and ces2) by lc-ms/ms proteomics: Application to pbpk modeling of oseltamivir in vivo pharmacokinetics in infants. Drug Metab. Dispos. 2017, 45, 216–223. [Google Scholar] [CrossRef]
- Duan, P.; Wu, F.; Moore, J.N.; Fisher, J.; Crentsil, V.; Gonzalez, D.; Zhang, L.; Burckart, G.J.; Wang, J. Assessing cyp2c19 ontogeny in neonates and infants using physiologically based pharmacokinetic models: Impact of enzyme maturation versus inhibition. CPT: Pharmacometrics Syst. Pharmacol. 2019, 8, 158–166. [Google Scholar]
- Emoto, C.; Johnson, T.N.; Neuhoff, S.; Hahn, D.; Vinks, A.A.; Fukuda, T. Pbpk model of morphine incorporating developmental changes in hepatic oct1 and ugt2b7 proteins to explain the variability in clearances in neonates and small infants. CPT:Pharmacometrics Syst. Pharmacol. 2018, 7, 464–473. [Google Scholar] [CrossRef]
- Hahn, D.; Emoto, C.; Euteneuer, J.C.; Mizuno, T.; Vinks, A.A.; Fukuda, T. Influence of oct1 ontogeny and genetic variation on morphine disposition in critically ill neonates: Lessons from pbpk modeling and clinical study. Clin. Pharmacol. Ther. 2019, 105, 761–768. [Google Scholar] [CrossRef]
- Johnson, T.N.; Bonner, J.J.; Tucker, G.T.; Turner, D.B.; Jamei, M. Development and applications of a physiologically-based model of paediatric oral drug absorption. Eur. J. Pharm. Sci. 2018, 115, 57–67. [Google Scholar] [CrossRef]
- Mahmood, I.; Tegenge, M.A. A comparative study between allometric scaling and physiologically based pharmacokinetic modeling for the prediction of drug clearance from neonates to adolescents. J. Clin. Pharmacol. 2019, 59, 189–197. [Google Scholar] [CrossRef]
- Michelet, R.; Van Bocxlaer, J.; Allegaert, K.; Vermeulen, A. The use of pbpk modeling across the pediatric age range using propofol as a case. J. Pharmacokinet. Pharmacodyn. 2018, 45, 765–785. [Google Scholar] [CrossRef]
- T’jollyn, H.; Vermeulen, A.; Van Bocxlaer, J. Pbpk and its virtual populations: The impact of physiology on pediatric pharmacokinetic predictions of tramadol. AAPS J. 2019, 21, 8. [Google Scholar] [CrossRef]
- Troutman, J.A.; Sullivan, M.C.; Carr, G.J.; Fisher, J. Development of growth equations from longitudinal studies of body weight and height in the full term and preterm neonate: From birth to four years postnatal age. Birth Defects Res. 2018, 110, 916–932. [Google Scholar] [CrossRef]
- Allegaert, K.; Simons, S.H.P.; Tibboel, D.; Krekels, E.H.; Knibbe, C.A.; van den Anker, J.N. Non-maturational covariates for dynamic systems pharmacology models in neonates, infants, and children: Filling the gaps beyond developmental pharmacology. Eur. J. Pharm. Sci. 2017, 109, S27–S31. [Google Scholar] [CrossRef]
- Smits, A.; De Cock, P.; Vermeulen, A.; Allegaert, K. Physiologically based pharmacokinetic (pbpk) modeling and simulation in neonatal drug development: How clinicians can contribute. Expert Opin. Drug Metab. Toxicol. 2019, 15, 25–34. [Google Scholar] [CrossRef]
- European Medicines Agency. 10-Year Report to the European Commission: General Report on the Experience Acquired as a Result of the Application of the Paediatric Regulation. Available online: https://ec.europa.eu/health/sites/health/files/files/paediatrics/2016_pc_report_2017/ema_10_year_report_for_consultation.pdf (accessed on 19 March 2019).
- Zisowsky, J.; Krause, A.; Dingemanse, J. Drug development for pediatric populations: Regulatory aspects. Pharmaceutics 2010, 2, 364–388. [Google Scholar] [CrossRef] [PubMed]
- Ivanovska, V.; Rademaker, C.M.A.; van Dijk, L.; Mantel-Teeuwisse, A.K. Pediatric drug formulations: A review of challenges and progress. Pediatrics 2014, 134, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Ernest, T.B.; Craig, J.; Nunn, A.; Salunke, S.; Tuleu, C.; Breitkreutz, J.; Alex, R.; Hempenstall, J. Preparation of medicines for children—A hierarchy of classification. Int. J. Pharm. 2012, 435, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Richey, R.H.; Hughes, C.; Craig, J.V.; Shah, U.U.; Ford, J.L.; Barker, C.E.; Peak, M.; Nunn, A.J.; Turner, M.A. A systematic review of the use of dosage form manipulation to obtain required doses to inform use of manipulation in paediatric practice. Int. J. Pharm. 2017, 518, 155–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Medicines Agency. Paediatric-Use Marketing Authorisations. Available online: https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation/paediatric-medicines/paediatric-use-marketing-authorisations (accessed on 22 February 2019).
- EDQM; Council of Europe. European Paediatric Formulary. Available online: https://paedform.edqm.eu/home (accessed on 22 February 2019).





| Type of Catheter | Characteristics | Issues |
|---|---|---|
| Peripheral Venous Catheters | ||
| Peripheral venous catheter | Application: Most IV drugs, isotonic IV fluids, blood transfusions Low flow rates Physicochemical irritation with some drugs results in phlebitis Dwell time: Most need to be removed within three days due to complications | Difficult to insert in the neonates due to the small and hard to visualize vessels |
| Central Venous Catheters (CVC) | ||
| Umbilical venous catheter (UVC) | Application: For diagnostic and therapeutic purposes—infusion of medication, TPN, hypertonic IV fluids, central venous pressure and venous blood gas monitoring, blood transfusions Dwell time: Up to 14 days | Suitable for neonates only as the umbilical vein remains for up to two weeks after birth UVC usually inserted within 12 hours of birth if indicated, for parenteral nutrition and/or inotropic support. |
| Peripherally inserted central catheter (PICC) | Application: Medication and IV fluid administration, TPN, blood sampling Suitable for irritant drugs Not suitable for large volume administration in emergency situations (for 28G 20 cm long catheter the max flow is 1 mL/min) Available multi-lumen catheters Made of polyurethane or silicone | Links the benefits of peripheral and central catheter PICC inserted at any time and used for all drugs (in conjunction with UVC helps reduce risk of drug incompatibilities). |
| Chemical and physical compatibility of drug formulation used in multi-drug administration [28] including generic brands |
| Chemical and physical compatibility of drug formulation used in combination with neonatal TPN [108,109] |
| Compatibility of drug with diluents typically used in the NICU and stability after dilution |
| Compatibility of drug formulation while mixing at Y-site junction at different mixing ratios [108,109] |
| Stability of drug formulation over extended period of time (e.g., over 24 h infusion) |
| Stability of drug formulation exposed to different environmental conditions (high temperature, strong light, high oxygen levels) [110] |
| Stability and compatibility of excipients used in drug formulation |
| Stability and compatibility of excipients used in drug formulation with IV administration set and container |
| Compatibility of drug formulation with IV administration set and container |
| Strength(s)/concentration of drug that can cover neonatal weight- or age-bands as well as fluid restricted patients |
| Performance of medical equipment delivering drug—volumetric and smart pumps, syringe drivers |
| Design of IV administration set minimising drug delivery delays |
| Degree of Concern Associated with the Route of Administration | Likelihood of Packaging Component–Dosage Form Interaction | ||
|---|---|---|---|
| High | Medium | Low | |
| Highest | Inhalation aerosol and spray | Injections and injectable suspensions, inhalation solution | Sterile powders and powders for injection, inhalation powders |
| High | Transdermal ointment and patches | Ophthalmic solutions and suspension, nasal aerosol and spray | |
| Low | Topic solutions and suspensions, topical and lingual aerosol, oral solutions and suspensions | Oral tablets and oral (hard and soft gelatin) capsules, topical powders, oral powders | |
| Route of Administration | Impact on Absorption/Distribution | Reasons |
|---|---|---|
| Oral | Altered absorption | Neonatal pH is elevated in the stomach (increased for basic drugs and reduced for acidic drugs) Immature ontogeny of transporter expression |
| Reduced absorption | Slower gastric emptying Reduced relative surface area in the intestine | |
| Increased absorption | Slower intestinal transit Reduced intestinal P-glycoprotein expression | |
| Rectal | Decreased surface area | Reduced relative surface area of rectum |
| Respiratory | Decreased absorption | Immature lung branching and development Reduced lung capacity and inspiratory flow |
| Nasal | No data shown | Potential for irritation in the nasal mucosa in neonates |
| Dermal and transdermal | Increased absorption | Higher BSA/kg ratio Thinner stratum cornea layer More hydrated stratum corneum Higher relative surface area to bodyweight |
| IV | Reduced distribution | Reduced blood volume |
| Intramuscular | Reduced distribution | Reduced muscle mass |
| Altered distribution | Variable muscle blood flow | |
| Subcutaneous | Reduced distribution | Reduced subcutaneous fat |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Brien, F.; Clapham, D.; Krysiak, K.; Batchelor, H.; Field, P.; Caivano, G.; Pertile, M.; Nunn, A.; Tuleu, C. Making Medicines Baby Size: The Challenges in Bridging the Formulation Gap in Neonatal Medicine. Int. J. Mol. Sci. 2019, 20, 2688. https://doi.org/10.3390/ijms20112688
O’Brien F, Clapham D, Krysiak K, Batchelor H, Field P, Caivano G, Pertile M, Nunn A, Tuleu C. Making Medicines Baby Size: The Challenges in Bridging the Formulation Gap in Neonatal Medicine. International Journal of Molecular Sciences. 2019; 20(11):2688. https://doi.org/10.3390/ijms20112688
Chicago/Turabian StyleO’Brien, Fiona, David Clapham, Kamelia Krysiak, Hannah Batchelor, Peter Field, Grazia Caivano, Marisa Pertile, Anthony Nunn, and Catherine Tuleu. 2019. "Making Medicines Baby Size: The Challenges in Bridging the Formulation Gap in Neonatal Medicine" International Journal of Molecular Sciences 20, no. 11: 2688. https://doi.org/10.3390/ijms20112688
APA StyleO’Brien, F., Clapham, D., Krysiak, K., Batchelor, H., Field, P., Caivano, G., Pertile, M., Nunn, A., & Tuleu, C. (2019). Making Medicines Baby Size: The Challenges in Bridging the Formulation Gap in Neonatal Medicine. International Journal of Molecular Sciences, 20(11), 2688. https://doi.org/10.3390/ijms20112688