New Polymeric Films with Antibacterial Activity Obtained by UV-induced Copolymerization of Acryloyloxyalkyltriethylammonium Salts with 2-Hydroxyethyl Methacrylate
Abstract
:1. Introduction
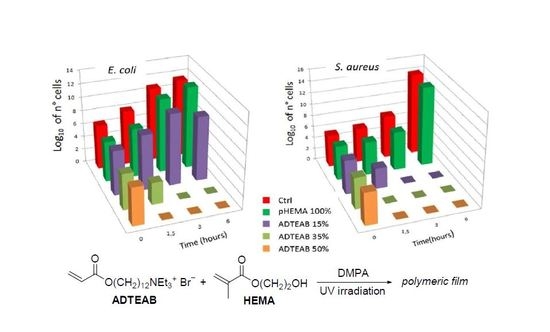
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of Polymeric Films
3.2. Microorganisms and General Growth Conditions
3.3. Antibacterial Assessment of Polymeric Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AATEABs | acryloyloxydodecyltriethylammonium bromide (ω-(acryloyloxy)-N,N,N-triethylalcan-1-aminium bromides) |
| ADTEAB | acryloyloxydodecyltriethylammonium bromide (12-(acryloyloxy)-N,N,N-triethylundecan-1-aminium bromide) |
| AUTEAB | acryloyloxyundecyltriethylammonium bromide(11-(acryloyloxy)-N,N,N-triethyldodecan-1-aminium bromide) |
| CFU | colony-forming unit |
| DMPA | 2,2-dimethoxy-2-phenylacetophenone (2,2-dimethoxy-1,2-diphenylethan-1-one) |
| HEMA | 2-hydroxyethyl methacrylate |
| LB | Luria-Bertani |
| OD | optical density |
| pHEMA | poly-2-hydroxyethyl methacrylate |
| PQASs | polymerizable quaternary ammonium salts |
| QASs | quaternary ammonium salts |
References
- Almakki, A.; Jumas-Bilak, E.; Marchandin, H.; Licznar-Fajardo, P. Antibiotic resistance in urban runoff. Sci. Total Environ. 2019, 667, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Canica, M.; Manageiro, V.; Abriouel, H.; Moran-Gilad, J.; Franz, C.M.A.P. Antibiotic resistance in foodborne bacteria. Trends Food Sci. Technol. 2019, 84, 41–44. [Google Scholar] [CrossRef]
- Li, R.; Jay, J.A.; Stenstrom, M.K. Fate of antibiotic resistance genes and antibiotic-resistant bacteria in water resource recovery facilities. Water Environ. Res. 2019, 91, 5–20. [Google Scholar] [CrossRef]
- Peterson, E.; Kaur, P. Antibiotic resistance mechanisms in bacteria: Relationships between resistance determinants of antibiotic producers, environmental Bacteria, and clinical pathogens. Front. Microbiol. 2018, 9, 2928. [Google Scholar] [CrossRef] [PubMed]
- Babakhani, S.; Oloomi, M. Transposons: The agents of antibiotic resistance in bacteria. J. Basic Microbiol. 2018, 58, 905–917. [Google Scholar] [CrossRef]
- Xing, H.; Lu, M.; Yang, T.; Liu, H.; Sun, Y.; Zhao, X.; Xu, H.; Yang, L.; Ding, P. Structure-function relationships of nonviral gene vectors: Lessons from antimicrobial polymers. Acta Biomater. 2019, 86, 15–20. [Google Scholar] [CrossRef]
- Munoz-Bonilla, A.; Echeverria, C.; Sonseca, A.; Arrieta, M.P.; Fernandez-Garcia, M. Bio-based polymers with antimicrobial properties towards sustainable development. Materials 2019, 12, 641. [Google Scholar] [CrossRef]
- Konai, M.M.; Bhattacharjee, B.; Ghosh, S.; Haldar, J. Recent progress in polymer research to tackle infections and antimicrobial resistance. Biomacromolecules 2018, 19, 1888–1917. [Google Scholar] [CrossRef] [PubMed]
- Ergene, C.; Yasuhara, K.; Palermo, E.F. Biomimetic antimicrobial polymers: Recent advances in molecular design. Polym. Chem. 2018, 9, 2407–2427. [Google Scholar] [CrossRef]
- Al-Jumaili, A.; Kumar, A.; Bazaka, K.; Jacon, M.V. Plant secondary metabolite-derived polymers: A potential approach to develop antimicrobial films. Polymers 2018, 10, 515. [Google Scholar] [CrossRef] [PubMed]
- Ergene, C.; Palermo, E.F. Antimicrobial synthetic polymers: An update on structure-activity relationships. Curr. Pharm. Des. 2018, 24, 855–865. [Google Scholar] [CrossRef]
- Yang, Y.; Cai, Z.; Huang, Z.; Tang, X.; Zhang, X. Antimicrobial cationic polymers: From structural design to functional control. Polym. J. 2018, 50, 33–44. [Google Scholar] [CrossRef]
- Lam, S.J.; Wong, E.H.H.; Boyer, C.; Qiao, G.G. Antimicrobial polymeric nanoparticles. Progr. Polym. Sci. 2018, 76, 40–64. [Google Scholar] [CrossRef]
- Alvarez-Paino, M.; Munoz-Bonilla, A.; Fernandez-Garcia, M. Antimicrobial polymers in the nano-world. Nanomaterials 2017, 7, 48. [Google Scholar] [CrossRef]
- Huang, K.-S.; Yang, C.-H.; Huang, S.-L.; Chan, C.-Y.; Lu, Y.-Y.; Lin, Y.-S. Recent advances in antimicrobial polymers: A mini-review. Int. J. Mol. Sci. 2016, 17, 1578. [Google Scholar] [CrossRef]
- Gonzalez-Henriquez, C.M.; Sarabia-Vallejos, M.A.; Rodriguez Hernandez, J. Antimicrobial polymers for additive manufacturing. Int. J. Mol. Sci. 2019, 20, 1210. [Google Scholar] [CrossRef]
- Feldman, D. Polymer nanocomposites for tissue engineering, antimicrobials and drug delivery. Biointerface Res. Appl. Chem. 2018, 8, 3153–3160. [Google Scholar]
- Gahruie, H.H.; Niakousari, M. Antioxidant, antimicrobial, cell viability and enzymatic inhibitory of antioxidant polymers as biological macromolecules. Int. J. Biol. Part A Macromol. 2017, 104, 606–617. [Google Scholar]
- Hartlieb, M.; Williams, E.G.L.; Kuroki, A.; Perrier, S.; Locock, K.E.S. Antimicrobial polymers: Mimicking amino acid functionality, sequence control and three-dimensional structure of host-defense peptides. Curr. Med. Chem. 2017, 24, 2115–2140. [Google Scholar] [CrossRef]
- Wo, Y.; Brisbois, E.J.; Bartlett, R.H.; Meyerhoff, M.E. Recent advances in thromboresistant and antimicrobial polymers for biomedical applications: Just say yes to nitric oxide (NO). Biomater. Sci. 2016, 4, 1161–1183. [Google Scholar] [CrossRef]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current advancements in chitosan-based film production for food technology; A review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C. Polymeric nanocomposites and nanocoatings for food packaging: A review. Materials 2018, 11, 1834. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.C.P.; Sousa, R.C.S.; Otoni, C.G.; Moraes, A.R.F.; Souza, V.G.L.; Medeiros, E.A.A.; Espita, P.J.P.; Pires, A.C.S.; Coimbra, J.S.R.; Soares, N.F.F. Nisin and other antimicrobial peptides: Production, mechanisms of action, and application in active food packaging. Innov. Food Sci. Emerg. Technol. 2018, 48, 179–194. [Google Scholar] [CrossRef]
- Galie, S.; Garcia-Gutierrez, C.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Gafri, H.F.S.; Zuki, F.M.; Aroua, M.K.; Hashim, N.A. Mechanism of bacterial adhesion on ultrafiltration membrane modified by natural antimicrobial polymers (Chitosan) and combination with activated carbon (PAC). Rev. Chem. Eng. 2019, 35, 421–443. [Google Scholar] [CrossRef]
- Zhu, J.; Hou, J.; Zhang, Y.; Tian, M.; He, T.; Liu, J.; Chen, V. Polymeric antimicrobial membranes enabled by nanomaterials for water treatment. J. Membr. Sci. 2018, 550, 173–197. [Google Scholar] [CrossRef]
- Mukherjee, M.; De, S. Antibacterial polymeric membranes: A short review. Environm. Sci. Water Res. Technol. 2018, 4, 1078–1104. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, J.; Hou, J.; Zhang, Y.; Liu, J.; Ven der Bruggen, B. Graphene-based antimicrobial polymeric membranes: A review. J. Mater. Sci. A 2017, 5, 6776–6793. [Google Scholar] [CrossRef]
- Akbari, S.; Kozlowski, R.M. A review of application of amine-terminated dendritic materials in textile engineering. J. Text. Inst. 2019, 110, 460–467. [Google Scholar] [CrossRef]
- Emam, H.E. Antimicrobial cellulosic textiles based on organic compounds. 3 Biothech. 2019, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Ul-Islam, S.; Butola, B.S. Recent advances in chitosan polysaccharide and its derivatives in antimicrobial modification of textile materials. Int. J. Biol. Macromol. 2019, 121, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Morais, D.S.; Guedes, R.M.; Lopes, M.A. Antimicrobial approaches for textiles: From research to market. Materials 2016, 9, 498. [Google Scholar] [CrossRef]
- Oblak, E.; Piecuch, A.; Rewak-Soroczynska, A.; Paluch, E. Activity of gemini quaternary ammonium aalts against microorganisms. Appl. Microbiol. Biotechnol. 2019, 103, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Fait, M.E.; Bakas, L.; Garrote, G.L.; Morcelle, S.R.; Saparrat, M.C.N. Cationic surfactants as antifungal agents. Appl. Microbiol. Biothechnol. 2019, 103, 97–112. [Google Scholar] [CrossRef]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Mulder, I.; Siemens, J.; Sentek, V.; Amelung, W.; Smalla, K.; Jachalke, S. Quaternary ammonium compounds in soil: Implications for antibiotic resistance development. Rev. Environ. Sci. Bio-Technol. 2018, 17, 159–185. [Google Scholar] [CrossRef]
- Jiao, Y.; Niu, L.-n.; Ma, S.; Li, J.; Tay, F.R.; Chen, J.-h. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Progr. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef]
- Jennings, M.C.; Minbiole, K.P.C.; Wuest, W.M. Quaternary ammonium compounds: An antimicrobial mainstay and platform for innovation to address bacterial resistance. Acs Infect. Dis. 2015, 1, 288–303. [Google Scholar] [CrossRef]
- Gerba, C.P. Quaternary ammonium biocides: Efficacy in application. Appl. Environ. Microbiol. 2015, 81, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Buffet-Bataillon, S.; Tattevin, P.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Emergence of resistance to antibacterial agents: The role of quaternary ammonium compounds-a critical review. Int. J. Antimicrob. Agents 2012, 39, 381–389. [Google Scholar] [CrossRef]
- Yoo, J.H. Review of disinfection and sterilization – Back to the basics. Infect Chemother. 2018, 50, 101–109. [Google Scholar] [CrossRef]
- Inacio, A.S.; Domingues, N.S.; Nunes, A.; Martins, P.T.; Moreno, M.J.; Estronca, L.M.; Fernandes, R.; Moreno, A.J.M.; Borrego, M.J.; Gomes, J.P.; Vaz, W.L.C.; Vieira, O.V. Quaternary ammonium surfactant structure determines selective toxicity towards bacteria: Mechanisms of action and clinical implications in antibacterial prophylaxis. J. Antimicrob. Chemother. 2016, 71, 641–654. [Google Scholar] [CrossRef]
- Zubris, D.L.; Minbiole, K.P.C.; Wuest, W.M. Polymeric quaternary ammonium compounds: Versatile antimicrobial materials. Curr. Top. Med. Chem. 2017, 17, 305–318. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, J.J.; Dai, X.L. Preparation and characterization of reactive chitosan quaternary ammonium salt and its application in antibacterial finishing of cotton fabric. Text. Res. J. 2017, 87, 759–765. [Google Scholar] [CrossRef]
- Xue, Y.; Xiao, H.N.; Zhang, Y. Antimicrobial polymeric materials with quaternary ammonium and phosphonium salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef]
- Mancuso, R.; Amuso, R.; Armentano, B.; Grasso, G.; Rago, V.; Cappello, A.R.; Galiano, F.; Figoli, A.; De Luca, G.; Hoinkis, J.; Gabriele, B. Synthesis and antibacterial activity of plymerizable acryloyloxytriethyl ammonium salts. ChemPlusChem 2017, 82, 1235–1244. [Google Scholar] [CrossRef]
- Deowan, S.A.; Galiano, F.; Hoinkis, J.; Johnson, D.; Altinkaya, S.A.; Gabriele, B.; Hilal, N.; Drioli, E.; Figoli, A. Novel low-fouling membrane bioreactor (MBR) for industrial wastewater treatment. J. Membr. Sci. 2016, 510, 524–532. [Google Scholar] [CrossRef] [Green Version]
- Galiano, F.; Figoli, A.; Deowan, S.A.; Johnson, D.; Altinkaya, S.A.; Veltri, L.; De Luca, G.; Mancuso, R.; Hilal, N.; Gabriele, B.; Hoinkis, J. A step forward to a more efficient wastewater treatment by membrane surface modification via polymerizable biocontinuous microemulsion. J. Membr. Sci. 2015, 482, 103–114. [Google Scholar] [CrossRef]
- Galiano, F.; Schmidt, S.A.; Ye, X.; Kumar, R.; Mancuso, R.; Curcio, E.; Gabriele, B.; Hoinkis, J.; Figoli, A. UV-LED induced bicontinuous microemulsion polymerisation for surface modification of commercial membranes – Enhancing the antifouling properties. Sep. Pur. Technol. 2018, 194, 149–160. [Google Scholar] [CrossRef]
- De Luca, G.; Amuso, R.; Figoli, A.; Mancuso, R.; Lucadamo, L.; Gabriele, B. Modeling of structure-property relashionships of polymerizable surfactants with antimicrobial activity. Appl. Sci. 2018, 8, 1972. [Google Scholar] [CrossRef]
- Tomar, N.; Tomar, M.; Gulati, N.; Nagaich, U. pHEMA hydrogels: Devices for ocular drug delivery. Int. J. Heal. Allied Sci. 2012, 1, 224. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Sundararaman, M.; Kumar, R.R.; Venkatesan, P.; Ilangovan, A. 1-Alkyl-(N,N-dimethylamino)pyridinium bromides: Inhibitory effect on virulence factors of Candida Albicans and on the growth of bacterial pathogens. J. Med. Microbiol. 2013, 62, 241–248. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Söderling, E.; Österblad, M.; Vallittu, P.K.; Lassila, L.V.J. Synthesis of methacrylate monomers with antibacterial effects against S. mutans. Molecules 2011, 16, 9755–9763. [Google Scholar] [CrossRef] [PubMed]
- Sezonov, G.; Joseleau-Petit, D.; D’Ari, R. Escherichia Coli physiology in Luria-Bertani broth. J. Bacteriol. 2007, 189, 8746–8749. [Google Scholar] [CrossRef] [PubMed]







| Film | AATEAB (wt%) | HEMA (wt%) | Water (wt%) |
|---|---|---|---|
| AUTEAB 15% | AUTEAB (15) | (85) | (0) |
| AUTEAB 35% | AUTEAB (35) | (65) | (0) |
| AUTEAB 50% | AUTEAB (50) | (50) | (0) |
| AUTEAB 100% | AUTEAB (75) | (0) | (25) 1 |
| ADTEAB 15% | ADTEAB (15) | (85) | (0) |
| ADTEAB 35% | ADTEAB (35) | (65) | (0) |
| ADTEAB 50% | ADTEAB (50) | (50) | (0) |
| pHEMA | (0) | (100) | (0) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galiano, F.; Mancuso, R.; Guzzo, M.G.; Lucente, F.; Gukelberger, E.; Losso, M.A.; Figoli, A.; Hoinkis, J.; Gabriele, B. New Polymeric Films with Antibacterial Activity Obtained by UV-induced Copolymerization of Acryloyloxyalkyltriethylammonium Salts with 2-Hydroxyethyl Methacrylate. Int. J. Mol. Sci. 2019, 20, 2696. https://doi.org/10.3390/ijms20112696
Galiano F, Mancuso R, Guzzo MG, Lucente F, Gukelberger E, Losso MA, Figoli A, Hoinkis J, Gabriele B. New Polymeric Films with Antibacterial Activity Obtained by UV-induced Copolymerization of Acryloyloxyalkyltriethylammonium Salts with 2-Hydroxyethyl Methacrylate. International Journal of Molecular Sciences. 2019; 20(11):2696. https://doi.org/10.3390/ijms20112696
Chicago/Turabian StyleGaliano, Francesco, Raffaella Mancuso, Maria Grazia Guzzo, Fabrizio Lucente, Ephraim Gukelberger, Maria Adele Losso, Alberto Figoli, Jan Hoinkis, and Bartolo Gabriele. 2019. "New Polymeric Films with Antibacterial Activity Obtained by UV-induced Copolymerization of Acryloyloxyalkyltriethylammonium Salts with 2-Hydroxyethyl Methacrylate" International Journal of Molecular Sciences 20, no. 11: 2696. https://doi.org/10.3390/ijms20112696
APA StyleGaliano, F., Mancuso, R., Guzzo, M. G., Lucente, F., Gukelberger, E., Losso, M. A., Figoli, A., Hoinkis, J., & Gabriele, B. (2019). New Polymeric Films with Antibacterial Activity Obtained by UV-induced Copolymerization of Acryloyloxyalkyltriethylammonium Salts with 2-Hydroxyethyl Methacrylate. International Journal of Molecular Sciences, 20(11), 2696. https://doi.org/10.3390/ijms20112696