Esculetin, a Coumarin Derivative, Prevents Thrombosis: Inhibitory Signaling on PLCγ2–PKC–AKT Activation in Human Platelets
Abstract
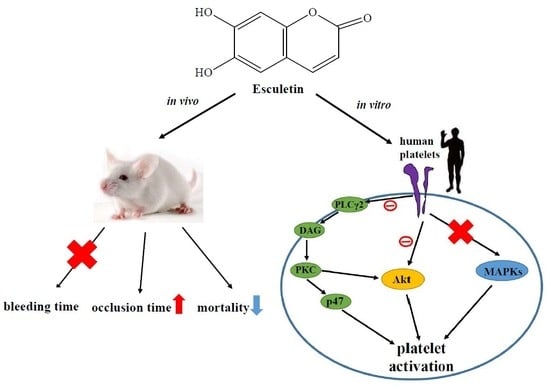
:1. Introduction
2. Results
2.1. Effects of Esculetin on the Aggregation of Washed Human Platelets Stimulated by Various Agonists
2.2. Influence of Esculetin on Surface P-Selectin Expression, ATP Release Reaction, and Cytotoxicity in Washed Human Platelets
2.3. Regulatory Roles of Esculetin in Akt Activation and the PLCγ2–PKC Cascade
2.4. Effects of Esculetin on MAPK Activation
2.5. Esculetin Scavenges OH
2.6. Effects of Esculetin on Antithrombotic Activity Ex Vivo and In Vivo
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Platelet Aggregation
4.3. Lactate Dehydrogenase (LDH) Activity
4.4. Immunoblotting
4.5. Detection of Hydroxyl Radical Formation
4.6. Measurement of Closure Time of Human Whole Blood Using a PFA-100™ Platelet Function Analyzer
4.7. Fluorescein-Induced Platelet Thrombi in Mesenteric Microvessels of Mice
4.8. Bleeding Time in Mice Tail Veins
4.9. ADP-Induced Acute Pulmonary Thromboembolism in Mice
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, T.Y.; White, J.A.; Tricoci, P.; Giugliano, R.P.; Zeymer, U.; Harrington, R.A.; Montalescot, G.; James, S.K.; Van de Werf, F.; Armstrong, P.W.; et al. Upstream clopidogrel use and the efficacy and safety of early eptifibatide treatment in patients with acute coronary syndrome: An analysis from the early glycoprotein IIb/IIIa inhibition in patients with non-ST-segment elevation acute coronary syndrome (EARLY ACS) trial. Circulation 2011, 123, 722–730. [Google Scholar]
- Gasparovic, H.; Petricevic, M.; Kopjar, T.; Djuric, Z.; Svetina, L.; Biocina, B. Impact of dual antiplatelet therapy on outcomes among aspirin-resistant patients following coronary artery bypass grafting. Am. J. Cardiol. 2014, 113, 1660–1667. [Google Scholar] [CrossRef]
- Olivier, C.B.; Diehl, P.; Schnabel, K.; Weik, P.; Zhou, Q.; Bode, C.; Moser, M. Third generation P2Y12 antagonists inhibit platelet aggregation more effectively than clopidogrel in a myocardial infarction registry. Thromb. Haemost. 2014, 111, 266–272. [Google Scholar] [PubMed]
- Ostrowska, M.; Adamski, P.; Koziński, M.; Navarese, E.P.; Fabiszak, T.; Grześk, G.; Paciorek, P.; Kubica, J. Off-target effects of glycoprotein IIb/IIIa receptor inhibitors. Cardiol. J. 2014, 21, 458–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieswandt, B.; Watson, S.P. Platelet-collagen interaction: Is GPVI the central receptor? Blood 2003, 102, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Dütting, S.; Bender, M.; Nieswandt, B. Platelet GPVI: A target for antithrombotic therapy?! Trends Pharmacol. Sci. 2012, 33, 583–590. [Google Scholar] [CrossRef]
- Julián-Almárcegui, C.; Vandevijvere, S.; Gottrand, F.; Beghin, L.; Dallongeville, J.; Sjöstrom, M.; Leclercq, C.; Manios, Y.; Widhalm, K.; Ferreira De Morares, A.C.; et al. Association of heart rate and blood pressure among European adolescents with usual food consumption: The HELENA study. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Ju, W.; Pei, S.; Tang, Y.; Xiao, Y. Pharmacological activities and synthesis of esculetin and its derivatives: A mini-review. Molecules 2017, 22, 387. [Google Scholar] [CrossRef]
- Kadakol, A.; Sharmaa, N.; Kulkarnib, Y.A.; Gaikwada, A.B. Esculetin: A phytochemical endeavor fortifying effect against non-communicable diseases. Biomed. Pharmacother. 2016, 84, 1442–1448. [Google Scholar] [CrossRef]
- Karthika, P.; Rajadurai, M.; Ganapathy, P.; Kanchana, G. Preventive effect of esculetin on lipid peroxides and antioxidants in isoproterenol-induced myocardial infarction in Wistar rats. J. Pharm. Res. 2012, 5, 915–918. [Google Scholar]
- Pan, S.L.; Huang, Y.W.; Guh, J.H.; Chang, Y.L.; Peng, C.Y.; Teng, C.M. Esculetin inhibits Ras-mediated cell proliferation and attenuates vascular restenosis following angioplasty in rats. Biochem. Pharmacol. 2003, 65, 1897–1905. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, U.S.; Kim, W.J.; Moon, S.K. Inhibitory effect of esculetin on migration, invasion and matrix metalloproteinase-9 expression in TNF-α-induced vascular smooth muscle cells. Mol. Med. Reports 2011, 4, 337–341. [Google Scholar]
- Sibbing, D.; von Beckerath, N.; Morath, T.; Stegherr, J.; Mehilli, J.; Sarafoff, N.; Braun, S.; Schulz, S.; Schömig, A.; Kastrati, A. Oral anticoagulation with coumarin derivatives and antiplatelet effects of clopidogrel. Eur. Heart J. 2010, 31, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. Biomed. Res. Int. 2013, 2013, 963248. [Google Scholar] [CrossRef] [PubMed]
- Kodaira, H.; Ishikawa, M.; Komoda, Y.; Nakajima, T. Isolation and identification of anti-platelet aggregation principles from the bark of Fraxinus japonica BLUME. Chem. Pharm. Bull. 1983, 31, 2262–2268. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, K.; Okuda, H.; Arichi, S. Selective inhibition of platelet lipoxygenase by esculetin. Biochim. Biophys. Acta. 1982, 713, 68–72. [Google Scholar]
- Lu, W.J.; Wu, M.P.; Lin, K.H.; Lin, Y.C.; Chou, H.C.; Sheu, J.R. Hinokitiol is a novel glycoprotein VI antagonist on human platelets. Platelets 2014, 25, 595–602. [Google Scholar] [CrossRef]
- Kim, K.; Li, J.; Barazia, A.; Tseng, A.; Youn, S.W.; Abbadessa, G.; Yu, Y.; Schwartz, B.; Andrews, R.K.; Gordeuk, V.R.; et al. ARQ 092, an orally-available, selective AKT inhibitor, attenuates neutrophil-platelet interactions in sickle cell disease. Haematologica 2017, 102, 246–259. [Google Scholar] [CrossRef]
- Gilio, K.; Harper, M.T.; Cosemans, J.M.; Konopatskaya, O.; Munnix, I.C.; Prinzen, L.; Leitges, M.; Liu, Q.; Molkentin, J.D.; Heemskerk, J.W.; et al. Functional divergence of platelet protein kinase C (PKC) isoforms in thrombus formation on collagen. J. Biol. Chem. 2010, 285, 23410–23419. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef]
- Kwon, S.U.; Cha, J.Y.; Lee, H.Y.; Xin, M.; Ji, S.J.; Kim, D.K.; Park, D.S.; Pyo, M.K.; Lee, Y.M. Chloroform fraction of Euphorbia maculata has antiplatelet activity via suppressing thromboxane B2 formation. Mol. Med. Rep. 2015, 11, 4255–4261. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Ha, T.Y.; Ahn, J.; Kim, S. Analysis and distribution of esculetin in plasma and tissues of rats after oral administration. Prev. Nutr. Food Sci. 2014, 19, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Rao, G.N. Emerging role of 12/15-Lipoxygenase (ALOX15) in human pathologies. Prog. Lipid Res. 2019, 73, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, T.; Ding, K.; Liu, Z.; Li, Y.; He, T.; Zhang, W.; Fan, Y.; Ma, W.; Cui, L.; et al. Phospholipase Cγ2 Signaling Cascade Contribute to the Antiplatelet Effect of Notoginsenoside Fc. Front. Pharmacol. 2018, 9, 1293. [Google Scholar] [CrossRef] [PubMed]
- Béziau, D.M.; Toussaint, F.; Blanchette, A.; Dayeh, N.R.; Charbel, C.; Tardif, J.C.; Dupuis, J.; Ledoux, J. Expression of phosphoinositide-specific phospholipase C isoforms in native endothelial cells. PLoS ONE 2015, 10, e0123769. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Adams, T.; Zhi, H.; Yu, M.; Wen, R.; Newman, P.J.; Wang, D.; Newman, D.K. Restoration of responsiveness of phospholipase Cγ2-deficient platelets by enforced expression of phospholipase Cγ1. PLoS ONE 2015, 10, e0119739. [Google Scholar] [CrossRef] [PubMed]
- Séverin, S.; Ghevaert, C.; Mazharian, A. The mitogen-activated protein kinase signaling pathways: Role in megakaryocyte differentiation. J. Thromb. Haemost. 2010, 8, 17–26. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Q.; Liu, Y.Y.; Sun, K.; Fan, J.Y.; Wang, C.S.; Han, J.Y. Inhibitory effect of caffeic acid on ADP-induced thrombus formation and platelet activation involves mitogen-activated protein kinases. Sci. Rep. 2015, 5, 13824. [Google Scholar] [CrossRef] [Green Version]
- Rukoyatkina, N.; Mindukshev, I.; Walter, U.; Gambaryan, S. Dual role of the p38 MAPK/cPLA2 pathway in the regulation of platelet apoptosis induced by ABT-737 and strong platelet agonists. Cell Death Dis. 2013, 4, e931. [Google Scholar] [CrossRef]
- Morihara, N.; Hino, A. Aged garlic extract suppresses platelet aggregation by changing the functional property of platelets. J. Nat. Med. 2017, 71, 249–256. [Google Scholar] [CrossRef]
- Adam, F.; Kauskot, A.; Nurden, P.; Sulpice, E.; Hoylaerts, M.F.; Davis, R.J.; Rosa, J.P.; Bryckaert, M. Platelet JNK1 is involved in secretion and thrombus formation. Blood 2010, 115, 4083–4092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guidetti, G.F.; Canobbio, I.; Torti, M. PI3K/Akt in platelet integrin signaling and implications in thrombosis. Adv. Biol. Regul. 2015, 59, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; De, S.; Damron, D.S.; Chen, W.S.; Hay, N.; Byzova, T.V. Impaired platelet responses to thrombin and collagen in AKT-1-deficient mice. Blood 2004, 104, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.A.; Gartner, T.K.; Hay, N.; Du, X. ADP-stimulated activation of Akt during integrin outside-in signaling promotes platelet spreading by inhibiting glycogen synthase kinase-3β. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2232–2240. [Google Scholar] [CrossRef] [PubMed]
- Jayakumara, T.; Chen, W.F.; Lu, W.J.; Chou, D.S.; Hsiao, G.; Hsu, C.Y.; Sheu, J.R.; Hsieh, C.Y. A novel antithrombotic effect of sulforaphane via activation of platelet adenylate cyclase: Ex vivo and in vivo studies. J. Nutr. Biochem. 2013, 24, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Yang, P.S.; Tsao, J.T.; Jayakumar, T.; Wang, M.J.; Sheu, J.R.; Chou, D.S. Mechanism of free radical generation in platelets and primary hepatocytes: A novel electron spin resonance study. Mol. Med. Rep. 2018, 17, 2061–2069. [Google Scholar] [CrossRef]
- Sheu, J.R.; Lee, C.R.; Lin, C.H.; Hsiao, G.; Ko, W.C.; Chen, Y.C.; Yen, M.H. Mechanisms involved in the antiplatelet activity of Staphylococcus aureus lipoteichoic acid in human platelets. Thromb. Haemost. 2000, 83, 777–784. [Google Scholar] [CrossRef]
- Chou, D.S.; Hsiao, G.; Shen, M.Y.; Tsai, Y.J.; Chen, T.F.; Sheu, J.R. ESR spin trapping of a carbon-centered free radical from agonist-stimulated human platelets. Free Radic. Biol. Med. 2005, 39, 237–248. [Google Scholar] [CrossRef]
- Jilma, B. Platelet function analyzer (PFA-100): A tool to quantify congenital or acquired platelet dysfunction. J. Lab. Clin. Med. 2001, 138, 152–163. [Google Scholar] [CrossRef]
- Hsiao, G.; Lin, K.H.; Chang, Y.; Chen, T.L.; Tzu, N.H.; Chou, D.S.; Sheu, J.R. Protective mechanisms of inosine in platelet activation and cerebral ischemic damage. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1998–2004. [Google Scholar] [CrossRef]
- Sheu, J.R.; Hung, W.C.; Wu, C.H.; Lee, Y.M.; Yen, M.H. Antithrombotic effect of rutaecarpine, an alkaloid isolated from Evodia rutaecarpa, on platelet plug formation in in vivo experiments. Br. J. Haematol. 2000, 110, 110–115. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsia, C.-W.; Lin, K.-C.; Lee, T.-Y.; Hsia, C.-H.; Chou, D.-S.; Jayakumar, T.; Velusamy, M.; Chang, C.-C.; Sheu, J.-R. Esculetin, a Coumarin Derivative, Prevents Thrombosis: Inhibitory Signaling on PLCγ2–PKC–AKT Activation in Human Platelets. Int. J. Mol. Sci. 2019, 20, 2731. https://doi.org/10.3390/ijms20112731
Hsia C-W, Lin K-C, Lee T-Y, Hsia C-H, Chou D-S, Jayakumar T, Velusamy M, Chang C-C, Sheu J-R. Esculetin, a Coumarin Derivative, Prevents Thrombosis: Inhibitory Signaling on PLCγ2–PKC–AKT Activation in Human Platelets. International Journal of Molecular Sciences. 2019; 20(11):2731. https://doi.org/10.3390/ijms20112731
Chicago/Turabian StyleHsia, Chih-Wei, Kao-Chang Lin, Tzu-Yin Lee, Chih-Hsuan Hsia, Duen-Suey Chou, Thanasekaran Jayakumar, Marappan Velusamy, Chao-Chien Chang, and Joen-Rong Sheu. 2019. "Esculetin, a Coumarin Derivative, Prevents Thrombosis: Inhibitory Signaling on PLCγ2–PKC–AKT Activation in Human Platelets" International Journal of Molecular Sciences 20, no. 11: 2731. https://doi.org/10.3390/ijms20112731
APA StyleHsia, C. -W., Lin, K. -C., Lee, T. -Y., Hsia, C. -H., Chou, D. -S., Jayakumar, T., Velusamy, M., Chang, C. -C., & Sheu, J. -R. (2019). Esculetin, a Coumarin Derivative, Prevents Thrombosis: Inhibitory Signaling on PLCγ2–PKC–AKT Activation in Human Platelets. International Journal of Molecular Sciences, 20(11), 2731. https://doi.org/10.3390/ijms20112731