Protective Effect of Acteoside on Ovariectomy-Induced Bone Loss in Mice
Abstract
:1. Introduction
2. Results
2.1. Body, Uterine, and Vaginal Weights
2.2. Acteoside Improved the Bone Biomechanical Properties
2.3. Acteoside Prevented Bone Loss in Ovariectomized Mice
2.4. Acteoside Promoted Bone Formation and Inhibited Resorption Markers
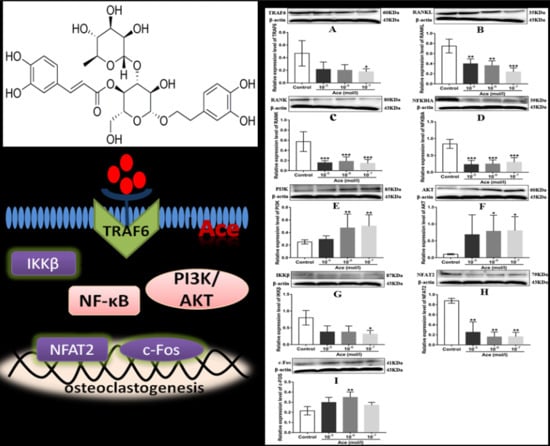
2.5. Acteoside Influenced the Protein Expression Levels of TRAF6, RANKL, RANK, NF-κB, PI3K, AKT, IKKβ, NFAT2, and C-Fos
3. Discussion
4. Materials and Methods
4.1. Preparation of Acteoside
4.2. Materials and Reagents
4.3. Laboratory Animals
4.4. Biomechanical Testing
4.5. Bone Structure Analysis
4.6. Biochemical Parameter Analysis
4.7. Protein Extraction and Western Blot Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| Ace | acteoside |
| AKT | protein kinase B |
| ALP | alkaline phosphatase |
| BCA | bicinchoninic acid |
| BGP | bone gla-protein |
| BMC | bone mineral content |
| BMD | bone mineral density |
| BVF | bone volume fraction |
| CK | cathepsin K |
| DMEM | Dulbecco’s modified eagle media |
| DMSO | dimethyl sulfoxide |
| DPD | deoxypyridinoline |
| EV | estradiol valerate |
| IKKβ | IκB kinase β |
| LC | liquid chromatography |
| MCSF | macrophage colony-stimulating factor |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NFAT2 | nuclear factor of activated T-cells c2 |
| NF-κB | nuclear factor kappa B |
| NMR | nuclear magnetic resonance |
| OVX | ovariectomized |
| PI3K | phosphoinositide 3-kinase |
| RANK | receptor activator of nuclear factor kappa B |
| RANKL | receptor activator of nuclear factor kappa B ligand |
| SD | Standard deviation |
| Tb. N | trabecular number |
| Tb. Sp | trabecular separation |
| Tb.Th | trabecular thickness |
| TCM | traditional Chinese medicine |
| TMC | tissue mineral content |
| TMD | tissue mineral density |
| TNF | tumor-necrosis factor |
| TRAF6 | tumor-necrosis factor receptor-associated factor 6 |
| TRAP | tartrate-resistant acid phosphatase |
| UHPLC | ultra high performance liquid chromatography |
References
- Hennemann, A. Osteoporosis Prevention, Diagnosis, and Therapy. Med. Monatsschr. Pharm. 2002, 25, 164. [Google Scholar] [PubMed]
- Van den Bergh, J.P.; van Geel, T.A.; Geusens, P.P. Osteoporosis, frailty and fracture: Implications for case finding and therapy. Nat. Rev. Rheumatol. 2012, 8, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Banu, J.; Varela, E.; Fernandes, G. Alternative therapies for the prevention and treatment of osteoporosis. Nutr. Rev. 2012, 1, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lee, S.M.Y.L.; Wong, Y.M.; Ching, P.L.; Pang, C.S.; Qin, L.; Leung, P.C.; Fung, K.P. Dosing Effects of an Antiosteoporosis Herbal Formula—A Preclinical Investigation Using a Rat Model. Phytother. Res. 2007, 22, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Nakashima, T.; Hiroshi, N.; Penninger, J.M. RANKL–RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 2006, 12, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, Q.; Zhou, L.; Yuan, J.B.; Lin, X.; Zeng, R.; Liang, X.; Zhao, J.; Xu, J. Andrographolide Inhibits Ovariectomy-Induced Bone Loss via the Suppression of RANKL Signaling Pathways. Int. J. Mol. Sci. 2015, 16, 27470–27481. [Google Scholar] [CrossRef] [Green Version]
- Zou, W.; Teitelbaum, S.L. Integrins, growth factors, and the osteoclast cytoskeleton. Ann. N. Y. Acad. Sci. 2010, 1192, 27–31. [Google Scholar] [CrossRef]
- Tan, E.M.; Li, L.; Indran, I.R.; Chew, N.; Yong, E.-L. TRAF6 Mediates Suppression of Osteoclastogenesis and Prevention of Ovariectomy-Induced Bone Loss by a Novel Prenylflavonoid. J. Bone Miner. Res. 2016, 32, 846. [Google Scholar] [CrossRef]
- Yuan, F.; Xu, R.; Jiang, D.; He, X.; Su, Q.; Jin, C.; Li, X. Leonurine hydrochloride inhibits osteoclastogenesis and prevents osteoporosis associated with estrogen deficiency by inhibiting the NF-κB and PI3K/Akt signaling pathways. Bone 2015, 75, 128–137. [Google Scholar] [CrossRef]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and Activation of the Transcription Factor NFATc1 (NFAT2) Integrate RANKL Signaling in Terminal Differentiation of Osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef] [Green Version]
- Chiou, W.F.; Lin, L.C.; Chen, C.F. Acteoside protects endothelial cells against free radical-induced oxidative stress. J. Pharm. Pharmacol. 2004, 56, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Pastore, S.; Potapovich, A.; Kostyuk, V.; Mariani, V.; Lulli, D.; de LucaL, C.; Korkina, L. Plant Polyphenols Effectively Protect HaCaT Cells from Ultraviolet C-Triggered Necrosis and Suppress Inflammatory Chemokine Expression. Ann. N. Y. Acad. Sci. 2009, 1171, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.Q.; Zhang, J.R.; Pu, X.P.; Ma, J.; Li, C.L. Protective effect of verbascoside on 1-methyl−4-phenylpyridinium ion-induced neurotoxicity in PC12 cells. Eur. J. Pharmacol. 2002, 451, 119–124. [Google Scholar] [CrossRef]
- Kodani, A.; Kikuchi, T.; Tohda, C. Acteoside improves muscle atrophy and motor function by inducing new myokine secretion in chronic spinal cord injury. J. Neurotraum. 2018, 36, 12. [Google Scholar] [CrossRef] [PubMed]
- Editorial Committee of Chinese Pharmacopoeia. Pharmacopoeia of the People’s Republic of China, 10th ed.; China Medical Science and Technology Press: Beijing, China, 2015; pp. 178–179. [Google Scholar]
- Wang, T.; Zhang, X.; Xie, W. Cistanche deserticola Y. C. Ma, “Desert Ginseng”: A Review. Am. J. Chin. Med. 2012, 40, 1123–1141. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.C.; Fan, Y.G.; Liu, J.R.; Zeng, Y.R.; Yi, C.Z.; Yan, L. Experimental study of directional differentiation of bone mesenchymal stem cells (BMSCs) to osteoblasts guided by serum containing cistanche deserticola. Chin. J. Orthop. Traumatol. 2010, 23, 606–608. [Google Scholar]
- Song, D.; Cao, Z.; Liu, Z.; Tickner, J.; Qiu, H.; Wang, C.; Chen, K.; Wang, Z.; Dong, S.; Xu, J. Cistanche deserticola polysaccharide attenuates osteoclastogenesis and bone resorption via inhibiting RANKL signaling and reactive oxygen species production. J. Cell. Physiol. 2018, 233, 9674–9684. [Google Scholar] [CrossRef] [PubMed]
- Li, T.M.; Huang, H.C.; Su, C.M.; Ho, T.Y.; Wu, C.M.; Chen, W.C.; Fong, Y.C.; Tang, C.H. Cistanche deserticola extract increases bone formation in osteoblasts. J. Pharm. Pharmacol. 2012, 64, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, Z.; Wang, W.; Yao, H.; Ma, X. Therapeutic Effect of Cistanoside A on Bone Metabolism of Ovariectomized Mice. Molecules 2017, 22, 197. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, K.S.; Yi, S.H.; Kook, S.H.; Lee, J.C. Acteoside suppresses RANKL-mediated osteoclastogenesis by inhibiting c-Fos induction and NF-kappaB pathway and attenuating ROS production. PLoS ONE 2013, 8, e80873. [Google Scholar] [CrossRef]
- He, J.; Chen, M.; Lin, D. New insights into the tonifying kidney-yin herbs and formulas for the treatment of osteoporosis. Arch. Osteoporos 2017, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Alipieva, K.; Korkina, L.; OrhanM, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef]
- Schilling, T.; Ebert, R.; Raaijmakers, N.; Schütze, N.; Jakob, F. Effects of phytoestrogens and other plant-derived compounds on mesenchymal stem cells, bone maintenance and regeneration. J. Steroid Biochem. Mol. Biol. 2014, 139, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Kim, K.J.; Kim, S.J.; Mun, S.K.; Hong, S.G.; Son, Y.J.; Yee, S.T. Suppression Effect of Astaxanthin on Osteoclast Formation In Vitro and Bone Loss In Vivo. Int. J. Mol. Sci. 2018, 19, 912. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.E.; Kumer, J.L.; Majumdar, S.; Khan, M.; Lotz, J.; Stevens, R.E.; Klein, R.; Phelps, K.V. The effects of synthetic conjugated estrogens, a (cenestin) on trabecular bone structure and strength in the ovariectomized rat model. Osteoporosis Int. 2002, 13, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Teong, B.; Kuo, S.M.; Tsai, W.H.; Ho, M.L.; Chen, C.H.; Huang, H.H. Liposomal encapsulation for systemic delivery of propranolol via transdermal iontophoresis improves bone microarchitecture in ovariectomized rats. Int. J. Mol. Sci. 2017, 18, 822. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Z.G.; Li, C.; Hu, S.J.; Liu, L.; Wang, J.P.; Mei, Q.B. Du-Zhong (Eucommia ulmoides Oliv.) cortex extract prevent OVX-induced osteoporosis in rats. Bone 2009, 45, 553–559. [Google Scholar] [CrossRef]
- Fraher, L.J. Biochemical Markers of Bone Turnover. Clin. Biochem. 1993, 26, 431–432. [Google Scholar] [CrossRef]
- Lomaga, M.A.; Yeh, W.C.; Sarosi, I.; Duncan, G.S.; Furlonger, C.; Ho, A.; Morony, S.; Capparelli, C.; Van, G.; Kaufman, S.; et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999, 13, 1015–1024. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Wang, T.; Yan, X.; Liu, Z.; Yan, Y.; Yang, K.; Qi, J.; Zhou, H.; Qian, N.; Zhou, Q.; et al. A novel rhein derivative modulates bone formation and resorption and ameliorates estrogen-dependent bone loss. J. Bone Miner. Res. 2018, 34, 361–374. [Google Scholar] [CrossRef]
- Nakashima, T.; Takayanagi, H. New regulation mechanisms of osteoclast differentiation. Ann. N. Y. Acad. Sci. 2011, 1240, E13–E18. [Google Scholar] [CrossRef] [PubMed]
- Theill, L.E.; Boyle, W.J.; Penninger, J.M. RANK-L AND RANK: T Cells, Bone Loss, and Mammalian Evolution. Annu. Rev. Immunol. 2002, 20, 795–823. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lee, J.; Jang, J.H.; Lee, S.; Nan, M.H.; Oh, B.; Lee, S.G.; Kim, H.H.; Soung, N.K.; Ahn, J.S.; et al. Ginsenoside Rh2 inhibits osteoclastogenesis through down-regulation of NF-κB, NFATc1 and c-Fos. Bone 2012, 50, 1207–1213. [Google Scholar] [CrossRef] [PubMed]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Zhang, B.; Liu, J.; Dong, Y.; Li, Y.; Li, N.; Zhao, X.; Snooks, H.; Hu, C.; Ma, X. Protective Effect of Acteoside on Ovariectomy-Induced Bone Loss in Mice. Int. J. Mol. Sci. 2019, 20, 2974. https://doi.org/10.3390/ijms20122974
Yang L, Zhang B, Liu J, Dong Y, Li Y, Li N, Zhao X, Snooks H, Hu C, Ma X. Protective Effect of Acteoside on Ovariectomy-Induced Bone Loss in Mice. International Journal of Molecular Sciences. 2019; 20(12):2974. https://doi.org/10.3390/ijms20122974
Chicago/Turabian StyleYang, Lingling, Bo Zhang, Jingjing Liu, Yanhong Dong, Yanting Li, Nan Li, Xiaojun Zhao, Hunter Snooks, Changling Hu, and Xueqin Ma. 2019. "Protective Effect of Acteoside on Ovariectomy-Induced Bone Loss in Mice" International Journal of Molecular Sciences 20, no. 12: 2974. https://doi.org/10.3390/ijms20122974
APA StyleYang, L., Zhang, B., Liu, J., Dong, Y., Li, Y., Li, N., Zhao, X., Snooks, H., Hu, C., & Ma, X. (2019). Protective Effect of Acteoside on Ovariectomy-Induced Bone Loss in Mice. International Journal of Molecular Sciences, 20(12), 2974. https://doi.org/10.3390/ijms20122974