Slowly Reducible Genetically Encoded Green Fluorescent Indicator for In Vivo and Ex Vivo Visualization of Hydrogen Peroxide
Abstract
:1. Introduction
2. Results
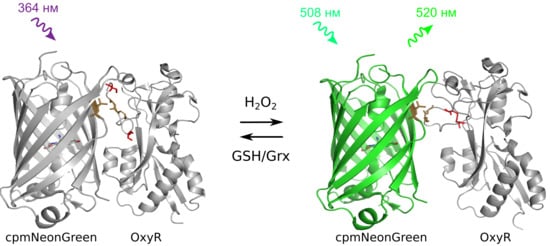
2.1. Developing a Slowly-Reducible Green Indicator for hydrogen peroxide in a Bacterial System
2.2. In vitro Characterization of NeonOxIrr
2.3. Response of NeonOxIrr to external H2O2 in Different Organelles of Mammalian Cells
2.4. Response of NeonOxIrr to Endogenously Produced ROS in the Cytoplasm, on Plasma Membrane and in Mitochondria of Mammalian Cells
2.5. Visualization of Optogenetically Generated ROS in Mammalian Cells
2.6. Engineering of the Ratiometric Version of the NeonOxIrr Peroxide Indicator
2.7. Optimization of Chemical Fixation Protocol for Bacteria Expressing the Ratiometric NeonOxIrr-P2A-mCherry Indicator
2.8. Response of the Ratiometric Versions of the NeonOxIrr Indicator to External H2O2 in Mammalian Cells
2.9. Ex vivo H2O2 Detection in HeLa Cells Expressing Ratiometric NeonOxIrr-P2A-mCherry
2.10. In vivo Response of Ratiometric NeonOxIrr-P2A-mCherry Indicator to External H2O2 in Living Dissociated Neuronal Cultures
2.11. Development of the Alkylation-Fixation Protocol with NEM and Various Detergents for H2O2 Detection in Neuronal Cultures Expressing Ratiometric NeonOxIrr-P2A-mCherry
2.12. Ex vivo H2O2 Detection in the Mouse brain Expressing Ratiometric NeonOxIrr-P2A-mCherry using a Modified Fixation Protocol
2.13. Ex vivo Response of NeonOxIrr-P2A-mCherry to Endogenously Produced ROS in the Brain after γ-Irradiation.
3. Discussion
4. Materials and Methods
4.1. Fixation of the NeonOxIrr-P2A-mCherry Indicator in Bacteria Pretreated with Alkylating Reagents
4.2. Expression in Mammalian Cells
4.3. Alkylation-Fixation of Mammalian Cells and Neuronal Cultures
4.4. Construction of Vectors for the Preparation of Adeno-Associated Virus Particles
4.5. Preparation of rAAV Particles
4.6. Isolation, Transduction, and Imaging of Neuronal Cultures
4.7. Introduction of rAAV Particles in the Mouse central Nervous System (CNS)
4.8. Oxidation of the Indicator with H2O2 Followed by its Fixation ex vivo
4.9. Irradiation with γ-radiation
4.10. Preparation of Brain Tissue Samples and Visualization
4.11. Statistical Processing of Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethics approval and Consent to Participate
References
- Lee, C.R.; Patel, J.C.; O’Neill, B.; Rice, M.E. Inhibitory and excitatory neuromodulation by hydrogen peroxide: Translating energetics to information. J. Physiol. 2015, 593, 3431–3446. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.E. H2O2: A dynamic neuromodulator. Neuroscientist 2011, 17, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.C.; Rice, M.E. Classification of H2O2 as a neuromodulator that regulates striatal dopamine release on a subsecond time scale. ACS Chem. Neurosci. 2012, 3, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Beckhauser, T.F.; Francis-Oliveira, J.; De Pasquale, R. Reactive Oxygen Species: Physiological and Physiopathological Effects on Synaptic Plasticity. J. Exp. Neurosci. 2016, 10, 23–48. [Google Scholar] [CrossRef] [PubMed]
- Belousov, V.V.; Fradkov, A.F.; Lukyanov, K.A.; Staroverov, D.B.; Shakhbazov, K.S.; Terskikh, A.V.; Lukyanov, S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat. Methods 2006, 3, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, Y.G.; Bilan, D.S.; Matlashov, M.E.; Mishina, N.M.; Markvicheva, K.N.; Subach, O.M.; Subach, F.V.; Bogeski, I.; Hoth, M.; Enikolopov, G.; et al. Red fluorescent genetically encoded indicator for intracellular hydrogen peroxide. Nat. Commun. 2014, 5, 5222. [Google Scholar] [CrossRef]
- Albrecht, S.C.; Barata, A.G.; Grosshans, J.; Teleman, A.A.; Dick, T.P. In vivo mapping of hydrogen peroxide and oxidized glutathione reveals chemical and regional specificity of redox homeostasis. Cell Metab. 2011, 14, 819–829. [Google Scholar] [CrossRef]
- Barata, A.G.; Dick, T.P. In vivo imaging of H2O2 production in Drosophila. Methods Enzymol. 2013, 526, 61–82. [Google Scholar]
- Gutscher, M.; Pauleau, A.L.; Marty, L.; Brach, T.; Wabnitz, G.H.; Samstag, Y.; Meyer, A.J.; Dick, T.P. Real-time imaging of the intracellular glutathione redox potential. Nat. Methods 2008, 5, 553–559. [Google Scholar] [CrossRef]
- Morgan, B.; Van Laer, K.; Owusu, T.N.; Ezerina, D.; Pastor-Flores, D.; Amponsah, P.S.; Tursch, A.; Dick, T.P. Real-time monitoring of basal H2O2 levels with peroxiredoxin-based probes. Nat. Chem. Biol. 2016, 12, 437–443. [Google Scholar] [CrossRef]
- Subach, F.V.; Piatkevich, K.D.; Verkhusha, V.V. Directed molecular evolution to design advanced red fluorescent proteins. Nat. Methods 2011, 8, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Shaner, N.C.; Lambert, G.G.; Chammas, A.; Ni, Y.; Cranfill, P.J.; Baird, M.A.; Sell, B.R.; Allen, J.R.; Day, R.N.; Israelsson, M.; et al. A bright monomeric green fluorescent protein derived from Branchiostoma lanceolatum. Nat. Methods 2013, 10, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Aslund, F.; Zheng, M.; Beckwith, J.; Storz, G. Regulation of the OxyR transcription factor by hydrogen peroxide and the cellular thiol-disulfide status. Proc. Natl. Acad. Sci. USA 1999, 96, 6161–6165. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.L.; Liebler, D.C. Mitochondrial protein targets of thiol-reactive electrophiles. Chem. Res. Toxicol. 2008, 21, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.K.; Leinweber, B.L.; Smith, C.V. Detection of reversible protein thiol modifications in tissues. Anal. Biochem. 2006, 358, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.; Clavreul, N.; Sethuraman, M.; Adachi, T.; Cohen, R.A. Thiol oxidation in signaling and response to stress: Detection and quantification of physiological and pathophysiological thiol modifications. Free Radic Biol. Med. 2007, 43, 1099–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, N.Y.; Liu, Q.; Stamer, S.L.; Liebler, D.C. Protein targets of reactive electrophiles in human liver microsomes. Chem. Res. Toxicol. 2007, 20, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.N.; Hunt, H.D.; Horton, R.M.; Pullen, J.K.; Pease, L.R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 1989, 77, 51–59. [Google Scholar] [CrossRef]
- Grabe, M.; Oster, G. Regulation of organelle acidity. J. Gen. Physiol. 2001, 117, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Santo-Domingo, J.; Demaurex, N. Perspectives on: SGP symposium on mitochondrial physiology and medicine: The renaissance of mitochondrial pH. J. Gen. Physiol. 2012, 139, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Roma, L.P.; Duprez, J.; Takahashi, H.K.; Gilon, P.; Wiederkehr, A.; Jonas, J.C. Dynamic measurements of mitochondrial hydrogen peroxide concentration and glutathione redox state in rat pancreatic beta-cells using ratiometric fluorescent proteins: Confounding effects of pH with HyPer but not roGFP1. Biochem. J. 2012, 441, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Bagiyan, G.A.; Koroleva, I.K.; Soroka, N.V.; Ufimtsev, A.V. Oxidation of thiol compounds by molecular oxygen in aqueous solutions. Russ. Chem. Bull. Int. Ed. 2003, 52, 1135–1141. [Google Scholar] [CrossRef]
- Huang, B.K.; Sikes, H.D. Quantifying intracellular hydrogen peroxide perturbations in terms of concentration. Redox Biol. 2014, 2, 955–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antunes, F.; Cadenas, E. Estimation of H2O2 gradients across biomembranes. FEBS Lett. 2000, 475, 121–126. [Google Scholar] [CrossRef]
- Treiman, M.; Caspersen, C.; Christensen, S.B. A tool coming of age: Thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca(2+)-ATPases. Trends Pharmacol. Sci. 1998, 19, 131–135. [Google Scholar] [CrossRef]
- Carter, R.E.; Sorkin, A. Endocytosis of functional epidermal growth factor receptor-green fluorescent protein chimera. J. Biol. Chem. 1998, 273, 35000–35007. [Google Scholar] [CrossRef]
- Nicolier, M.; Decrion-Barthod, A.Z.; Launay, S.; Pretet, J.L.; Mougin, C. Spatiotemporal activation of caspase-dependent and -independent pathways in staurosporine-induced apoptosis of p53wt and p53mt human cervical carcinoma cells. Biol. Cell. 2009, 101, 455–467. [Google Scholar] [CrossRef]
- Fisher, A.B. Redox signaling across cell membranes. Antioxid Redox Signal. 2009, 11, 1349–1356. [Google Scholar] [CrossRef]
- Groeger, G.; Quiney, C.; Cotter, T.G. Hydrogen peroxide as a cell-survival signaling molecule. Antioxid. Redox Signal. 2009, 11, 2655–2671. [Google Scholar] [CrossRef]
- Bulina, M.E.; Chudakov, D.M.; Britanova, O.V.; Yanushevich, Y.G.; Staroverov, D.B.; Chepurnykh, T.V.; Merzlyak, E.M.; Shkrob, M.A.; Lukyanov, S.; Lukyanov, K.A. A genetically encoded photosensitizer. Nat. Biotechnol. 2006, 24, 95–99. [Google Scholar] [CrossRef]
- De Felipe, P.; Luke, G.A.; Hughes, L.E.; Gani, D.; Halpin, C.; Ryan, M.D. E unum pluribus: Multiple proteins from a self-processing polyprotein. Trends Biotechnol. 2006, 24, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Soderberg, M.; Edlund, C.; Kristensson, K.; Dallner, G. Lipid compositions of different regions of the human brain during aging. J. Neurochem. 1990, 54, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Jamur, M.C.; Oliver, C. Permeabilization of cell membranes. Methods Mol. Biol. 2010, 588, 63–66. [Google Scholar] [PubMed]
- Azzam, E.I.; Jay-Gerin, J.P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.M.; Yang, H.S.; Kang, S.W.; Ho, J.N.; Lee, S.B.; Um, H.D. Amplification of the gamma-irradiation-induced cell death pathway by reactive oxygen species in human U937 cells. Cell Signal. 2008, 20, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Kobashigawa, S.; Suzuki, K.; Yamashita, S. Ionizing radiation accelerates Drp1-dependent mitochondrial fission, which involves delayed mitochondrial reactive oxygen species production in normal human fibroblast-like cells. Biochem. Biophys. Res. Commun. 2011, 414, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Patrushev, M.; Kasymov, V.; Patrusheva, V.; Ushakova, T.; Gogvadze, V.; Gaziev, A.I. Release of mitochondrial DNA fragments from brain mitochondria of irradiated mice. Mitochondrion 2006, 6, 43–47. [Google Scholar] [CrossRef]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef]
- Barykina, N.V.; Subach, O.M.; Doronin, D.A.; Sotskov, V.P.; Roshchina, M.A.; Kunitsyna, T.A.; Malyshev, A.Y.; Smirnov, I.V.; Azieva, A.M.; Sokolov, I.S.; et al. A new design for a green calcium indicator with a smaller size and a reduced number of calcium-binding sites. Sci. Rep. 2016, 6, 34447. [Google Scholar] [CrossRef]
- Hwang, C.; Sinskey, A.J.; Lodish, H.F. Oxidized redox state of glutathione in the endoplasmic reticulum. Science 1992, 257, 1496–1502. [Google Scholar] [CrossRef]
- Shen, Y.; Dana, H.; Abdelfattah, A.S.; Patel, R.; Shea, J.; Molina, R.S.; Rawal, B.; Rancic, V.; Chang, Y.F.; Wu, L.; et al. A genetically encoded Ca2+ indicator based on circularly permutated sea anemone red fluorescent protein eqFP578. BMC Biol. 2018, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Cuervo, A.M. The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.K.; Burns, L.D.; Cocker, E.D.; Nimmerjahn, A.; Ziv, Y.; Gamal, A.E.; Schnitzer, M.J. Miniaturized integration of a fluorescence microscope. Nat. Methods 2011, 8, 871–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziv, Y.; Burns, L.D.; Cocker, E.D.; Hamel, E.O.; Ghosh, K.K.; Kitch, L.J.; El Gamal, A.; Schnitzer, M.J. Long-term dynamics of CA1 hippocampal place codes. Nat. Neurosci. 2013, 16, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, Y.; Roma, L.P.; Sobotta, M.C.; Rose, A.J.; Diaz, M.B.; Locatelli, G.; Breckwoldt, M.O.; Misgeld, T.; Kerschensteiner, M.; Herzig, S.; et al. Mouse redox histology using genetically encoded probes. Sci. Signal. 2016, 9, rs1. [Google Scholar] [CrossRef] [PubMed]
- Patterson, G.H.; Knobel, S.M.; Sharif, W.D.; Kain, S.R.; Piston, D.W. Use of the green fluorescent protein and its mutants in quantitative fluorescence microscopy. Biophys. J. 1997, 73, 2782–2790. [Google Scholar] [CrossRef] [Green Version]







| Protein | NeonOxIrr | HyPer3 | mEGFP | ||
|---|---|---|---|---|---|
| Red | Ox | Red | Ox | ||
| Absorbance maximum (nm) | 508 | 498 | 490 | ||
| Extinction coefficient (M−1cm−1)1 | ND | 110,000 | ND | 65,000 | 64,000 |
| Emission maximum (nm) | 520 | 518 | 512 | ||
| Quantum yield2 | 0.57 | 0.17 | 0.60 | ||
| Relative brightness (%) | ND | 170 | ND | 29 | 100 |
| pKa3 | 5.9 | 5.9 | ≥7.9 | ≥7.6 | 5.9 |
| pH-range (pH changes impact to contrast)4 | pH 5.5–8.5 (6–52%) | pH 5.5–8.5 (62–596%) | NA | ||
| Maturation t0.5 at 37 °C (min)5 | 4.2 | 25 | 14 | ||
| Photobleaching t0.5 (s)6 | 168 ± 30 | 354 ± 3 | 174 ± 8 | ||
| Fluorescence contrast on bacterial suspension (fold)7 | 2.8 ± 0.6 | 2.5 ± 0.5 | NA | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subach, O.M.; Kunitsyna, T.A.; Mineyeva, O.A.; Lazutkin, A.A.; Bezryadnov, D.V.; Barykina, N.V.; Piatkevich, K.D.; Ermakova, Y.G.; Bilan, D.S.; Belousov, V.V.; et al. Slowly Reducible Genetically Encoded Green Fluorescent Indicator for In Vivo and Ex Vivo Visualization of Hydrogen Peroxide. Int. J. Mol. Sci. 2019, 20, 3138. https://doi.org/10.3390/ijms20133138
Subach OM, Kunitsyna TA, Mineyeva OA, Lazutkin AA, Bezryadnov DV, Barykina NV, Piatkevich KD, Ermakova YG, Bilan DS, Belousov VV, et al. Slowly Reducible Genetically Encoded Green Fluorescent Indicator for In Vivo and Ex Vivo Visualization of Hydrogen Peroxide. International Journal of Molecular Sciences. 2019; 20(13):3138. https://doi.org/10.3390/ijms20133138
Chicago/Turabian StyleSubach, Oksana M., Tatiana A. Kunitsyna, Olga A. Mineyeva, Alexander A. Lazutkin, Dmitri V. Bezryadnov, Natalia V. Barykina, Kiryl D. Piatkevich, Yulia G. Ermakova, Dmitry S. Bilan, Vsevolod V. Belousov, and et al. 2019. "Slowly Reducible Genetically Encoded Green Fluorescent Indicator for In Vivo and Ex Vivo Visualization of Hydrogen Peroxide" International Journal of Molecular Sciences 20, no. 13: 3138. https://doi.org/10.3390/ijms20133138
APA StyleSubach, O. M., Kunitsyna, T. A., Mineyeva, O. A., Lazutkin, A. A., Bezryadnov, D. V., Barykina, N. V., Piatkevich, K. D., Ermakova, Y. G., Bilan, D. S., Belousov, V. V., Anokhin, K. V., Enikolopov, G. N., & Subach, F. V. (2019). Slowly Reducible Genetically Encoded Green Fluorescent Indicator for In Vivo and Ex Vivo Visualization of Hydrogen Peroxide. International Journal of Molecular Sciences, 20(13), 3138. https://doi.org/10.3390/ijms20133138