Real Microgravity Influences the Cytoskeleton and Focal Adhesions in Human Breast Cancer Cells
Abstract
:1. Introduction
2. Results
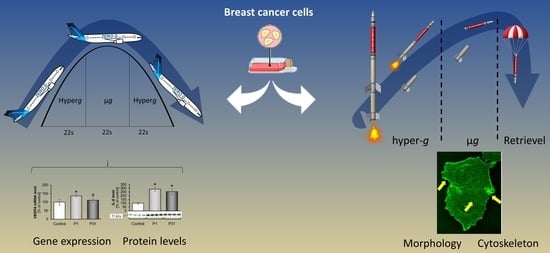
2.1. TEXUS 54 Sounding Rocket Mission: “Live-Cell Imaging of Human Breast Cancer Cells in Short-Term Weightlessness”
2.2. Immunostaining of MCF 7 Cells Exposed to r-µg during the TEXUS 54 Sounding Rocket Mission and Fixed in Orbit
2.3. Results of the 31st DLR Parabolic Flight Campaign: “Effects Of Short-Term Microgravity on Human Breast Cancer Cells”
2.3.1. Studies on Cytoskeletal Genes
2.3.2. Altered Expression of Genes of the Focal Adhesion Complex
2.3.3. Changes of Extracellular Matrix and Cytokine Gene Expression
2.3.4. Pathway Analyses
3. Discussion
3.1. Cytoskeletal Alterations Visualized during the TEXUS 54 Mission
3.2. MCF-7 Breast Cancer Cells Exposed to PF Maneuvers during the 31st DLR PFC
3.3. Microgravity-Induced Cytoskeleton Changes and Potent Physiological Responses
4. Materials and Methods
4.1. Cell Culture
4.2. Construction of An Expression Cassette to Visualize F-actin and α-tubulin
4.3. Generation of MCF 7 Cells Expressing Lifeact-eGFP-IRES-mCherry-Tubulin
4.4. Live Cell Imaging by the FLUMIAS Microscope
4.5. TEXUS 54 Sounding Rocket Mission
4.6. FLUFIX and Immunocytochemistry of MCF-7 Fixed Cells during TEXUS 54
4.7. 31st DLR Parabolic Flight Campaign
4.8. RNA Isolation and qPCR
4.9. Western Blot Analysis
4.10. Immunostaining of Fixed MCF-7 Cells Collected during the 31st Parabolic Flight Campaign
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | two-dimensional |
| 3D | three-dimensional |
| ACTB | Beta-actin |
| CAV1 | Caveolin-1 |
| CAV2 | Caveolin-2 |
| CDH1 | E-cadherin |
| COL1A1 | Collagen type 1 alpha 1 |
| CXCL8 | Interleukin 8 |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DLR | German Aerospace Center |
| ECM | Extracellular matrix |
| ESRANGE | European Space and Sounding Rocket Range |
| EZR | Ezrin |
| FLUMIAS | spinning-disc Fluorescence Microscopy Analysis System |
| FN1 | Fibronectin |
| FTC-133 | Follicular thyroid cancer cell line 133 |
| GFP | Green fluorescent protein |
| GLOBOCAN | Global Cancer Observatory |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| ISS | International Space Station |
| ITGB1 | Integrin beta 1 |
| KRT8 | Cytokeratin 8 |
| LAMA1 | Laminin alpha 1 |
| LAMA3 | Laminin alpha 3 |
| LIMA1 | LIM domain and actin-binding protein 1 |
| MCF-7 | Michigan cancer foundation |
| MCS | Multicellular spheroids |
| MMP9 | Matrix metalloproteinases 9 |
| MSN | Moesin |
| MTSS1 | Metastasis suppressor protein 1 |
| P | Parabola |
| PAI1 | Plasminogen activator inhibitor 1 |
| PF | Parabolic flight |
| PFA | Paraformaldehyde |
| PFC | Parabolic flight campaign |
| pLAGICT | pcDNA3.1 LifeAct-eGFP-IRES-mCherry-Tubulin |
| pSB-LAGICT | Sleeping Beauty LifeAct-eGFP-IRES-mCherry-Tubulin |
| PTK2 | Protein tyrosine kinase 2; Focal adhesion kinase 1 |
| r-µg | real microgravity |
| RDX | Radixin |
| RPM | Random positioning machine |
| s-µg | simulated microgravity |
| SB | Sleeping beauty |
| TIMP1 | Metalloproteinase inhibitor 1 |
| TLN1 | Talin-1 |
| TUBB | Beta tubulin |
| TX (TEXUS) | ‘Technische Experimente unter Schwerelosigkeit’ |
| VCL | Vinculin |
| VEGFA | Vascular endothelial growth factor A |
| WHO | World health organization |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Cancer.net. Breast cancer: Statistics. Available online: https://www.cancer.net/cancer-types/breast-cancer (accessed on 14 May 2019).
- Becker, J.L.; Souza, G.R. Using space-based investigations to inform cancer research on earth. Nat. Rev. Cancer 2013, 13, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D.; Wehland, M.; Pietsch, J.; Aleshcheva, G.; Wise, P.; van Loon, J.; Ulbrich, C.; Magnusson, N.E.; Infanger, M.; Bauer, J. Growing tissues in real and simulated microgravity: New methods for tissue engineering. Tissue Eng. Part B Rev. 2014, 20, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D.; Bauer, J.; Wise, P.; Kruger, M.; Simonsen, U.; Wehland, M.; Infanger, M.; Corydon, T.J. The role of sox family members in solid tumours and metastasis. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Vorselen, D.; Roos, W.H.; MacKintosh, F.C.; Wuite, G.J.; van Loon, J.J. The role of the cytoskeleton in sensing changes in gravity by nonspecialized cells. FASEB J. 2014, 28, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Corydon, T.J.; Mann, V.; Slumstrup, L.; Kopp, S.; Sahana, J.; Askou, A.L.; Magnusson, N.E.; Echegoyen, D.; Bek, T.; Sundaresan, A.; et al. Reduced expression of cytoskeletal and extracellular matrix genes in human adult retinal pigment epithelium cells exposed to simulated microgravity. Cell Physiol Biochem 2016, 40, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hughes-Fulford, M. Function of the cytoskeleton in gravisensing during spaceflight. Adv. Space Res. 2003, 32, 1585–1593. [Google Scholar] [CrossRef]
- Maier, J.A.; Cialdai, F.; Monici, M.; Morbidelli, L. The impact of microgravity and hypergravity on endothelial cells. Biomed. Res. Int. 2015, 2015, 434803. [Google Scholar] [CrossRef]
- Carlsson, S.I.; Bertilaccio, M.T.; Ballabio, E.; Maier, J.A. Endothelial stress by gravitational unloading: Effects on cell growth and cytoskeletal organization. Biochim. Biophys. Acta 2003, 1642, 173–179. [Google Scholar] [CrossRef]
- Corydon, T.J.; Kopp, S.; Wehland, M.; Braun, M.; Schutte, A.; Mayer, T.; Hulsing, T.; Oltmann, H.; Schmitz, B.; Hemmersbach, R.; et al. Alterations of the cytoskeleton in human cells in space proved by life-cell imaging. Sci. Rep. 2016, 6, 20043. [Google Scholar] [CrossRef]
- Kopp, S.; Warnke, E.; Wehland, M.; Aleshcheva, G.; Magnusson, N.E.; Hemmersbach, R.; Corydon, T.J.; Bauer, J.; Infanger, M.; Grimm, D. Mechanisms of three-dimensional growth of thyroid cells during long-term simulated microgravity. Sci. Rep. 2015, 5, 16691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopp, S.; Kruger, M.; Feldmann, S.; Oltmann, H.; Schutte, A.; Schmitz, B.; Bauer, J.; Schulz, H.; Saar, K.; Huebner, N.; et al. Thyroid cancer cells in space during the texus-53 sounding rocket mission—the thyroid project. Sci. Rep. 2018, 8, 10355. [Google Scholar] [CrossRef] [PubMed]
- Kopp, S.; Kruger, M.; Bauer, J.; Wehland, M.; Corydon, T.J.; Sahana, J.; Nassef, M.Z.; Melnik, D.; Bauer, T.J.; Schulz, H.; et al. Microgravity affects thyroid cancer cells during the texus-53 mission stronger than hypergravity. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D.; Egli, M.; Kruger, M.; Riwaldt, S.; Corydon, T.J.; Kopp, S.; Wehland, M.; Wise, P.; Infanger, M.; Mann, V.; et al. Tissue engineering under microgravity conditions-use of stem cells and specialized cells. Stem Cells Dev. 2018, 27, 787–804. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Pietsch, J.; Wehland, M.; Schulz, H.; Saar, K.; Hübner, N.; Bauer, J.; Braun, M.; Schwarzwälder, A.; Segerer, J.; et al. Differential gene expression profile and altered cytokine secretion of thyroid cancer cells in space. FASEB J. 2014, 28, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Vassy, J.; Portet, S.; Beil, M.; Millot, G.; Fauvel-Lafeve, F.; Gasset, G.; Schoevaert, D. Weightlessness acts on human breast cancer cell line mcf-7. Adv Space Res 2003, 32, 1595–1603. [Google Scholar] [CrossRef]
- Vassy, J.; Portet, S.; Beil, M.; Millot, G.; Fauvel-Lafeve, F.; Karniguian, A.; Gasset, G.; Irinopoulou, T.; Calvo, F.; Rigaut, J.P.; et al. The effect of weightlessness on cytoskeleton architecture and proliferation of human breast cancer cell line mcf-7. FASEB J. 2001, 15, 1104–1106. [Google Scholar] [CrossRef] [PubMed]
- Tewinkel, M.; Burfeindt, J.; Rank, P.; Volkmann, D. Automatic fixation facility for plant seedlings in the texus sounding rocket programme. Microgravity Sci. Technol. 1991, 4, 216–220. [Google Scholar] [PubMed]
- Kopp, S.; Slumstrup, L.; Corydon, T.J.; Sahana, J.; Aleshcheva, G.; Islam, T.; Magnusson, N.E.; Wehland, M.; Bauer, J.; Infanger, M.; et al. Identifications of novel mechanisms in breast cancer cells involving duct-like multicellular spheroid formation after exposure to the random positioning machine. Sci. Rep. 2016, 6, 26887. [Google Scholar] [CrossRef]
- Kopp, S.; Sahana, J.; Islam, T.; Petersen, A.G.; Bauer, J.; Corydon, T.J.; Schulz, H.; Saar, K.; Huebner, N.; Slumstrup, L.; et al. The role of nfkappab in spheroid formation of human breast cancer cells cultured on the random positioning machine. Sci. Rep. 2018, 8, 921. [Google Scholar] [CrossRef]
- Aleshcheva, G.; Wehland, M.; Sahana, J.; Bauer, J.; Corydon, T.J.; Hemmersbach, R.; Frett, T.; Egli, M.; Infanger, M.; Grosse, J.; et al. Moderate alterations of the cytoskeleton in human chondrocytes after short-term microgravity produced by parabolic flight maneuvers could be prevented by up-regulation of bmp-2 and sox-9. FASEB J. 2015, 29, 2303–2314. [Google Scholar] [CrossRef] [PubMed]
- Wehland, M.; Ma, X.; Braun, M.; Hauslage, J.; Hemmersbach, R.; Bauer, J.; Grosse, J.; Infanger, M.; Grimm, D. The impact of altered gravity and vibration on endothelial cells during a parabolic flight. Cell Physiol. Biochem. 2013, 31, 432–451. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, J.; Gass, S.; Nebuloni, S.; Echegoyen, D.; Riwaldt, S.; Baake, C.; Bauer, J.; Corydon, T.J.; Egli, M.; Infanger, M.; et al. Three-dimensional growth of human endothelial cells in an automated cell culture experiment container during the spacex crs-8 iss space mission - the spheroids project. Biomaterials 2017, 124, 126–156. [Google Scholar] [CrossRef] [PubMed]
- Strauch, S.M.; Grimm, D.; Corydon, T.J.; Kruger, M.; Bauer, J.; Lebert, M.; Wise, P.; Infanger, M.; Richter, P. Current knowledge about the impact of microgravity on the proteome. Expert. Rev. Proteomics 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hader, D.P.; Braun, M.; Grimm, D.; Hemmersbach, R. Gravireceptors in eukaryotes-a comparison of case studies on the cellular level. NPJ Microgravity 2017, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Guignandon, A.; Faure, C.; Neutelings, T.; Rattner, A.; Mineur, P.; Linossier, M.T.; Laroche, N.; Lambert, C.; Deroanne, C.; Nusgens, B.; et al. Rac1 gtpase silencing counteracts microgravity-induced effects on osteoblastic cells. FASEB J. 2014, 28, 4077–4087. [Google Scholar] [CrossRef] [PubMed]
- Mann, V.; Grimm, D.; Corydon, T.J.; Kruger, M.; Wehland, M.; Riwaldt, S.; Sahana, J.; Kopp, S.; Bauer, J.; Reseland, J.E.; et al. Changes in human foetal osteoblasts exposed to the random positioning machine and bone construct tissue engineering. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Tan, X.; Xu, A.; Zhao, T.; Zhao, Q.; Zhang, J.; Fan, C.; Deng, Y.; Freywald, A.; Genth, H.; Xiang, J. Simulated microgravity inhibits cell focal adhesions leading to reduced melanoma cell proliferation and metastasis via fak/rhoa-regulated mtorc1 and ampk pathways. Sci. Rep. 2018, 8, 3769. [Google Scholar] [CrossRef]
- Thiel, C.S.; Tauber, S.; Seebacher, C.; Schropp, M.; Uhl, R.; Lauber, B.; Polzer, J.; Neelam, S.; Zhang, Y.; Ullrich, O. Real-time 3d high-resolution microscopy of human cells on the international space station. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Moos, P.J.; Graft, K.; Edwards, M.D.; Stodieck, L.S.; Einhorn, R.; Luttges, M.W. Gravity-induced changes in microtubule formation. USGS Bull. 1988, 2, 55. [Google Scholar]
- Papaseit, C.; Pochon, N.; Tabony, J. Microtubule self-organization is gravity-dependent. Proc. Natl. Acad. Sci. USA 2000, 97, 8364–8368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piepmeier, E.H.; Kalns, J.E.; McIntyre, K.M.; Lewis, M.L. Prolonged weightlessness affects promyelocytic multidrug resistance. Exp. Cell Res. 1997, 237, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, J. Growth factor-induced signal transduction in adherent mammalian cells is sensitive to gravity. FASEB J. 1999, 13, S35–S42. [Google Scholar] [CrossRef]
- Leung, D.W.; Cachianes, G.; Kuang, W.J.; Goeddel, D.V.; Ferrara, N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989, 246, 1306–1309. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Hou, L.; Li, J.; Shao, S.; Huang, S.; Meng, D.; Liu, L.; Feng, L.; Xia, P.; Qin, T.; et al. Vegf/nrp-1axis promotes progression of breast cancer via enhancement of epithelial-mesenchymal transition and activation of nf-kappab and beta-catenin. Cancer Lett. 2016, 373, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, R.M.; Fleisher, M.; Francis, G.L.; Robbins, R.J. Serum vascular endothelial growth factor levels are elevated in metastatic differentiated thyroid cancer but not increased by short-term tsh stimulation. J. Clin. Endocrinol. Metab. 2002, 87, 1737–1742. [Google Scholar] [CrossRef]
- Grosse, J.; Wehland, M.; Pietsch, J.; Ma, X.; Ulbrich, C.; Schulz, H.; Saar, K.; Hubner, N.; Hauslage, J.; Hemmersbach, R.; et al. Short-term weightlessness produced by parabolic flight maneuvers altered gene expression patterns in human endothelial cells. FASEB J. 2012, 26, 639–655. [Google Scholar] [CrossRef]
- Ulbrich, C.; Pietsch, J.; Grosse, J.; Wehland, M.; Schulz, H.; Saar, K.; Hubner, N.; Hauslage, J.; Hemmersbach, R.; Braun, M.; et al. Differential gene regulation under altered gravity conditions in follicular thyroid cancer cells: Relationship between the extracellular matrix and the cytoskeleton. Cell Physiol. Biochem. 2011, 28, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Sun, S.; Zhang, F.; Luo, C.; Zheng, L.; Wu, Y.; Li, N.; Zhang, C.; Wang, C.; Chen, Q.; et al. Microgravity-induced hepatogenic differentiation of rbmscs on board the sj-10 satellite. FASEB J. 2019, 33, 4273–4286. [Google Scholar] [CrossRef]
- dos Santos, P.B.; Zanetti, J.S.; Ribeiro-Silva, A.; Beltrao, E.I. Beta 1 integrin predicts survival in breast cancer: A clinicopathological and immunohistochemical study. Diagn Pathol. 2012, 7, 104. [Google Scholar] [CrossRef]
- Theodosiou, M.; Widmaier, M.; Bottcher, R.T.; Rognoni, E.; Veelders, M.; Bharadwaj, M.; Lambacher, A.; Austen, K.; Muller, D.J.; Zent, R.; et al. Kindlin-2 cooperates with talin to activate integrins and induces cell spreading by directly binding paxillin. Elife 2016, 5, e10130. [Google Scholar] [CrossRef] [PubMed]
- Shinde, A.; Libring, S.; Alpsoy, A.; Abdullah, A.; Schaber, J.A.; Solorio, L.; Wendt, M.K. Autocrine fibronectin inhibits breast cancer metastasis. Mol. Cancer Res. 2018, 16, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Ehling, M.; Kato, K.; Kanai, K.; van Lessen, M.; Frye, M.; Zeuschner, D.; Nakayama, M.; Vestweber, D.; Adams, R.H. Integrin beta1 controls ve-cadherin localization and blood vessel stability. Nat. Commun. 2015, 6, 6429. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Seng, S.; Li, H.; Kennel, S.J.; Avraham, H.K.; Avraham, S. Integrin regulation by vascular endothelial growth factor in human brain microvascular endothelial cells: Role of alpha6beta1 integrin in angiogenesis. J. Biol. Chem. 2006, 281, 40450–40460. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Ithychanda, S.; Qin, J.; Plow, E.F. Mechanisms of talin-dependent integrin signaling and crosstalk. Biochim Biophys Acta 2014, 1838, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Grosse, J.; Wehland, M.; Pietsch, J.; Schulz, H.; Saar, K.; Hubner, N.; Eilles, C.; Bauer, J.; Abou-El-Ardat, K.; Baatout, S.; et al. Gravity-sensitive signaling drives 3-dimensional formation of multicellular thyroid cancer spheroids. FASEB J. 2012, 26, 5124–5140. [Google Scholar] [CrossRef] [PubMed]
- Ilic, D.; Kovacic, B.; Johkura, K.; Schlaepfer, D.D.; Tomasevic, N.; Han, Q.; Kim, J.B.; Howerton, K.; Baumbusch, C.; Ogiwara, N.; et al. Fak promotes organization of fibronectin matrix and fibrillar adhesions. J. Cell Sci. 2004, 117, 177–187. [Google Scholar] [CrossRef]
- Guan, J.L. Integrin signaling through fak in the regulation of mammary stem cells and breast cancer. IUBMB Life 2010, 62, 268–276. [Google Scholar] [CrossRef]
- Sullivan, N.J.; Sasser, A.K.; Axel, A.E.; Vesuna, F.; Raman, V.; Ramirez, N.; Oberyszyn, T.M.; Hall, B.M. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene 2009, 28, 2940–2947. [Google Scholar] [CrossRef]
- Kitadai, Y.; Haruma, K.; Mukaida, N.; Ohmoto, Y.; Matsutani, N.; Yasui, W.; Yamamoto, S.; Sumii, K.; Kajiyama, G.; Fidler, I.J.; et al. Regulation of disease-progression genes in human gastric carcinoma cells by interleukin 8. Clin. Cancer Res. 2000, 6, 2735–2740. [Google Scholar]
- Cohen, T.; Nahari, D.; Cerem, L.W.; Neufeld, G.; Levi, B.Z. Interleukin 6 induces the expression of vascular endothelial growth factor. J. Biol. Chem. 1996, 271, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Galisteo, R.; Gutkind, J.S. Cxcl8/il8 stimulates vascular endothelial growth factor (vegf) expression and the autocrine activation of vegfr2 in endothelial cells by activating nfkappab through the cbm (carma3/bcl10/malt1) complex. J. Biol. Chem. 2009, 284, 6038–6042. [Google Scholar] [CrossRef] [PubMed]
- Lecuit, T.; Yap, A.S. E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat. Cell Biol. 2015, 17, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Sahana, J.; Nassef, M.Z.; Wehland, M.; Kopp, S.; Kruger, M.; Corydon, T.J.; Infanger, M.; Bauer, J.; Grimm, D. Decreased e-cadherin in mcf7 human breast cancer cells forming multicellular spheroids exposed to simulated microgravity. Proteomics 2018, 18, e1800015. [Google Scholar] [CrossRef] [PubMed]
- Hazan, R.B.; Kang, L.; Roe, S.; Borgen, P.I.; Rimm, D.L. Vinculin is associated with the e-cadherin adhesion complex. J. Biol. Chem. 1997, 272, 32448–32453. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Sun, M.; Jin, F.; Xiao, Q.; He, M.; Wu, H.; Ren, J.; Zhao, L.; Zhao, H.; Yao, W.; et al. Combined expression of ezrin and e-cadherin is associated with lymph node metastasis and poor prognosis in breast cancer. Oncol. Rep. 2015, 34, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Thapa, N.; Tan, X.; Choi, S.; Wise, T.; Anderson, R.A. Pipkigamma and talin couple phosphoinositide and adhesion signaling to control the epithelial to mesenchymal transition. Oncogene 2017, 36, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Dudek, M.C.; Wong, K.E.; Bassa, L.M.; Mora, M.C.; Ser-Dolansky, J.; Henneberry, J.M.; Crisi, G.M.; Arenas, R.B.; Schneider, S.S. Antineoplastic effects of rhodiola crenulata treatment on b16-f10 melanoma. Tumour Biol. 2015, 36, 9795–9805. [Google Scholar] [CrossRef] [PubMed]
- Cowden Dahl, K.D.; Symowicz, J.; Ning, Y.; Gutierrez, E.; Fishman, D.A.; Adley, B.P.; Stack, M.S.; Hudson, L.G. Matrix metalloproteinase 9 is a mediator of epidermal growth factor-dependent e-cadherin loss in ovarian carcinoma cells. Cancer Res. 2008, 68, 4606–4613. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, M.; Avraham, I.; Dor, Y.; Bachar-Lustig, E.; Itin, A.; Jung, S.; Chimenti, S.; Landsman, L.; Abramovitch, R.; Keshet, E. Vegf-induced adult neovascularization: Recruitment, retention, and role of accessory cells. Cell 2006, 124, 175–189. [Google Scholar] [CrossRef]
- Zheng, P.S.; Wen, J.; Ang, L.C.; Sheng, W.; Viloria-Petit, A.; Wang, Y.; Wu, Y.; Kerbel, R.S.; Yang, B.B. Versican/pg-m g3 domain promotes tumor growth and angiogenesis. FASEB J. 2004, 18, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Takahashi, H.; Murai, Y.; Cui, Z.; Nomoto, K.; Niwa, H.; Tsuneyama, K.; Takano, Y. Expressions of mmp-2, mmp-9 and vegf are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res. 2006, 26, 3579–3583. [Google Scholar] [PubMed]
- Davis, G.E.; Bayless, K.J.; Mavila, A. Molecular basis of endothelial cell morphogenesis in three-dimensional extracellular matrices. Anat. Rec. 2002, 268, 252–275. [Google Scholar] [CrossRef] [PubMed]
- Krüger, M.; Melnik, D.; Kopp, S.; Buken, C.; Sahana, J.; Bauer, J.; Wehland, M.; Hemmersbach, R.; Corydon, T.J.; Infanger, M.; et al. Fighting thyroid cancer with microgravity research. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Mattila, P.K.; Lappalainen, P. Filopodia: Molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 2008, 9, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Jacquemet, G.; Hamidi, H.; Ivaska, J. Filopodia in cell adhesion, 3d migration and cancer cell invasion. Curr. Opin. Cell Biol. 2015, 36, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Jacquemet, G.; Green, D.M.; Bridgewater, R.E.; von Kriegsheim, A.; Humphries, M.J.; Norman, J.C.; Caswell, P.T. Rcp-driven alpha5beta1 recycling suppresses rac and promotes rhoa activity via the racgap1-iqgap1 complex. J. Cell Biol. 2013, 202, 917–935. [Google Scholar] [CrossRef]
- Paul, N.R.; Allen, J.L.; Chapman, A.; Morlan-Mairal, M.; Zindy, E.; Jacquemet, G.; Fernandez del Ama, L.; Ferizovic, N.; Green, D.M.; Howe, J.D.; et al. Alpha5beta1 integrin recycling promotes arp2/3-independent cancer cell invasion via the formin fhod3. J. Cell Biol. 2015, 210, 1013–1031. [Google Scholar] [CrossRef]
- Shibue, T.; Brooks, M.W.; Inan, M.F.; Reinhardt, F.; Weinberg, R.A. The outgrowth of micrometastases is enabled by the formation of filopodium-like protrusions. Cancer Discov 2012, 2, 706–721. [Google Scholar] [CrossRef]
- Shibue, T.; Brooks, M.W.; Weinberg, R.A. An integrin-linked machinery of cytoskeletal regulation that enables experimental tumor initiation and metastatic colonization. Cancer Cell 2013, 24, 481–498. [Google Scholar] [CrossRef]
- Lindberg, U.; Karlsson, R.; Lassing, I.; Schutt, C.E.; Höglund, A.S. The microfilament system and malignancy. Semin. Cancer Biol. 2008, 18, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D. How cells (might) sense microgravity. FASEB J. 1999, 13, S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Aleshcheva, G.; Sahana, J.; Ma, X.; Hauslage, J.; Hemmersbach, R.; Egli, M.; Infanger, M.; Bauer, J.; Grimm, D. Changes in morphology, gene expression and protein content in chondrocytes cultured on a random positioning machine. PLoS One 2013, 8, e79057. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D.; Bauer, J.; Kossmehl, P.; Shakibaei, M.; Schoberger, J.; Pickenhahn, H.; Schulze-Tanzil, G.; Vetter, R.; Eilles, C.; Paul, M.; et al. Simulated microgravity alters differentiation and increases apoptosis in human follicular thyroid carcinoma cells. FASEB J. 2002, 16, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Aronovich, E.L.; McIvor, R.S.; Hackett, P.B. The sleeping beauty transposon system: A non-viral vector for gene therapy. Hum. Mol. Genet. 2011, 20, R14–R20. [Google Scholar] [CrossRef] [PubMed]
- Askou, A.L.; Aagaard, L.; Kostic, C.; Arsenijevic, Y.; Hollensen, A.K.; Bek, T.; Jensen, T.G.; Mikkelsen, J.G.; Corydon, T.J. Multigenic lentiviral vectors for combined and tissue-specific expression of mirna- and protein-based antiangiogenic factors. Mol. Ther. Methods Clin. Dev. 2015, 2, 14064. [Google Scholar] [CrossRef] [PubMed]
- Pihlmann, M.; Askou, A.L.; Aagaard, L.; Bruun, G.H.; Svalgaard, J.D.; Holm-Nielsen, M.H.; Dagnaes-Hansen, F.; Bek, T.; Mikkelsen, J.G.; Jensen, T.G.; et al. Adeno-associated virus-delivered polycistronic microrna-clusters for knockdown of vascular endothelial growth factor in vivo. J. Gene Med. 2012, 14, 328–338. [Google Scholar] [CrossRef]
- Staunstrup, N.H.; Sharma, N.; Bak, R.O.; Svensson, L.; Petersen, T.K.; Aarenstrup, L.; Kristiansen, K.; Bolund, L.; Mikkelsen, J.G. A sleeping beauty DNA transposon-based genetic sensor for functional screening of vitamin d3 analogues. BMC Biotechnol. 2011, 11, 33. [Google Scholar] [CrossRef]














| Antibody Name | Class | Type | Company | Reference no. | Dilution |
|---|---|---|---|---|---|
| Matrix Metallopeptidase 9 (MMP9) (2C3) | mouse monoclonal AB | Primary Antibody | Santa Cruz | sc-21733 | (1:100) |
| interleukin 6 (IL6) (E-4) | mouse monoclonal AB | Primary Antibody | Santa Cruz | sc-28343 | (1:100) |
| interleukin 8 (IL8) (C-11) | mouse monoclonal AB | Primary Antibody | Santa Cruz | sc-376750 | (1:100) |
| Vascular Endothelial Growth Factor (VEGF) (c‑term) | Rabbit monoclonal | Primary Antibody | Epitomics, Inc | #1909-1 | (1:250) |
| Alexa fluor plus 488 goat anti-mouse IgG (H + L) | Goat polyclonal | Secondary Antibody | Invitrogen by Thermo Fischer Scientific | A32723 | (1:400) |
| Alexa fluor 488 F(ab’) 2 frangment of goat anti-rabbit igG (H + L) | Goat polyclonal | Secondary Antibody | Invitrogen by Thermo Fischer Scientific | A11070 | (1:500) |
| Alexa Fluor 568 phalloidin | bicyclic peptide toxin | Toxin | Invitrogen by Thermo Fischer Scientific | A12380 | one unit in 200 µL per slide |
| Fluoroshield with DAPI | fluorescent stain | fluorescent stain | Sigma Life science | F6057 | no dilution |
| Factor | Primer Name | Sequence 5′–3′ |
|---|---|---|
| 18S | 18s-F | GGAGCCTGCGGCTTAATTT |
| 18s-R | CAACTAAGAACGGCCATGCA | |
| ACTB | ACTB-F | TGCCGACAGGATGCAGAAG |
| ACTB-R | GCCGATCCACACGGAGTACT | |
| CAV1 | CAV1-F | CCTCCTCACAGTTTTCATCCA |
| CAV1-R | TGTAGATGTTGCCCTGTTCC | |
| CAV2 | CAV2-F | GATCCCCACCGGCTCAAC |
| CAV2-R | CACCGGCTCTGCGATCA | |
| COL1A1 | COL1A1-F | ACGAAGACATCCCACCAATCAC |
| COL1A1-R | CGTTGTCGCAGACGCAGAT | |
| EZR | EZR-F | GCAATCCAGCCAAATACAACTG |
| EZR-R | CCACATAGTGGAGGCCAAAGTAC | |
| FN1 | FN1-F | TGAGGAGCATGGTTTTAGGAGAA |
| FN1-R | TCCTCATTTACATTCGGCGTATAC | |
| ICAM1 | ICAM1-F | CGGCTGACGTGTGCAGTAAT |
| ICAM1-R | CTTCTGAGACCTCTGGCTTCGT | |
| IL6 | IL6-F | CGGGAACGAAAGAGAAGCTCTA |
| IL6-R | GAGCAGCCCCAGGGAGAA | |
| CXCL8 | IL8-F | TGGCAGCCTTCCTGATTTCT |
| IL8-R | GGGTGGAAAGGTTTGGAGTATG | |
| KRT8 | KRT8-F | GATCTCTGAGATGAACCGGAACA |
| KRT8-R | GCTCGGCATCTGCAATGG | |
| LAMA1 | LAMA1-F | TGACTGACCTGGGTTCAGGA |
| LAMA1-R | TGCTAGCACTCCTTGCTTCC | |
| LAMA3 | LAMA3-F | AAAGCAAGAAGTCAGTCCAGC |
| LAMA3-R | TCCCATGAAGACCATCTCGG | |
| MMP9 | MMP9-F | CCTGGAGACCTGAGAACCAATC |
| MMP9-R | TTCGACTCTCCACGCATCTCT | |
| MSN | MSN-F | GAAATTTGTCATCAAGCCCATTG |
| MSN-R | CCATGCACAAGGCCAAGAT | |
| TBP | TBP-F | GTGACCCAGCATCACTGTTTC |
| TBP-R | GCAAACCAGAAACCCTTGCG | |
| TIMP1 | TIMP1-F | GCCATCGCCGCAGATC |
| TIMP1-R | GCTATCAGCCACAGCAACAACA | |
| TLN1 | TLN1-F | GATGGCTATTACTCAGTACAGACAACTGA |
| TLN1-R | CATAGTAGACTCCTCATCTCCTTCCA | |
| TUBB | TUBB-F | CTGGACCGCATCTCTGTGTACTAC |
| TUBB-R | GACCTGAGCGAACAGAGTCCAT | |
| VEGFA | VEGFA-F | GCGCTGATAGACATCCATGAAC |
| VEGFA-R | CTACCTCCACCATGCCAAGTG | |
| VCL | VCL-F | GTCTCGGCTGCTCGTATCTT |
| VCL-R | GTCCACCAGCCCTGTCATTT | |
| PTK2 | FAK1-F | TGTGGGTAAACCAGATCCTGC |
| FAK1-R | CTGAAGCTTGACACCCTCGT | |
| RDX | RDX-F | GAAAATGCCGAAACCAATCAA |
| RDX-R | GTATTGGGCTGAATGGCAAATT | |
| PAI1 | PAI1-F | AGGCTGACTTCACGAGTCTTTCA |
| PAI1-R | CACTCTCGTTCACCTCGATCTTC | |
| CDH1 | CDH1-F | GCTGGACCGAGAGAGTTTCC |
| CDH1-R | CAGCTGTTGCTGTTGTGCTT | |
| ITGB1 | ITGB1-F | GAAAACAGCGCATATCTGGAAATT |
| ITGB1-R | CAGCCAATCAGTGATCCACAA |
| Antibody Name | Source | Company | Reference no. | MW kDa | Dilution |
|---|---|---|---|---|---|
| Anti-Cyclophilin B | Rabbit monoclonal | Abcam | #178397 | 24 | 1: 1000 |
| Anti-Cytokeratin | Mouse monoclonal | Sigma | #C1801 | 68 | 1: 1000 |
| Anti-E Cadherin | Mouse monoclonal | Abcam | ab1416 | 97 | 1:500 |
| Anti-FAK | Rabbit monoclonal | Abcam | ab40794 | 125 | 1:1000 |
| Anti-IL-8 | Rabbit polyclonal | Abcam | ab7747 | 11 | 1:500 |
| Anti-Integrin beta 1 | Rabbit monoclonal | Abcam | #134179 | 88 | 1: 1000 |
| Anti-Laminin | Rabbit polyclonal | Sigma | #L9393 | 220 | 1: 1000 |
| Anti-PAI1 | Rabbit polyclonal | Abcam | Ab66705 | 45 | 1:1000 |
| Anti-Vinculin | Mouse monoclonal | Abcam | Ab18058 | 124 | 1:1000 |
| Anti-β-Actin | Mouse monoclonal | Sigma | A5316 | 42 | 1:2000 |
| Beta Tubulin Antibody | Rabbit Polyclonal | Santa Cruz Biotechnology | sc-9104 | 55 | 1: 1000 |
| Ezrin | Rabbit polyclonal | Cell Signaling | #3145 | 81 | 1:500 |
| Fibronectin | Mouse monoclonal | Invitrogen | #MA5-11981 | 250 | 1:1000 |
| GAPDH (14C10) | Rabbit monoclonal | Cell signaling | #5014S | 37 | 1:1000 |
| MMP9 | Mouse monoclonal | ThermoFisher | #MA5-14220 | 92 | 1: 500 |
| Moesin (Q480) | Rabbit polyclonal | Cell signaling | #3150 | 78 | 1:500 |
| Radixin | Rabbit monoclonal | Cell Signaling | #2636S | 80 | 1: 1000 |
| TIMP1 | Mouse monoclonal | ThermoFisher | #MA5-13688 | 28 | 1: 500 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nassef, M.Z.; Kopp, S.; Wehland, M.; Melnik, D.; Sahana, J.; Krüger, M.; Corydon, T.J.; Oltmann, H.; Schmitz, B.; Schütte, A.; et al. Real Microgravity Influences the Cytoskeleton and Focal Adhesions in Human Breast Cancer Cells. Int. J. Mol. Sci. 2019, 20, 3156. https://doi.org/10.3390/ijms20133156
Nassef MZ, Kopp S, Wehland M, Melnik D, Sahana J, Krüger M, Corydon TJ, Oltmann H, Schmitz B, Schütte A, et al. Real Microgravity Influences the Cytoskeleton and Focal Adhesions in Human Breast Cancer Cells. International Journal of Molecular Sciences. 2019; 20(13):3156. https://doi.org/10.3390/ijms20133156
Chicago/Turabian StyleNassef, Mohamed Zakaria, Sascha Kopp, Markus Wehland, Daniela Melnik, Jayashree Sahana, Marcus Krüger, Thomas J. Corydon, Hergen Oltmann, Burkhard Schmitz, Andreas Schütte, and et al. 2019. "Real Microgravity Influences the Cytoskeleton and Focal Adhesions in Human Breast Cancer Cells" International Journal of Molecular Sciences 20, no. 13: 3156. https://doi.org/10.3390/ijms20133156
APA StyleNassef, M. Z., Kopp, S., Wehland, M., Melnik, D., Sahana, J., Krüger, M., Corydon, T. J., Oltmann, H., Schmitz, B., Schütte, A., Bauer, T. J., Infanger, M., & Grimm, D. (2019). Real Microgravity Influences the Cytoskeleton and Focal Adhesions in Human Breast Cancer Cells. International Journal of Molecular Sciences, 20(13), 3156. https://doi.org/10.3390/ijms20133156