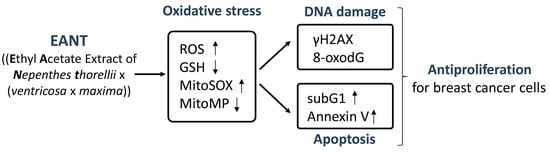
Antiproliferation for Breast Cancer Cells by Ethyl Acetate Extract of Nepenthes thorellii x (ventricosa x maxima)
Abstract
:1. Introduction
2. Results
2.1. The Identified Components from Fingerprint Profiles of EANT
2.2. EANT Preferentially Inhibits Viability of Breast Cancer Cells
2.3. EANT Changes Cell Cycle Distribution in Breast Cancer Cells
2.4. EANT Induces Apoptosis in Breast Cancer Cells
2.5. EANT Induces ROS Production and GSH Depletion in Breast Cancer Cells
2.6. EANT Induces MitoSOX Production and MMP Reduction in Breast Cancer Cells
2.7. EANT Induces DNA Damage in Breast Cancer Cells
3. Discussion
3.1. EANT Preferentially Inhibits Proliferation of Breast Cancer Cells
3.2. EANT Induces Oxidative Stress on Breast Cancer Cells
3.3. EANT Induces Oxidative Stress-Mediated Apoptosis and DNA Damage on Breast Cancer Cells
3.4. Conclusion
4. Materials and Methods
4.1. Nepenthes Extraction and Inhibitors
4.2. HPLC Fingerprint Profile of EANT
4.3. Cell Culture and Viability
4.4. Cell Cycle Assay
4.5. Annexin V/7AAD Assay for Apoptosis
4.6. ROS Assay
4.7. GSH Assay
4.8. Mitochondrial Superoxide Assay
4.9. Mitochondrial Membrane Potential Assay
4.10. γH2AX Assay
4.11. 8-Oxo-2′deoxyguanosine Assay
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Teoh, P.L.; Liau, M.; Cheong, B.E. Phyla nodiflora L. Extracts induce apoptosis and cell cycle arrest in human breast cancer cell line, MCF-7. Nutr. Cancer 2019, 71, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Aung, T.N.; Qu, Z.; Kortschak, R.D.; Adelson, D.L. Understanding the effectiveness of natural compound mixtures in cancer through their molecular mode of action. Int. J. Mol. Sci. 2017, 18, 656. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Samani, M.; Rafieian-Kopaei, M.; Lorigooini, Z.; Shirzad, H. The effect of Euphorbia szovitsii Fisch. & C.A.Mey extract on the viability and the proliferation of MDA-MB-231 cell line. Biosci. Rep. 2019, 39, BSR20181538. [Google Scholar] [PubMed]
- Jiang, X.; Cao, C.; Sun, W.; Chen, Z.; Li, X.; Nahar, L.; Sarker, S.D.; Georgiev, M.I.; Bai, W. Scandenolone from Cudrania tricuspidata fruit extract suppresses the viability of breast cancer cells (MCF-7) in vitro and in vivo. Food Chem. Toxico.l 2019, 126, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.R.; Chiu, C.F.; Hu, J.L.; Feng, C.H.; Huang, C.Y.; Bai, L.Y.; Sheu, J.H. A sterol from soft coral induces apoptosis and autophagy in MCF-7 breast cancer cells. Ma.r Drugs 2018, 16, 238. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Tang, J.Y.; Ou-Yang, F.; Wang, H.R.; Guan, P.Y.; Huang, C.Y.; Chen, C.Y.; Hou, M.F.; Sheu, J.H.; Chang, H.W. Sinularin selectively kills breast cancer cells showing G2/M arrest, apoptosis, and oxidative DNA damage. Molecules 2018, 23, 849. [Google Scholar] [CrossRef] [PubMed]
- Sanusi, S.B.; Bakar, M.F.A.; Mohamed, M.; Sabran, S.F.; Mainasara, M.M. Ethnobotanical, phytochemical, and pharmacological properties of Nepenthes species: A review. Asian. J. Pharm. Clin. Res. 2017, 10, 16–19. [Google Scholar] [CrossRef]
- Ismail, N.A.; Kamariah, A.S.; Lim, L.B.; Ahmad, A. Phytochemical and pharmacological evaluation of methanolic extracts of the leaves of Nepenthes bicalcarata Hook. F. Int. J. Pharma. Phyto. Res. 2015, 7, 1127–1138. [Google Scholar]
- Shin, K.S.; Lee, S.; Cha, B.J. Suppression of phytopathogenic fungi by hexane extract of Nepenthes ventricosa x maxima leaf. Fitoterapia 2007, 78, 585–586. [Google Scholar] [CrossRef]
- Thao, N.P.; Luyen, B.T.; Koo, J.E.; Kim, S.; Koh, Y.S.; Thanh, N.V.; Cuong, N.X.; Kiem, P.V.; Minh, C.V.; Kim, Y.H. In vitro anti-inflammatory components isolated from the carnivorous plant Nepenthes mirabilis (Lour.) Rafarin. Pharm. Biol. 2016, 54, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Sugata, M.; Lin, C.Y.; Shih, Y.C. Anti-inflammatory and anticancer activities of taiwanese purple-fleshed sweet potatoes (Ipomoea batatas L. Lam) extracts. Biomed. Res. Int. 2015, 2015, 768093. [Google Scholar] [CrossRef] [PubMed]
- Orlikova, B.; Legrand, N.; Panning, J.; Dicato, M.; Diederich, M. Anti-inflammatory and anticancer drugs from nature. Cancer Treat Re.s 2014, 159, 123–143. [Google Scholar]
- Akunne, T.C.; Akah, P.A.; Nwabunike, I.A.; Nworu, C.S.; Okereke, E.K.; Okereke, N.C.; Okeke, F.C.; Hsu, T.-C. Anti-inflammatory and anticancer activities of extract and fractions of Rhipsalis neves-armondii (Cactaceae) aerial parts. Cogent Biol. 2016, 2, 1237259. [Google Scholar] [CrossRef]
- De, U.; Son, J.Y.; Jeon, Y.; Ha, S.Y.; Park, Y.J.; Yoon, S.; Ha, K.T.; Choi, W.S.; Lee, B.M.; Kim, I.S.; et al. Plumbagin from a tropical pitcher plant (Nepenthes alata Blanco) induces apoptotic cell death via a p53-dependent pathway in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2019, 123, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.C.; Serpa, J. Glutathione in ovarian cancer: A double-edged sword. Int. J. Mol. Sci. 2018, 19, 1882. [Google Scholar] [CrossRef] [PubMed]
- Richter, C. Reactive oxygen and DNA damage in mitochondria. Mutat Res. 1992, 275, 249–255. [Google Scholar] [CrossRef]
- Likhitwitayawuid, K.; Kaewamatawong, R.; Ruangrungsi, N.; Krungkrai, J. Antimalarial naphthoquinones from Nepenthes Thorelii. Planta Med. 1998, 64, 237–241. [Google Scholar] [CrossRef]
- Schlauer, J.; Nerz, J.; Rischer, H. Carnivorous plant chemistry. Acta Botanica Gallica 2005, 152, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Eilenberg, H.; Pnini-Cohen, S.; Rahamim, Y.; Sionov, E.; Segal, E.; Carmeli, S.; Zilberstein, A. Induced production of antifungal naphthoquinones in the pitchers of the carnivorous plant Nepenthes khasiana. J. Exp. Bot. 2010, 61, 911–922. [Google Scholar] [CrossRef]
- Sobhani, M.; Abbas-Mohammadi, M.; Ebrahimi, S.N.; Aliahmadi, A. Tracking leading anti-Candida compounds in plant samples; Plumbago europaea. Iran. J. Microbiol. 2018, 10, 187–193. [Google Scholar] [PubMed]
- Aronsson, P.; Munissi, J.J.E.; Gruhonjic, A.; Fitzpatrick, P.A.; Landberg, G.; Nyandoro, S.S.; Erdelyi, M. Phytoconstituents with radical scavenging and cytotoxic activities from Diospyros Shimbaensis. Dis. 2016, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Moawad, A.; Owis, A.; AbouZid, S.; Ahmed, O. Flavonoids of Calligonum polygonoides and their cytotoxicity. Pharm. Biol. 2016, 54, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.E.; Park, J.Y.; Kim, W.K. In vitro histoculture drug response assay and in vivo blood chemistry of a novel Pt(IV) compound, K104. Anticancer Res. 2007, 27, 321–326. [Google Scholar] [PubMed]
- Poljsak, B.; Suput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef] [PubMed]
- Sznarkowska, A.; Kostecka, A.; Meller, K.; Bielawski, K.P. Inhibition of cancer antioxidant defense by natural compounds. Oncotarget 2017, 8, 15996–16016. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.C.; Tseng, C.N.; Yang, J.I.; Huang, H.W.; Fang, Y.; Tang, J.Y.; Chang, F.R.; Chang, H.W. Antiproliferation and induction of apoptosis in Ca9-22 oral cancer cells by ethanolic extract of Gracilaria tenuistipitata. Molecules 2012, 17, 10916–10927. [Google Scholar] [CrossRef] [PubMed]
- Inbaraj, J.J.; Chignell, C.F. Cytotoxic action of juglone and plumbagin: A mechanistic study using HaCaT keratinocytes. Chem. Res. Toxicol. 2004, 17, 55–62. [Google Scholar] [CrossRef]
- Castro, F.A.; Mariani, D.; Panek, A.D.; Eleutherio, E.C.; Pereira, M.D. Cytotoxicity mechanism of two naphthoquinones (menadione and plumbagin) in Saccharomyces cerevisiae. PLoS One 2008, 3, e3999. [Google Scholar] [CrossRef]
- Kong, X.; Luo, J.; Xu, T.; Zhou, Y.; Pan, Z.; Xie, Y.; Zhao, L.; Lu, Y.; Han, X.; Li, Z.; et al. Plumbagin enhances TRAIL-induced apoptosis of human leukemic Kasumi1 cells through upregulation of TRAIL death receptor expression, activation of caspase-8 and inhibition of cFLIP. Oncol. Rep. 2017, 37, 3423–3432. [Google Scholar] [CrossRef]
- Gaascht, F.; Teiten, M.H.; Cerella, C.; Dicato, M.; Bagrel, D.; Diederich, M. Plumbagin modulates leukemia cell redox status. Molecules 2014, 19, 10011–10032. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Sharma, A.; Garg, V.; Tuli, H.S.; Kumar, G.; Kumar, M.; Mukherjee, T. Reactive oxygen species (ROS): An activator of apoptosis and autophagy in cancer. J. Biol. Chem. Sci. 2016, 3, 256–264. [Google Scholar]
- Semaan, J.; Pinon, A.; Rioux, B.; Hassan, L.; Limami, Y.; Pouget, C.; Fagnere, C.; Sol, V.; Diab-Assaf, M.; Simon, A.; et al. Resistance to 3-HTMC-induced apoptosis through activation of PI3K/Akt, MEK/ERK, and p38/COX-2/PGE2 pathways in human HT-29 and HCT116 colorectal cancer cells. J. Cell. Biochem. 2016, 117, 2875–2885. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, J.; Huang, H. Oxidative stress induces H2AX phosphorylation in human spermatozoa. FEBS Lett 2006, 580, 6161–6168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banath, J.P.; Klokov, D.; MacPhail, S.H.; Banuelos, C.A.; Olive, P.L. Residual gammaH2AX foci as an indication of lethal DNA lesions. BMC Cancer 2010, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J 2003, 17, 1195–1214. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Yeh, J.M.; Chan, W.H. Hazardous impacts of silver nanoparticles on mouse oocyte maturation and fertilization and fetal development through induction of apoptotic processes. Env. Toxicol 2018, 33, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.S.; Lin, C.P.; Chen, Y.P.; Chao, M.R.; Li, C.C.; Liu, K.L. CYP450-mediated mitochondrial ROS production involved in arecoline N-oxide-induced oxidative damage in liver cell lines. Env. Toxicol 2018, 33, 1029–1038. [Google Scholar] [CrossRef]
- Chen, C.Y.; Yen, C.Y.; Wang, H.R.; Yang, H.P.; Tang, J.Y.; Huang, H.W.; Hsu, S.H.; Chang, H.W. Tenuifolide B from Cinnamomum tenuifolium stem selectively inhibits proliferation of oral cancer cells via apoptosis, ROS generation, mitochondrial depolarization, and DNA damage. Toxins 2016, 8, 319. [Google Scholar] [CrossRef]
- Vignon, C.; Debeissat, C.; Georget, M.T.; Bouscary, D.; Gyan, E.; Rosset, P.; Herault, O. Flow cytometric quantification of all phases of the cell cycle and apoptosis in a two-color fluorescence plot. PLoS ONE 2013, 8, e68425. [Google Scholar] [CrossRef]
- Yeh, C.C.; Yang, J.I.; Lee, J.C.; Tseng, C.N.; Chan, Y.C.; Hseu, Y.C.; Tang, J.Y.; Chuang, L.Y.; Huang, H.W.; Chang, F.R.; et al. Anti-proliferative effect of methanolic extract of Gracilaria tenuistipitata on oral cancer cells involves apoptosis, DNA damage, and oxidative stress. BMC Complement Altern. Med. 2012, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.Y.; Chung, C.H.; Ciou, J.S.; Su, P.F.; Wang, P.W.; Shieh, D.B.; Wang, T.C. Molecular damage and responses of oral keratinocyte to hydrogen peroxide. BMC Oral Health 2019, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.T.; Huang, C.Y.; Tang, J.Y.; Liaw, C.C.; Li, R.N.; Liu, J.R.; Sheu, J.H.; Chang, H.W. Reactive oxygen species mediate soft corals-derived sinuleptolide-induced antiproliferation and DNA damage in oral cancer cells. Onco. Targets Ther. 2017, 10, 3289–3297. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.S.; Tang, J.Y.; Yen, C.Y.; Huang, H.W.; Wu, C.Y.; Chung, Y.A.; Wang, H.R.; Chen, I.S.; Huang, M.Y.; Chang, H.W. Antiproliferation of Cryptocarya concinna-derived cryptocaryone against oral cancer cells involving apoptosis, oxidative stress, and DNA damage. BMC Complement Altern. Med. 2016, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Haung, J.W.; Chang, F.R.; Huang, K.J.; Huang, H.M.; Huang, H.W.; Chou, C.K.; Wu, Y.C.; Chang, H.W. Golden berry-derived 4beta-hydroxywithanolide E for selectively killing oral cancer cells by generating ROS, DNA damage, and apoptotic pathways. PLoS One 2013, 8, e64739. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou-Yang, F.; Tsai, I.-H.; Tang, J.-Y.; Yen, C.-Y.; Cheng, Y.-B.; Farooqi, A.A.; Chen, S.-R.; Yu, S.-Y.; Kao, J.-K.; Chang, H.-W. Antiproliferation for Breast Cancer Cells by Ethyl Acetate Extract of Nepenthes thorellii x (ventricosa x maxima). Int. J. Mol. Sci. 2019, 20, 3238. https://doi.org/10.3390/ijms20133238
Ou-Yang F, Tsai I-H, Tang J-Y, Yen C-Y, Cheng Y-B, Farooqi AA, Chen S-R, Yu S-Y, Kao J-K, Chang H-W. Antiproliferation for Breast Cancer Cells by Ethyl Acetate Extract of Nepenthes thorellii x (ventricosa x maxima). International Journal of Molecular Sciences. 2019; 20(13):3238. https://doi.org/10.3390/ijms20133238
Chicago/Turabian StyleOu-Yang, Fu, I-Hsuan Tsai, Jen-Yang Tang, Ching-Yu Yen, Yuan-Bin Cheng, Ammad Ahmad Farooqi, Shu-Rong Chen, Szu-Yin Yu, Jun-Kai Kao, and Hsueh-Wei Chang. 2019. "Antiproliferation for Breast Cancer Cells by Ethyl Acetate Extract of Nepenthes thorellii x (ventricosa x maxima)" International Journal of Molecular Sciences 20, no. 13: 3238. https://doi.org/10.3390/ijms20133238
APA StyleOu-Yang, F., Tsai, I. -H., Tang, J. -Y., Yen, C. -Y., Cheng, Y. -B., Farooqi, A. A., Chen, S. -R., Yu, S. -Y., Kao, J. -K., & Chang, H. -W. (2019). Antiproliferation for Breast Cancer Cells by Ethyl Acetate Extract of Nepenthes thorellii x (ventricosa x maxima). International Journal of Molecular Sciences, 20(13), 3238. https://doi.org/10.3390/ijms20133238