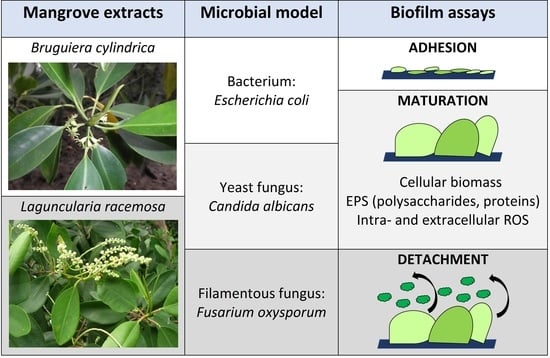
Promoting Beneficial and Inhibiting Undesirable Biofilm Formation with Mangrove Extracts
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Analysis of the Extracts
Total Phenol Content (TPC), Total Flavonoid Content (TFC) and Oxygen Radical Absorbance Capacity (ORAC)
2.2. LC-MS Analysis of Secondary Metabolites
2.3. Mangrove Extracts do not Have Toxic Effects
2.3.1. Mangrove Extracts do not Have a Biocidal Effect
2.3.2. Mangrove Extracts do not Modulate Planktonic Growth
2.3.3. Mangrove Extracts do not Affect F. oxysporum Conidia Germination
2.4. Mangrove Extracts are not a Carbon and Energy Source
2.5. Mangrove Extracts Affect Cells/Conidia Adhesion
2.6. Mangrove Extract Modulate Biofilm Maturation
2.6.1. Mangrove Extracts Affect Cellular Biomass Abundant
2.6.2. Mangrove Extracts Increase the Sum and Ratio of Extracellular Polysaccharides and Proteins
2.6.3. Mangrove Extracts Increase the Intracellular and Extracellular Amount of Reactive Oxygen Species (ROS)
2.7. Mangrove Extract Modulate the Biofilm Detachment
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extraction
4.2. Total Flavonoid Content (TFC)
4.3. Total Phenol Content (TPC)
4.4. Oxygen Radical Absorbance Capacity (ORAC)
4.5. LC-MS Analysis
4.6. Microbial Strains and Growth Media
4.7. Mangrove Extract Toxicity
4.7.1. Biocidal Activity
4.7.2. Modulation of Planktonic Growth
4.7.3. Effect on Conidia Germination
4.8. Mangrove Extracts as Carbon and Energy Source
4.9. Mangrove Effect on Cells/Conidia Adhesion
4.10. Mangrove Effect on Biofilm Maturation
4.10.1. Biofilm Culture
4.10.2. Biomass and EPS Extraction
4.10.3. Biomass Abundance
4.10.4. Extracellular Polymeric Substances (EPS) Composition
4.10.5. Level of Intracellular and Extracellular Oxidative Stress
4.11. Mangrove Effect on Biofilm Dispersion
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Galie, S.; Garcia-Gutierrez, C.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Pullerits, K.; Keucken, A.; Perssonz, K.M.; Paul, C.J.; Radstrom, P. Bacterial release from pipe biofilm in a full-scale drinking water distribution system. NPJ Biofilms Microbiomes 2019, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Di Pippo, F.; Di Gregorio, L.; Congestri, R.; Tandoi, V.; Rossetti, S. Biofilm growth and control in cooling water industrial systems. FEMS Microbiol. Ecol. 2018, 94, fiy044. [Google Scholar] [CrossRef] [PubMed]
- Jindal, S.; Anand, S.; Metzger, L.; Amamcharla, J. Short communication: A comparison of biofilm development on stainless steel and modified-surface plate heat exchangers during a 17-h milk pasteurization run. J. Dairy Sci. 2018, 101, 2921–2926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khouzani, M.K.; Bahrami, A.; Hosseini-Abari, A.; Khandouzi, M.; Taheri, P. Microbiologically influenced corrosion of a pipeline in a petrochemical plant. Metals 2019, 9, 459. [Google Scholar] [CrossRef]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Karn, S.K.; Duan, J.; Jenkinson, I.R. Book review: Role of biofilms in bioremediation. Front. Environ. Sci. 2017, 5, 22. [Google Scholar] [CrossRef]
- Turki, Y.; Mehri, I.; Lajnef, R.; Ben Rejab, A.; Khessairi, A.; Cherif, H.; Ouzari, H.; Hassen, A. Biofilms in bioremediation and wastewater treatment: Characterization of bacterial community structure and diversity during seasons in municipal wastewater treatment process. Environ. Sci. Pollut. Res. 2017, 24, 3519–3530. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, M. Beneficial biofilm formation by industrial bacteria Bacillus subtilis and related species. J. Biosci. Bioeng. 2006, 101, 1–8. [Google Scholar] [CrossRef]
- Angelaalincy, M.J.; Krishnaraj, R.N.; Shakambari, G.; Ashokkumar, B.; Kathiresan, S.; Varalakshmi, P. Biofilm engineering approaches for improving the performance of microbial fuel cells and bioelectrochemical systems. Front. Energy Res. 2018, 6, 63. [Google Scholar] [CrossRef]
- Van Duijkeren, E.; Schink, A.K.; Roberts, M.C.; Wang, Y.; Schwarz, S. Mechanisms of bacterial resistance to antimicrobial agents. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Peterson, E.; Kaur, P. Antibiotic resistance mechanisms in bacteria: Relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens. Front. Microbiol. 2018, 9, 2928. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, J.A.; Lopez, C.; Babanova, S.; Santoro, C.; Artyushkoya, K.; Ista, L.; Schuler, A.J.; Atanassov, P. Surface modification for enhanced biofilm formation and electron transport in shewanella anodes. J. Electrochem. Soc. 2015, 162, H597–H603. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Polo, A.; Foladori, P.; Ponti, B.; Bettinetti, R.; Gambino, M.; Villa, F.; Cappitelli, F. Evaluation of zosteric acid for mitigating biofilm formation of pseudomonas putida isolated from a membrane bioreactor system. Int. J. Mol. Sci. 2014, 15, 9497–9518. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Alshammari, E.; Patel, M.; Ashraf, S.A.; Khan, S.; Hadi, S. Significance and potential of marine microbial natural bioactive compounds against biofilms/biofouling: Necessity for green chemistry. PeerJ 2018, 6, e5049. [Google Scholar] [CrossRef]
- Bandaranayake, W.M. Bioactivities, bioactive compounds and chemical constituents of mangrove plants. Wetl. Ecol. Manag. 2002, 10, 421–452. [Google Scholar] [CrossRef]
- Rastegar, S.; Gozari, M. Effect of mangrove plant extract on growth of four fungal pathogens. J. Paramed. Sci. 2017, 8, 2008–4978. [Google Scholar] [CrossRef]
- Boik, J.; Kirakosyan, A.; Kaufman, P.B.; Mitchell Seymour, E.; Spelman, K. Interactions of bioactive plant metabolites: Synergism, antagonism, and additivity. In Recent Advances in Plant Biotechnology; Kirakosyan, A., Kaufman, P.B., Eds.; Springer: Boston, MA, USA, 2009; pp. 213–230. [Google Scholar]
- Glasenapp, Y.; Korth, I.; Nguyen, X.V.; Papenbrock, J. Sustainable use of mangroves as sources of valuable medicinal compounds: Species identification, propagation and secondary metabolite composition. S. Afr. J. Bot. 2019, 121, 317–328. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, C.M.; Ruiz-Ruiz, J.C.; Peraza-Echeverria, L.; Peraza-Sanchez, S.R.; Torres-Tapia, L.W.; Perez-Brito, D.; Tapia-Tussell, R.; Herrera-Chale, F.G.; Segura-Campos, M.R.; Quijano-Ramayo, A.; et al. Antioxidant, antihypertensive, anti-hyperglycemic, and antimicrobial activity of aqueous extracts from twelve native plants of the Yucatan coast. PLoS ONE 2019, 14, e0213493. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.H.; Chang, F.R.; Hua, M.Y.; Wu, Y.C.; Liu, S.T. Inhibitory effects of 1,2,3,4,6-Penta-O-Galloyl-beta-D-Glucopyranose on biofilm formation by Staphylococcus aureus. Antimicrob. Agents Chemother. 2011, 55, 1021–1027. [Google Scholar] [CrossRef]
- Das, M.C.; Sandhu, P.; Gupta, P.; Rudrapaul, P.; De, U.C.; Tribedi, P.; Akhter, Y.; Bhattacharjee, S. Attenuation of Pseudomonas aeruginosa biofilm formation by vitexin: A combinatorial study with azithromycin and gentamicin. Sci. Rep. 2016, 6, 23347. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.A.A.; Rodrigues, J.B.D.; Magnani, M.; de Souza, E.L.; de Siqueira, J.P. Inhibitory effects of flavonoids on biofilm formation by Staphylococcus aureus that overexpresses efflux protein genes. Microb. Pathog. 2017, 107, 193–197. [Google Scholar] [CrossRef]
- Arita-Morioka, K.; Yamanaka, K.; Mizunoe, Y.; Tanaka, Y.; Ogura, T.; Sugimoto, S. Inhibitory effects of myricetin derivatives on curli-dependent biofilm formation in Escherichia coli. Sci. Rep. 2018, 8, 8452. [Google Scholar] [CrossRef] [PubMed]
- Pejin, B.; Ciric, A.; Markovic, J.D.; Glamoclija, J.; Nikolic, M.; Sokovic, M. An insight into anti-biofilm and anti-quorum sensing activities of the selected anthocyanidins: The case study of Pseudomonas aeruginosa PAO1. Nat. Prod. Res. 2017, 31, 1177–1180. [Google Scholar] [CrossRef]
- Wu, C.Y.; Su, T.Y.; Wang, M.Y.; Yang, S.F.; Mar, K.; Hung, S.L. Inhibitory effects of tea catechin epigallocatechin-3-gallate against biofilms formed from Streptococcus mutans and a probiotic lactobacillus strain. Arch. Oral Biol. 2018, 94, 69–77. [Google Scholar] [CrossRef] [PubMed]
- De Vita, D.; Friggeri, L.; D’Auria, F.D.; Pandolfi, F.; Piccoli, F.; Panella, S.; Palamara, A.T.; Simonetti, G.; Scipione, L.; Santo, R.; et al. Activity of caffeic acid derivatives against Candida albicans biofilm. Bioorg. Med. Chem. Lett. 2014, 24, 1502–1505. [Google Scholar] [CrossRef] [PubMed]
- Lindow, S.E.; Brandl, M.T. Microbiology of the phyllosphere. Appl. Environ. Microbiol. 2003, 69, 1875–1883. [Google Scholar] [CrossRef]
- Harding, M.W.; Marques, L.L.R.; Howard, R.J.; Olson, M.E. Can filamentous fungi form biofilms? Trends Microbiol. 2009, 17, 475–480. [Google Scholar] [CrossRef]
- Borges, A.; Abreu, A.C.; Dias, C.; Saavedra, M.J.; Borges, F.; Simoes, M. New perspectives on the use of phytochemicals as an emergent strategy to control bacterial infections including biofilms. Molecules 2016, 21, 877. [Google Scholar] [CrossRef]
- Nebula, M.; Harisankar, H.S.; Chandramohanakumar, N. Metabolites and bioactivities of Rhizophoraceae mangroves. Nat. Prod. Bioprospect. 2013, 3, 207–232. [Google Scholar] [CrossRef] [Green Version]
- Lazarini, J.G.; Sardi, J.D.O.; Franchin, M.; Nani, B.D.; Freires, I.A.; Infante, J.; Paschoal, J.A.R.; de Alencar, S.M.; Rosalen, P.L. Bioprospection of Eugenia brasiliensis, a Brazilian native fruit, as a source of anti-inflammatory and antibiofilm compounds. Biomed. Pharmacother. 2018, 102, 132–139. [Google Scholar] [CrossRef]
- Wu, Y.P.; Bai, J.R.; Grosu, E.; Zhong, K.; Liu, L.J.; Tang, M.M.; Huang, Y.N.; Gao, H. Inhibitory effect of 2r,3r-dihydromyricetin on biofilm formation by Staphylococcus aureus. Foodborne Pathog. Dis. 2018, 15, 475–480. [Google Scholar] [CrossRef]
- Rocha, G.R.; Salamanca, E.J.F.; de Barros, A.L.; Lobo, C.I.V.; Klein, M.I. Effect of tt-farnesol and myricetin on in vitro biofilm formed by Streptococcus mutans and Candida albicans. BMC Complement Altern. Med. 2018, 18, 61. [Google Scholar] [CrossRef]
- Hung, C.; Zhou, Y.Z.; Pinkner, J.S.; Dodson, K.W.; Crowley, J.R.; Heuser, J.; Chapman, M.R.; Hadjifrangiskou, M.; Henderson, J.P.; Hultgren, S.J. Escherichia coli biofilms have an organized and complex extracellular matrix structure. MBio 2013, 4, e00645-13. [Google Scholar] [CrossRef]
- Yang, G.Q.; Lin, J.; Zeng, E.Y.; Zhuang, L. Extraction and characterization of stratified extracellular polymeric substances in Geobacter biofilms. Bioresour. Technol. 2019, 276, 119–126. [Google Scholar] [CrossRef]
- Pierce, C.G.; Vila, T.; Romo, J.A.; Montelongo-Jauregui, D.; Wall, G.; Ramasubramanian, A.; Lopez-Ribot, J.L. The Candida albicans biofilm matrix: Composition, structure and function. J. Fungi 2017, 3, 14. [Google Scholar] [CrossRef]
- Reichhardt, C.; Joubert, L.M.; Clemons, K.V.; Stevens, D.A.; Cegelski, L. Integration of electron microscopy and solid-state NMR analysis for new views and compositional parameters of Aspergillus fumigatus biofilms. Med. Mycol. 2019, 57, S239–S244. [Google Scholar] [CrossRef]
- Calvillo-Medina, R.P.; Reyes-Grajeda, J.P.; Barba-Escoto, L.; Bautista-Hernandez, L.A.; Campos-Guillen, J.; Jones, G.H.; Bautist-de Lucio, V.M. Proteome analysis of biofilm produced by a Fusarium falciforme keratitis infectious agent. Microb. Pathog. 2019, 130, 232–241. [Google Scholar] [CrossRef]
- Ahimou, F.; Semmens, M.J.; Haugstad, G.; Novak, P.J. Effect of protein, polysaccharide, and oxygen concentration profiles on biofilm cohesiveness. Appl. Environ. Microbiol. 2007, 73, 2905–2910. [Google Scholar] [CrossRef] [PubMed]
- Danese, P.N.; Pratt, L.A.; Kolter, R. Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture. J. Bacteriol. 2000, 182, 3593–3596. [Google Scholar] [CrossRef] [PubMed]
- Gambino, M.; Cappitelli, F. Mini-review: Biofilm responses to oxidative stress. Biofouling 2016, 32, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Villa, F.; Remelli, W.; Forlani, F.; Gambino, M.; Landini, P.; Cappitelli, F. Effects of chronic sub-lethal oxidative stress on biofilm formation by Azotobacter vinelandii. Biofouling 2012, 28, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Andrews, K.J.; Jeon, B. Enhanced biofilm formation by ferrous and ferric iron through oxidative stress in Campylobacter jejuni. Front. Microbiol. 2018, 9, 1204. [Google Scholar] [CrossRef] [PubMed]
- Svenningsen, N.B.; Martinez-Garcia, E.; Nicolaisen, M.H.; de Lorenzo, V.; Nybroe, O. The biofilm matrix polysaccharides cellulose and alginate both protect Pseudomonas putida mt-2 against reactive oxygen species generated under matric stress and copper exposure. Microbiology 2018, 164, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Whipps, J.M.; Hand, P.; Pink, D.; Bending, G.D. Phyllosphere microbiology with special reference to diversity and plant genotype. J. Appl. Microbiol. 2008, 105, 1744–1755. [Google Scholar] [CrossRef] [Green Version]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef]
- Rastogi, G.; Coaker, G.L.; Leveau, J.H.J. New insights into the structure and function of phyllosphere microbiota through high-throughput molecular approaches. FEMS Microbiol. Lett. 2013, 348, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, S.D.; Castillo, J.A. Influence of light on plant-phyllosphere interaction. Front. Plant Sci. 2018, 9, 1482. [Google Scholar] [CrossRef]
- Rosier, A.; Medeiros, F.H.V.; Bais, H.P. Defining plant growth promoting rhizobacteria molecular and biochemical networks in beneficial plant-microbe interactions. Plant Soil 2018, 428, 35–55. [Google Scholar] [CrossRef] [Green Version]
- Hunter, P.J.; Hand, P.; Pink, D.; Whipps, J.M.; Bending, G.D. Both leaf properties and microbe-microbe interactions influence within-species variation in bacterial population diversity and structure in the lettuce (Lactuca species) phyllosphere. Appl. Environ. Microbiol. 2010, 76, 8117–8125. [Google Scholar] [CrossRef] [PubMed]
- Bringel, F.; Couee, I. Pivotal roles of phyllosphere microorganisms at the interface between plant functioning and atmospheric trace gas dynamics. Front. Microbiol. 2015, 6, 486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, D.L.; Subramanian, S.; Lamont, J.R.; Bywater-Ekegard, M. Signaling in the phytomicrobiome: Breadth and potential. Front. Plant Sci. 2015, 6, 709. [Google Scholar] [CrossRef] [PubMed]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Merillon, J.M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Dewanto, V.; Wu, X.Z.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Huang, D.J.; Ou, B.X.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate flourescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Gillespie, K.M.; Chae, J.M.; Ainsworth, E.A. Rapid measurement of total antioxidant capacity in plants. Nat. Protoc. 2007, 2, 867–870. [Google Scholar] [CrossRef]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef]
- Sawada, Y.; Nakabayashi, R.; Yamada, Y.; Suzuki, M.; Sato, M.; Sakata, A.; Akiyama, K.; Sakurai, T.; Matsuda, F.; Aoki, T.; et al. RIKEN tandem mass spectral database (ReSpect) for phytochemicals: A plant-specific MS/MS-based data resource and database. Phytochemistry 2012, 82, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Faucher, S.P.; Charette, S.J. Editorial on: Bacterial pathogens in the non-clinical environment. Front. Microbiol. 2015, 6, 331. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Cattò, C.; Grazioso, G.; Dell’Orto, S.; Gelain, A.; Villa, S.; Marzano, V.; Vitali, A.; Villa, F.; Cappitelli, F.; Forlani, F. The response of Escherichia coli biofilm to salicylic acid. Biofouling 2017, 33, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; Vantriet, K. Modeling of the bacterial-growth curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [PubMed]
- Cattò, C.; Dell’Orto, S.; Villa, F.; Villa, S.; Gelain, A.; Vitali, A. Unravelling the structural and molecular basis responsible for the anti-biofilm activity of zosteric acid. PLoS ONE 2015, 10, e0136124. [Google Scholar] [CrossRef] [PubMed]
- Villa, F.; Borgonovo, G.; Cappitelli, F.; Giussani, B.; Bassoli, A. Sub-lethal concentrations of Muscari comosum bulb extract suppress adhesion and induce detachment of sessile yeast cells. Biofouling 2012, 28, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical review on biofilm methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Lukowicz, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Brooks, T.D.; Wilson, E.; et al. Quantitative and qualitative assessment methods for biofilm growth: A mini-review. Res. Rev. J. Eng. Technol. 2017, 6. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.I.; Lee, Y.C. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, W.; Bilinski, T.; Bartosz, G. Oxidative stress during aging of stationary cultures of the yeast Saccharomyces cerevisiae. Free Radic. Biol. Med. 2000, 28, 659–664. [Google Scholar] [CrossRef]
- Spadoni-Andreani, E.; Villa, F.; Cappitelli, F.; Krasowska, A.; Biniarz, P.; Lukaszewicz, M.; Secundo, F. Coating polypropylene surfaces with protease weakens the adhesion and increases the dispersion of Candida albicans cells. Biotechnol. Lett. 2017, 39, 423–428. [Google Scholar] [CrossRef] [PubMed]






| B. Cylindrica Leaf Extract | ||||||
| No | RT | Mass | MS/MS | Name | Accession | Source |
| 1 | 2.5 | 341.11 | 179.0, 161.0, 119.0, 89.0, 71.0 | Galactinol | PT211910 | ReSpect |
| 2 | 7.8 | 353.08 | 191.05, 179.03, 135.04 | Caffeic acid mod. | - | - |
| 3 | 9.1 | 372.13 | 132.04, 59.01 | n. i. | - | - |
| 4 | 11.0 | 427.18 | 367.16, 221.10 | Hirsutine | TY000056 | MassBank |
| 5 | 11.9 | 456.15 | 310.09, 148.04, 132.04, 121.03 | n. i. | - | - |
| 6 | 14.2 | 535.16 | 475.14, 323.09, 221.06, 179.05, 151.04 | n. i. | - | - |
| 7 | 15.8 | 445.2 | 385.18, 205.12, 161.04, 153.09 | 1-O-b-D-glucopyranosyl sinapate mod. | PS118108 | ReSpect |
| 8 | 17.1 | 475.18 | 415.16, 269.10, 161.04 | n. i. | - | - |
| 9 | 19.2 | 447.09 | 429.08, 357.06, 327.05, 285.04 | Homoorientin | PT204250 | ReSpect |
| 10 | 19.8 | 577.16 | 413.09, 311.05, 293.04 | Vitexin-2″-O-rhamnoside | PT208750 | ReSpect |
| 11 | 21.0 | 577.15 | 413.09, 311.05, 293.04 | Vitexin-2″-O-rhamnoside Isomer | PT208750 | ReSpect |
| 12 | 24.3 | 461.11 | 341.06, 299.05, 283.02 | n. i. | TY000253 | MassBank |
| 13 | 31.4 | 293.17 | 236.10, 221.15, 205.12 | n. i. | - | - |
| 14 | 32.0 | 339.22 | 307.19, 289.18, 245.19 | n. i. | - | - |
| L. racemosa Leaf Extract | ||||||
| No | RT | Mass | MS/MS | Name | Accession | Source |
| 1 | 2.7 | 181.07 | 163.06, 119.03, 101.02, 89.02, 71.01 | Mannitol | PT211960 | Respect |
| 2 | 2.9 | 341.10 | 179.05, 161.04, 119.03, 89.02 | Galactinol | PT211910 | Respect |
| 3 | 5.3 | 331.06 | 271.04, 211.02, 169.01, 151.00 | n. i. | ||
| 4 | 8.4 | 305.06 | 261.07, 219.06, 165.01, 125.02 | (–)-Epigallocatechin | ML000151 | MassBank |
| 5 | 10.7 | 467.03 | 458.03, 343.01, 301.00, 275.02, 249.04, 169.01, 125.02 | Casuarinin | - | [21] |
| 6 | 11.7 | 305.07 | 225.11, 96.96 | n. i. | - | - |
| 7 | 13.3 | 183.03 | 168.00, 124.01, 106.00, 78.01 | (galloyl) Methyl gallate | PM012531, PM012533 | Respect |
| 8 | 14.2 | 457.08 | 305.06, 169.01, 125.02 | Epigallocatechin 3,5-digallate | 43930 | Metlin |
| 9 | 15.0 | 319.04 | 301.03, 257.04, 215.03, 193.01, 175.00, 125.02 | Delphinidin | Standard | |
| 10 | 16.1 | 303.09 | 96.96, 79.95 | n. i. | - | - |
| 11 | 21.1 | 463.09 | 316.02, 287.02, 271.02 | Myricitrin | PR040144 | MassBank |
| 12 | 22.2 | 601.09 | 449.07, 316.02, 179.00 | Myricetin-3-xyloside glycoside | PS093009 | Respect |
| 13 | 22.9 | 615.10 | 463.08, 317.03, 179.00 | Myricitrin glycoside | PR040144 | MassBank |
| 14 | 24.8 | 423.00 | 343.05, 328.02, 313.00, 297.97 | Robinetin trimethyl ether mod. | BML01849 | MassBank |
| 15 | 31.4 | 293.17 | 236.10, 221.15, 205.12, 192.11 | n. i. | - | - |
| E. coli | C. albicans | F. oxysporum | ||||
|---|---|---|---|---|---|---|
| B. cyl. | L. rac. | B. cyl. | L. rac. | B. cyl. | L. rac. | |
| ADHESION | + | − | − | − | − | − |
| MATURATION | ||||||
| Cellular biomass | − | − | + | − | = | − |
| EPS (polysaccharides + proteins) | + | + | = | + | + | + |
| EPS (polysaccharides/proteins) | + | + | + | + | + | + |
| Intracellular ROS | + | + | + | + | + | + |
| Extracellular ROS | + | + | − | + | + | + |
| DETACHMENT | + | + | + | − | − | + |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glasenapp, Y.; Cattò, C.; Villa, F.; Saracchi, M.; Cappitelli, F.; Papenbrock, J. Promoting Beneficial and Inhibiting Undesirable Biofilm Formation with Mangrove Extracts. Int. J. Mol. Sci. 2019, 20, 3549. https://doi.org/10.3390/ijms20143549
Glasenapp Y, Cattò C, Villa F, Saracchi M, Cappitelli F, Papenbrock J. Promoting Beneficial and Inhibiting Undesirable Biofilm Formation with Mangrove Extracts. International Journal of Molecular Sciences. 2019; 20(14):3549. https://doi.org/10.3390/ijms20143549
Chicago/Turabian StyleGlasenapp, Yvana, Cristina Cattò, Federica Villa, Marco Saracchi, Francesca Cappitelli, and Jutta Papenbrock. 2019. "Promoting Beneficial and Inhibiting Undesirable Biofilm Formation with Mangrove Extracts" International Journal of Molecular Sciences 20, no. 14: 3549. https://doi.org/10.3390/ijms20143549
APA StyleGlasenapp, Y., Cattò, C., Villa, F., Saracchi, M., Cappitelli, F., & Papenbrock, J. (2019). Promoting Beneficial and Inhibiting Undesirable Biofilm Formation with Mangrove Extracts. International Journal of Molecular Sciences, 20(14), 3549. https://doi.org/10.3390/ijms20143549