Silencing of the Chitin Synthase Gene Is Lethal to the Asian Citrus Psyllid, Diaphorina citri
Abstract
:1. Introduction
2. Results
2.1. Analysis of the cDNA and Protein Sequences of DcCHS
2.2. Spatiotemporal Expression Profiles of DcCHS
2.3. Effect of DFB on D. citri Survival and DcCHS Expression Level
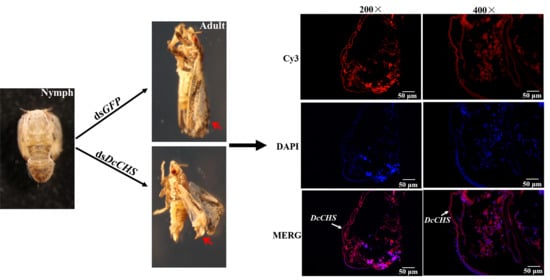
2.4. Localization of DcCHS Transcript by FISH
2.5. RNAi-Based Silencing of DcCHS and Epidermal Structure Analysis
3. Discussion
4. Materials and Methods
4.1. Diaphorina citri Rearing and Collection
4.2. RNA Isolation and cDNA Synthesis
4.3. Identification of DcCHS and Bioinformatics Analysis
4.4. RT-qPCR Analysis of DcCHS Expression Levels
4.5. Leaf-Dip Bioassay
4.6. dsRNA Synthesis and DcCHS RNAi Analysis
4.7. Fluorescence In Situ Hybridization (FISH) Analysis
4.8. Transmission Election Microscopy (TEM) Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CHS | Chitin synthase |
| TEM | Transmission electron microscopy |
| FISH | Fluorescence in situ hybridization |
| ACP | Asian citrus psyllid |
| CLas | Candidatus Liberibacter asiaticus |
| HLB | Huanglongbing |
| PM | Peritrophic membrane |
| RNAi | RNA interference |
| DFB | Diflubenzuron |
References
- Chen, X.D.; Ashfaq, M.; Stelinski, L.L. Susceptible of Asian citrus psyllid, Diaphorina citri (Hemiptera: Liviidae), to the insecticide afidopyropen: A new and potent modulator of insect transient receptor potential channels. Appl. Entomol. Zool. 2018, 53, 1–9. [Google Scholar] [CrossRef]
- Wang, N.; Pierson, E.A.; Setubal, J.C.; Xu, J.; Levy, J.G.; Zhang, Y.; Li, J.; Rangel, L.T.; Martins, J.J. The Candidatus liberibacter-host interface: Insights into pathogenesis mechanisms and disease control. Annu. Rev. Phytopathol. 2017, 55, 451–482. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.K.; Hu, R.F.; Pray, C.; Qiao, F.B.; Rozelle, S. Biotechnology as an alternative to chemical pesticides: A case study of Bt cotton in China. Agr. Econ. 2003, 29, 55–67. [Google Scholar] [CrossRef]
- Tiwari, S.; Clayson, P.J.; Kuhns, E.H.; Stelinski, L.L. Effects of buprofezin and diflubenzuron on various developmental stages of Asian citrus psyllid, Diaphorina citri. Pest. Manag. Sci. 2012, 68, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Merzendorfer, H.; Zimoch, L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 2003, 206, 4393–4412. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, H.W.; Huang, H.J.; Xue, J.; Wu, W.J.; Bao, Y.Y.; Xu, H.J.; Zhu, Z.R.; Cheng, J.A.; Zhang, C.X. Chitin synthase 1 gene and its two alternative splicing variants from two sap-sucking insects, Nilaparvata lugens and Laodelphax striatellus (Hemiptera: Delphacidae). Insect Biochem. Mol. Biol. 2012, 42, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Tobias, P.A.; Christie, N.; Naidoo, S.; Guest, D.I.; Kulheim, C. Identification of the Eucalyptus grandis chitinase gene family and expression characterization under different biotic stress challenges. Tree Physiol. 2017, 37, 565–582. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.Y.; Merzendorfer, H.; Zhang, W.Q.; Zhang, J.Z.; Muthukrishnan, S. Biosynthesis, turnover, and functions of chitin in insects. Annu. Rev. Entomol. 2016, 61, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Choi, K.H.; Kramer, K.J.; Muthukrishnan, S. Isolation and characterization of a genomic clone for the gene of an insect molting enzyme, chitinase. Insect Biochem. Mol. Biol. 1997, 27, 37–47. [Google Scholar] [CrossRef]
- Merzendorfer, H. Insect chitin synthases: A review. J. Comp. Physiol. B. 2006, 176, 1–15. [Google Scholar] [CrossRef]
- Mansur, J.F.; Alvarenga, E.S.; Figueira-Mansur, J.; Franco, T.A.; Ramos, I.B.; Masuda, H.; Melo, A.C.; Moreira, M.F. Effects of chitin synthase double-stranded RNA on molting and oogenesis in the Chagas disease vector Rhodnius prolixus. Insect Biochem. Mol. Biol. 2014, 51, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Zuber, R.; Oehl, K.; Norum, M.; Moussian, B. Report on Drosophila melanogaster larvae without functional tracheae. J. Zool. 2015, 296, 139–145. [Google Scholar] [CrossRef]
- Zhuo, W.W.; Fang, Y.; Kong, L.F.; Li, X.; Sima, Y.H.; Xu, S.Q. Chitin synthase A: A novel epidermal development regulation gene in the larvae of Bombyx mori. Mol. Biol. Rep. 2014, 41, 4177–4186. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, W.W.; Chu, F.; Kong, L.F.; Tao, H.; Sima, Y.H.; Xu, S.Q. Chitin synthase B: A midgut-specific gene induced by insect hormones and involved in food intake in Bombyx mori larvae. Arch. Insect Biochem. Physiol. 2014, 85, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Arakane, Y.; Muthukrishnan, S.; Kramer, K.J.; Specht, C.A.; Tomoyasu, Y.; Lorenzen, M.D.; Kanost, M.; Beeman, R.W. The Tribolium chitin synthase genes TcCHS1 and TcCHS2 are specialized for synthesis of epidermal cuticle and midgut peritrophic matrix. Insect Mol. Biol. 2005, 14, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.P.; Silva, J.R.; Vasconcelos, F.F.; Petretski, M.D.; Damatta, R.A.; Ribeiro, A.F.; Terra, W.R. Occurrence of midgut perimicrovillar membranes in paraneopteran insect orders with comments on their function and evolutionary significance. Arthropod Struct. Dev. 2004, 33, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E. Chitin synthesis and inhibition: A revisit. Pest. Manag. Sci. 2001, 57, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Mian, M.A.; Mittapalli, O.; Michel, A.P. Characterization of a chitin synthase encoding gene and effect of diflubenzuron in soybean aphid, Aphis glycines. Int. J. Biol. Sci. 2012, 8, 1323–1334. [Google Scholar] [CrossRef]
- Shang, F.; Xiong, Y.; Xia, W.K.; Wei, D.D.; Wei, D.; Wang, J.J. Identification, characterization and functional analysis of a chitin synthase gene in the brown citrus aphid, Toxoptera citricida (Hemiptera, Aphididae). Insect Mol. Biol. 2016, 25, 422–430. [Google Scholar] [CrossRef]
- Merzendorfer, H. The cellular basis of chitin synthesis in fungi and insects: Common principles and differences. Eur. J. Cell Biol. 2011, 90, 759–769. [Google Scholar] [CrossRef]
- Zimoch, L.; Merzendorfer, H. Immunolocaliation of chitin synthase in the tobacco hornworm. Cell Tissue Res. 2002, 308, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Terra, W.R.; Ferreira, C. Insect digestive enzymes: Properties, compartmentalization and function. Comp. Biochem. Phys. B. 1994, 109, 1–62. [Google Scholar] [CrossRef]
- Burkhard, P.; Stetefeld, J.; Strelkov, S.V. Coiled coils: A highly versatile protein folding motif. Trends Cell Biol. 2001, 11, 82–88. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Specht, C.A.; Dittmer, N.T.; Muthukrishnan, S.; Kanost, M.R.; Kramer, K.J. Sequence of a cDNA and expression of the gene encoding a putative epidermal chitin synthase of Manduca sexta. Insect Biochem. Mol. Biol. 2002, 32, 1497–1506. [Google Scholar] [CrossRef]
- Chen, X.F.; Yang, X.; Kumar, N.S.; Tang, B.; Sun, X.J.; Qiu, X.M.; Hu, J.; Zhang, W.Q. The class A chitin synthase gene of Spodoptera exigua: Molecular cloning and expression patterns. Insect Biochem. Mol. Biol. 2007, 37, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Arakane, Y.; Hogenkamp, D.G.; Zhu, Y.C.; Kramer, K.J.; Specht, C.A.; Beeman, R.W.; Kanost, M.R.; Muthukrishnan, S. Characterization of two chitin synthase genes of the red flour beetle, Tribolium castaneum, and alternate exon usage in one of the genes during development. Insect Biochem. Mol. Biol. 2004, 34, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, J.Z.; Zhu, K.Y. Chitosan/double-stranded RNA nanoparticle-mediated RNA interference to silencing chitin synthase genes through larval feeding in the African malaria mosquito (Anopheles gambiae). Insect Mol. Biol. 2010, 19, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, C.; Long, G.Y.; Yang, H.; Jin, D.C. Sublethal effects of buprofezin on development, reproduction, and chitin synthase 1 gene (SfCHS1) expression in the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). J. Asia Pac. Entomol. 2018, 21, 585–591. [Google Scholar] [CrossRef]
- Ashfaq, M.; Sonoda, S.; Tsumuki, H. Developmental and tissue-specific expression of CHS1 from Plutella xylostella and its response to chlorfluazuron. Pestic Biochem. Phys. 2007, 89, 20–30. [Google Scholar] [CrossRef]
- Boina, D.R.; Rogers, M.E.; Wang, N.; Stelinski, L.L. Effect of pyriproxyfen, a juvenile hormone mimic, on egg hatch, nymph development, adult emergence and reproduction of the Asian citrus psyllid, Diaphorina citri. Pest. Manag. Sci. 2010, 66, 349–357. [Google Scholar] [CrossRef]
- Mayer, R.T.; Chen, A.C.; Deloach, J.R. Chitin synthesis inhibiting insect growth regulators do not inhibit chitin synthase. Experientia 1981, 37, 337–338. [Google Scholar] [CrossRef]
- Xie, W.K.; Ding, T.B.; Niu, J.Z.; Liao, C.Y.; Zhong, R.; Yang, W.J.; Liu, B.; Dou, W.; Wang, J.J. Exposure to diflubenzuron results in an up-regulation of a chitin synthase 1 gene in citrus red mite, Panonychus citri (Acari: Tetranychidae). Int. J. Mol. Sci. 2014, 15, 3711–3728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z.; Zhu, K.Y. Characterization of a chitin synthase cDNA and its increased mRNA level associated with decreased chitin synthesis in Anopheles quadrimaculatus exposed to diflubenzuron. Insect Biochem. Mol. Biol. 2006, 36, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Huvenne, H.; Smagghe, G. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: A review. J. Insect Physiol. 2010, 56, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Song, H.F.; Fan, Y.H.; Zhang, J.Q.; Cooper, A.M.; Silver, K.; Li, D.Q.; Li, T.; Ma, E.B.; Zhu, K.Y.; Zhang, J.Z. Contributions of dsRNases to differential RNAi efficiencies between the injection and oral delivery of dsRNA in Locusta migratoria. Pest. Manag. Sci. 2019, 75, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.D.; Killiny, N. Effect of parental RNA interference of a transformer-2 homologue on female reproduction and offspring sex determination in Asian citrus psyllid. Physiol. Entomol. 2018, 43, 42–50. [Google Scholar] [CrossRef]
- Kishk, A.; Anber, H.A.; AbdEI-Raof, T.K.; EI-Sherbeni, A.D.; Hamed, S.; Gowda, S.; Killiny, N. RNA interference of carboxyesterases causes nymph mortality in the Asian citrus psyllid, Diaphorina citri. Arch. Insect Biochem. Physiol. 2017, 94, e21377. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.G.; Peng, H.; Yao, Q.; Chen, H.X.; Xie, Q.; Tang, B.; Zhang, W.Q. Developmental control of a Lepidopteran pest Spodoptera exigua by ingestion of bacteria expressing dsRNA of a non-midgut gene. PLoS ONE 2009, 4, e6225. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.B.; Yang, Q. A novel alternative splicing site of class A chitin synthase from the insect Ostrinia furnacalis-Gene organization, expression pattern and physiological significance. Insect Biochem. Mol. Biol. 2011, 41, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Rudall, K.M. The chitin/protein complexes of insect cuticle. Adv. Insect Physiol. 1963, 1, 257–313. [Google Scholar]
- Wu, Z.Z.; Zhang, H.; Bin, S.Y.; Chen, L.; Han, Q.X.; Lin, J.T. Antennal and abdominal transcriptomes reveal chemosensory genes in the Asian Citrus psyllid, Diaphorina citri. PLoS ONE 2016, 11, e0159372. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.J.; Zhou, C.H.; Yu, H.Z.; Huang, Y.L.; Liu, Y.X.; Xie, Y.X.; Wang, J.; Hu, P.; Hu, W.; Huang, A.J.; et al. Potential roles of insect tropomyosin1-X1 isoform in the process of Candidatus Liberibacter asiaticus infection of Diaphorina citri. J. Insect Physiol. 2019, 114, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, J.Z.; Park, Y.; Zhu, K.Y. Identification and characterization of two chitin synthase genes in African malaria mosquito, Anopheles gambiae. Insect Biochem. Mol. Biol. 2012, 42, 674–682. [Google Scholar] [CrossRef] [PubMed]








| Primers | Sequences | Purpose |
|---|---|---|
| DcCHS-RT-F | TCAGCATGGCGGGTTAAG | RT-qPCR |
| DcCHS-RT-R | CTCCGCGGAATGACATGAATA | |
| GAPDH-F | CATGGCAAGTTCAACGGTGA | |
| GAPDH-R | CGATGCCTTCTCAATGGTGG | |
| ds-DcCHS-F | TAATACGACTCACTATAGGGAGACAGGAAGGAGGTTATG | dsRNA synthesis |
| ds-DcCHS-R | TAATACGACTCACTATAGGGCATCTGGTGTAAGCGTCA | |
| ds-GFP-F | GGATCCTAATACGACTCACTATAGGCAGTGCTTCAGCCGCTACCC | |
| ds-GFP-R | GGATCCTAATACGACTCACTATAGGACTCCAGCAGGACCATGTGAT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.-J.; Huang, Y.-L.; Yu, H.-Z.; Li, N.-Y.; Xie, Y.-X.; Zhang, Q.; Zeng, X.-D.; Hu, H.; Huang, A.-J.; Yi, L.; et al. Silencing of the Chitin Synthase Gene Is Lethal to the Asian Citrus Psyllid, Diaphorina citri. Int. J. Mol. Sci. 2019, 20, 3734. https://doi.org/10.3390/ijms20153734
Lu Z-J, Huang Y-L, Yu H-Z, Li N-Y, Xie Y-X, Zhang Q, Zeng X-D, Hu H, Huang A-J, Yi L, et al. Silencing of the Chitin Synthase Gene Is Lethal to the Asian Citrus Psyllid, Diaphorina citri. International Journal of Molecular Sciences. 2019; 20(15):3734. https://doi.org/10.3390/ijms20153734
Chicago/Turabian StyleLu, Zhan-Jun, Yu-Ling Huang, Hai-Zhong Yu, Ning-Yan Li, Yan-Xin Xie, Qin Zhang, Xiang-Dong Zeng, Hao Hu, Ai-Jun Huang, Long Yi, and et al. 2019. "Silencing of the Chitin Synthase Gene Is Lethal to the Asian Citrus Psyllid, Diaphorina citri" International Journal of Molecular Sciences 20, no. 15: 3734. https://doi.org/10.3390/ijms20153734
APA StyleLu, Z.-J., Huang, Y.-L., Yu, H.-Z., Li, N.-Y., Xie, Y.-X., Zhang, Q., Zeng, X.-D., Hu, H., Huang, A.-J., Yi, L., & Su, H.-N. (2019). Silencing of the Chitin Synthase Gene Is Lethal to the Asian Citrus Psyllid, Diaphorina citri. International Journal of Molecular Sciences, 20(15), 3734. https://doi.org/10.3390/ijms20153734