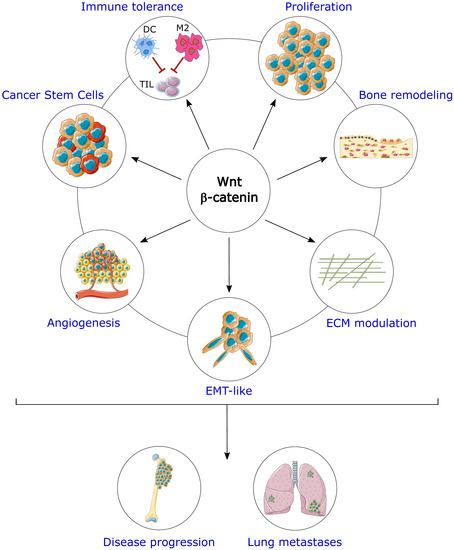
New Insights about the Wnt/β-Catenin Signaling Pathway in Primary Bone Tumors and Their Microenvironment: A Promising Target to Develop Therapeutic Strategies?
Abstract
:1. Primary Bone Tumors: Osteosarcoma and Ewing Sarcoma
2. Tumor Microenvironment: Crucial Role in Bone Sarcoma Tumor Growth and Metastatic Progression
2.1. Hijacking of the Bone Tumor Microenvironment by Bone Sarcoma Cells
2.2. Bone Sarcoma Microenvironment as a Prognostic Marker or Therapeutic Target
3. The Canonical Wnt/β-Catenin Signaling Pathway
4. Key Role of Wnt/β-Catenin Signaling Pathway in Osteosarcoma
5. Involvement of Wnt/β-Catenin Signaling Pathway in Ewing Sarcoma
6. Wnt/β-Catenin Pathway and the Bone Tumor Microenvironment
6.1. Wnt/β-Catenin Pathway and Bone Remodeling
6.2. Wnt/β-Catenin Pathway, Angiogenesis and Hypoxia
6.3. Wnt/β-Catenin Pathway and the Immune System
7. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| APC | Adenomatous Polyposis Coli |
| BCL-9 | B cell CLL/lymphoma 9 |
| β-TrCP | beta-Transducin-repeat-Containing Protein |
| BRG1 | Brahma-related gene 1 |
| CBP | CREB (cAMP-Response Element Binding protein) binding protein |
| CCL | C-C motif ligand |
| CK1α | Casein Kinase 1 alpha |
| CSC | Cancer Stem Cell |
| CXCL12 | C-X-C Motif Chemokine Ligand 12 |
| CXCR | C-X-C Motif Chemokine Receptor |
| DC | Dendritic cell |
| DKK | Dickkopf |
| Dvl | Dishevelled |
| EMT | Epithelial-Mesenchymal Transition |
| ETS | E26 Transformation-Specific |
| Fzd | Frizzled |
| GSK3β | Glycogen synthase kinase 3 beta |
| HDAC | histone deacetylase |
| HHLA2 | HERV-H LTR-associating 2 |
| HIF-1 | Hypoxia Inducible Factor 1 |
| IFNγ | Interferon γ |
| IGFBP4 | Insulin-like Growth Factor Binding Protein 4 |
| iNOS | inducible Nitric Oxide Synthase |
| LEF | Lymphoid Enhancer-binding Factor |
| LGR5 | Leucine Rich Repeat Containing G Protein-Coupled Receptor 5 |
| lncRNA | long non-coding RNA |
| LRP | Lipoprotein Receptor-related Protein |
| miR | miRNA |
| MMP | Matrix Metalloproteinase |
| MSC | Mesenchymal Stem Cell |
| MT1-MMP | Membrane type 1 metalloprotease |
| NK | Natural killer |
| NKD2 | Naked cuticle homolog 2 |
| PCP | Planar Cell Polarity |
| PD-1 | Programmed cell Death 1 |
| PDL-1 | Programmed cell Death Ligand 1 |
| PLOD2 | Procollagen-Lysine, 2-Oxoglutarate 5-Dioxygenase 2 |
| ROR | Receptor tyrosine-kinase-like Orphan Receptor |
| RSPO | R-spondin |
| Ryk | Related to tyrosine kinase |
| SFRP | Secreted-Fzd-Related Protein |
| SOST | Sclerostin |
| STAT3 | Signal transducer and activator of transcription 3 |
| TAF | Tumor Associated Fibroblast |
| TAM | Tumor Associated Macrophage |
| TAZ | Transcriptional co-Activator with a PDZ-binding domain |
| TCF | T-Cell Factor |
| TGFβ | Transforming Growth Factor beta |
| TLE | Transducin-Like Enhancer of split |
| TIL | Tumor Infiltrating Lymphocyte |
| TME | Tumor MicroEnvironment |
| VEGF | Vascular Endothelial Growth Factor |
| WIF1 | Wnt Inhibitory Factor 1 |
| WTX | Wilms tumor gene on X chromosome |
| YAP | Yes-Associated Protein |
References
- Ladenstein, R.; Pötschger, U.; Le Deley, M.C.; Whelan, J.; Paulussen, M.; Oberlin, O.; van den Berg, H.; Dirksen, U.; Hjorth, L.; Michon, J.; et al. Primary disseminated multifocal Ewing sarcoma: Results of the Euro-EWING 99 trial. J. Clin. Oncol. 2010, 28, 3284–3291. [Google Scholar] [CrossRef] [PubMed]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. International osteosarcoma incidence patterns in children and adolescents, middle ages, and elderly persons. Int. J. Cancer 2009, 125, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Galindo, C.; Navid, F.; Liu, T.; Billups, C.A.; Rao, B.N.; Krasin, M.J. Prognostic factors for local and distant control in Ewing sarcoma family of tumors. Ann. Oncol. 2008, 19, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Strauss, S.J.; Whelan, J.S. Current questions in bone sarcomas. Curr. Opin. Oncol. 2018, 30, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Delattre, O.; Zucman, J.; Plougastel, B.; Desmaze, C.; Melot, T.; Peter, M.; Kovar, H.; Joubert, I.; de Jong, P.; Rouleau, G. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature 1992, 359, 162–165. [Google Scholar] [CrossRef]
- Delattre, O.; Zucman, J.; Melot, T.; Garau, X.S.; Zucker, J.M.; Lenoir, G.M.; Ambros, P.F.; Sheer, D.; Turc-Carel, C.; Triche, T.J. The Ewing family of tumors—A subgroup of small-round-cell tumors defined by specific chimeric transcripts. N. Engl. J. Med. 1994, 331, 294–299. [Google Scholar] [CrossRef]
- Ginsberg, J.P.; de Alava, E.; Ladanyi, M.; Wexler, L.H.; Kovar, H.; Paulussen, M.; Zoubek, A.; Dockhorn-Dworniczak, B.; Juergens, H.; Wunder, J.S.; et al. EWS-FLI1 and EWS-ERG gene fusions are associated with similar clinical phenotypes in Ewing’s sarcoma. J. Clin. Oncol. 1999, 17, 1809–1814. [Google Scholar] [CrossRef]
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef]
- Crenn, V.; Biteau, K.; Amiaud, J.; Dumars, C.; Guiho, R.; Vidal, L.; Nail, L.-R.L.; Heymann, D.; Moreau, A.; Gouin, F.; et al. Bone microenvironment has an influence on the histological response of osteosarcoma to chemotherapy: Retrospective analysis and preclinical modeling. Am. J. Cancer Res. 2017, 7, 2333–2349. [Google Scholar]
- Alfranca, A.; Martinez-Cruzado, L.; Tornin, J.; Abarrategi, A.; Amaral, T.; de Alava, E.; Menendez, P.; Garcia-Castro, J.; Rodriguez, R. Bone microenvironment signals in osteosarcoma development. Cell. Mol. Life Sci. 2015, 72, 3097–3113. [Google Scholar] [CrossRef]
- Klemm, F.; Joyce, J.A. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015, 25, 198–213. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Jin, H.; Barrott, J.J.; Cable, M.G.; Monument, M.J.; Lerman, D.M.; Smith-Fry, K.; Nollner, D.; Jones, K.B. The Impact of Microenvironment on the Synovial Sarcoma Transcriptome. Cancer Microenviron 2017, 10, 1–7. [Google Scholar] [CrossRef]
- Goldstein, S.D.; Hayashi, M.; Albert, C.M.; Jackson, K.W.; Loeb, D.M. An orthotopic xenograft model with survival hindlimb amputation allows investigation of the effect of tumor microenvironment on sarcoma metastasis. Clin. Exp. Metastasis 2015, 32, 703–715. [Google Scholar] [CrossRef]
- Riemann, A.; Schneider, B.; Gündel, D.; Stock, C.; Gekle, M.; Thews, O. Acidosis Promotes Metastasis Formation by Enhancing Tumor Cell Motility. Adv. Exp. Med. Biol. 2016, 876, 215–220. [Google Scholar]
- Chattopadhyay, S.; Chaklader, M.; Chatterjee, R.; Law, A.; Law, S. Differential expression of mitotic regulators and tumor microenvironment influences the regional growth pattern of solid sarcoma along the cranio-caudal axis. Exp. Cell Res. 2016, 340, 91–101. [Google Scholar] [CrossRef]
- Lamoureux, F.; Richard, P.; Wittrant, Y.; Battaglia, S.; Pilet, P.; Trichet, V.; Blanchard, F.; Gouin, F.; Pitard, B.; Heymann, D.; et al. Therapeutic relevance of osteoprotegerin gene therapy in osteosarcoma: Blockade of the vicious cycle between tumor cell proliferation and bone resorption. Cancer Res. 2007, 67, 7308–7318. [Google Scholar] [CrossRef]
- Picarda, G.; Matous, E.; Amiaud, J.; Charrier, C.; Lamoureux, F.; Heymann, M.-F.; Tirode, F.; Pitard, B.; Trichet, V.; Heymann, D.; et al. Osteoprotegerin inhibits bone resorption and prevents tumor development in a xenogenic model of Ewing’s sarcoma by inhibiting RANKL. J. Bone Oncol. 2013, 2, 95–104. [Google Scholar] [CrossRef]
- Taylor, R.; Knowles, H.J.; Athanasou, N.A. Ewing sarcoma cells express RANKL and support osteoclastogenesis. J. Pathol. 2011, 225, 195–202. [Google Scholar] [CrossRef]
- Dass, C.R.; Choong, P.F.M. Zoledronic acid inhibits osteosarcoma growth in an orthotopic model. Mol. Cancer Ther. 2007, 6, 3263–3270. [Google Scholar] [CrossRef]
- Heymann, D.; Ory, B.; Blanchard, F.; Heymann, M.-F.; Coipeau, P.; Charrier, C.; Couillaud, S.; Thiery, J.P.; Gouin, F.; Redini, F. Enhanced tumor regression and tissue repair when zoledronic acid is combined with ifosfamide in rat osteosarcoma. Bone 2005, 37, 74–86. [Google Scholar] [CrossRef]
- Odri, G.A.; Dumoucel, S.; Picarda, G.; Battaglia, S.; Lamoureux, F.; Corradini, N.; Rousseau, J.; Tirode, F.; Laud, K.; Delattre, O.; et al. Zoledronic acid as a new adjuvant therapeutic strategy for Ewing’s sarcoma patients. Cancer Res. 2010, 70, 7610–7619. [Google Scholar] [CrossRef]
- Ory, B.; Heymann, M.-F.; Kamijo, A.; Gouin, F.; Heymann, D.; Redini, F. Zoledronic acid suppresses lung metastases and prolongs overall survival of osteosarcoma-bearing mice. Cancer 2005, 104, 2522–2529. [Google Scholar] [CrossRef]
- Han, Y.; Wu, C.; Wang, J.; Liu, N. CXCR7 maintains osteosarcoma invasion after CXCR4 suppression in bone marrow microenvironment. Tumour Biol. 2017, 39, 101042831770163. [Google Scholar] [CrossRef]
- Li, Y.-J.; Dai, Y.-L.; Zhang, W.-B.; Li, S.-J.; Tu, C.-Q. Clinicopathological and prognostic significance of chemokine receptor CXCR4 in patients with bone and soft tissue sarcoma: A meta-analysis. Clin. Exp. Med. 2017, 17, 59–69. [Google Scholar] [CrossRef]
- Perissinotto, E.; Cavalloni, G.; Leone, F.; Fonsato, V.; Mitola, S.; Grignani, G.; Surrenti, N.; Sangiolo, D.; Bussolino, F.; Piacibello, W.; et al. Involvement of chemokine receptor 4/stromal cell-derived factor 1 system during osteosarcoma tumor progression. Clin. Cancer Res. 2005, 11, 490–497. [Google Scholar]
- Cortini, M.; Massa, A.; Avnet, S.; Bonuccelli, G.; Baldini, N. Tumor-Activated Mesenchymal Stromal Cells Promote Osteosarcoma Stemness and Migratory Potential via IL-6 Secretion. PLoS ONE 2016, 11, e0166500. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Q.; Liu, T.; Guan, G.; Zhang, K.; Chen, J.; Jia, N.; Yan, S.; Chen, G.; Liu, S.; et al. Interleukin-6 suppression reduces tumour self-seeding by circulating tumour cells in a human osteosarcoma nude mouse model. Oncotarget 2016, 7, 446–458. [Google Scholar] [CrossRef]
- Qi, J.; Zhou, Y.; Jiao, Z.; Wang, X.; Zhao, Y.; Li, Y.; Chen, H.; Yang, L.; Zhu, H.; Li, Y. Exosomes Derived from Human Bone Marrow Mesenchymal Stem Cells Promote Tumor Growth Through Hedgehog Signaling Pathway. Cell. Physiol. Biochem. 2017, 42, 2242–2254. [Google Scholar] [CrossRef]
- Wang, Y.; Chu, Y.; Yue, B.; Ma, X.; Zhang, G.; Xiang, H.; Liu, Y.; Wang, T.; Wu, X.; Chen, B. Adipose-derived mesenchymal stem cells promote osteosarcoma proliferation and metastasis by activating the STAT3 pathway. Oncotarget 2017, 8, 23803–23816. [Google Scholar] [CrossRef]
- Heymann, M.-F.; Lézot, F.; Heymann, D. The contribution of immune infiltrates and the local microenvironment in the pathogenesis of osteosarcoma. Cell. Immunol. 2017. [Google Scholar] [CrossRef]
- Noy, R.; Pollard, J.W. Tumor-associated macrophages: From mechanisms to therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef]
- Gomez-Brouchet, A.; Illac, C.; Gilhodes, J.; Bouvier, C.; Aubert, S.; Guinebretiere, J.-M.; Marie, B.; Larousserie, F.; Entz-Werlé, N.; de Pinieux, G.; et al. CD163-positive tumor-associated macrophages and CD8-positive cytotoxic lymphocytes are powerful diagnostic markers for the therapeutic stratification of osteosarcoma patients: An immunohistochemical analysis of the biopsies fromthe French OS2006 phase 3 trial. Oncoimmunology 2017, 6, e1331193. [Google Scholar]
- Buddingh, E.P.; Kuijjer, M.L.; Duim, R.A.J.; Bürger, H.; Agelopoulos, K.; Myklebost, O.; Serra, M.; Mertens, F.; Hogendoorn, P.C.W.; Lankester, A.C.; et al. Tumor-infiltrating macrophages are associated with metastasis suppression in high-grade osteosarcoma: A rationale for treatment with macrophage activating agents. Clin. Cancer Res. 2011, 17, 2110–2119. [Google Scholar] [CrossRef]
- Dumars, C.; Ngyuen, J.-M.; Gaultier, A.; Lanel, R.; Corradini, N.; Gouin, F.; Heymann, D.; Heymann, M.-F. Dysregulation of macrophage polarization is associated with the metastatic process in osteosarcoma. Oncotarget 2016, 7, 78343–78354. [Google Scholar] [CrossRef]
- Zhou, Q.; Xian, M.; Xiang, S.; Xiang, D.; Shao, X.; Wang, J.; Cao, J.; Yang, X.; Yang, B.; Ying, M.; et al. All-Trans Retinoic Acid Prevents Osteosarcoma Metastasis by Inhibiting M2 Polarization of Tumor-Associated Macrophages. Cancer Immunol. Res. 2017, 5, 547–559. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, T.; Fukushi, J.; Yamamoto, S.; Matsumoto, Y.; Setsu, N.; Oda, Y.; Yamada, H.; Okada, S.; Watari, K.; Ono, M.; et al. Macrophage infiltration predicts a poor prognosis for human ewing sarcoma. Am. J. Pathol. 2011, 179, 1157–1170. [Google Scholar] [CrossRef]
- Handl, M.; Hermanova, M.; Hotarkova, S.; Jarkovsky, J.; Mudry, P.; Shatokhina, T.; Vesela, M.; Sterba, J.; Zambo, I. Clinicopathological correlation of tumor-associated macrophages in Ewing sarcoma. Biomed. Pap. Med. Fac. Palacky Univ. Olomouc 2018, 162, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Han, Q.; Shi, H.; Liu, F. CD163(+) M2-type tumor-associated macrophage support the suppression of tumor-infiltrating T cells in osteosarcoma. Int. Immunopharmacol. 2016, 34, 101–106. [Google Scholar] [CrossRef]
- Chang, C.-H.; Qiu, J.; O’Sullivan, D.; Buck, M.D.; Noguchi, T.; Curtis, J.D.; Chen, Q.; Gindin, M.; Gubin, M.M.; van der Windt, G.J.W.; et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell 2015, 162, 1229–1241. [Google Scholar] [CrossRef] [Green Version]
- Lewis, D.M.; Pruitt, H.; Jain, N.; Ciccaglione, M.; McCaffery, J.M.; Xia, Z.; Weber, K.; Eisinger-Mathason, T.S.K.; Gerecht, S. A Feedback Loop between Hypoxia and Matrix Stress Relaxation Increases Oxygen-Axis Migration and Metastasis in Sarcoma. Cancer Res. 2019, 79, 1981–1995. [Google Scholar] [CrossRef] [Green Version]
- Itoh, H.; Kadomatsu, T.; Tanoue, H.; Yugami, M.; Miyata, K.; Endo, M.; Morinaga, J.; Kobayashi, E.; Miyamoto, T.; Kurahashi, R.; et al. TET2-dependent IL-6 induction mediated by the tumor microenvironment promotes tumor metastasis in osteosarcoma. Oncogene 2018, 37, 2903–2920. [Google Scholar] [CrossRef]
- Hawkins, A.G.; Basrur, V.; da Veiga Leprevost, F.; Pedersen, E.; Sperring, C.; Nesvizhskii, A.I.; Lawlor, E.R. The Ewing Sarcoma Secretome and Its Response to Activation of Wnt/beta-catenin Signaling. Mol. Cell. Proteom. 2018, 17, 901–912. [Google Scholar] [CrossRef] [Green Version]
- Volchenboum, S.L.; Andrade, J.; Huang, L.; Barkauskas, D.A.; Krailo, M.; Womer, R.B.; Ranft, A.; Potratz, J.; Dirksen, U.; Triche, T.J.; et al. Gene expression profiling of Ewing sarcoma tumours reveals the prognostic importance of tumour–stromal interactions: A report from the Children’s Oncology Group. J. Pathol. Clin. Res. 2015, 1, 83–94. [Google Scholar] [CrossRef]
- Sand, L.G.L.; Berghuis, D.; Szuhai, K.; Hogendoorn, P.C.W. Expression of CCL21 in Ewing sarcoma shows an inverse correlation with metastases and is a candidate target for immunotherapy. Cancer Immunol. Immunother. 2016, 65, 995–1002. [Google Scholar] [CrossRef]
- Piperno-Neumann, S.; Le Deley, M.-C.; Rédini, F.; Pacquement, H.; Marec-Bérard, P.; Petit, P.; Brisse, H.; Lervat, C.; Gentet, J.-C.; Entz-Werlé, N.; et al. Zoledronate in combination with chemotherapy and surgery to treat osteosarcoma (OS2006): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016, 17, 1070–1080. [Google Scholar] [CrossRef]
- Paydas, S.; Bagir, E.K.; Deveci, M.A.; Gonlusen, G. Clinical and prognostic significance of PD-1 and PD-L1 expression in sarcomas. Med. Oncol. 2016, 33, 93. [Google Scholar] [CrossRef]
- Lussier, D.M.; O’Neill, L.; Nieves, L.M.; McAfee, M.S.; Holechek, S.A.; Collins, A.W.; Dickman, P.; Jacobsen, J.; Hingorani, P.; Blattman, J.N. Enhanced T-cell immunity to osteosarcoma through antibody blockade of PD-1/PD-L1 interactions. J. Immunother. 2015, 38, 96–106. [Google Scholar] [CrossRef]
- Shen, J.K.; Cote, G.M.; Choy, E.; Yang, P.; Harmon, D.; Schwab, J.; Nielsen, G.P.; Chebib, I.; Ferrone, S.; Wang, X.; et al. Programmed cell death ligand 1 expression in osteosarcoma. Cancer Immunol. Res. 2014, 2, 690–698. [Google Scholar] [CrossRef]
- Sundara, Y.T.; Kostine, M.; Cleven, A.H.G.; Bovée, J.V.M.G.; Schilham, M.W.; Cleton-Jansen, A.-M. Increased PD-L1 and T-cell infiltration in the presence of HLA class I expression in metastatic high-grade osteosarcoma: A rationale for T-cell-based immunotherapy. Cancer Immunol. Immunother. 2017, 66, 119–128. [Google Scholar] [CrossRef]
- Duchartre, Y.; Kim, Y.-M.; Kahn, M. The Wnt signaling pathway in cancer. Crit. Rev. Oncol. Hematol. 2016, 99, 141–149. [Google Scholar] [CrossRef]
- Polakis, P. Wnt Signaling in Cancer. Cold Spring Harb Perspect. Biol. 2012, 4, a008052. [Google Scholar] [CrossRef]
- Tai, D.; Wells, K.; Arcaroli, J.; Vanderbilt, C.; Aisner, D.L.; Messersmith, W.A.; Lieu, C.H. Targeting the WNT Signaling Pathway in Cancer Therapeutics. Oncologist 2015, 20, 1189–1198. [Google Scholar] [CrossRef] [Green Version]
- Major, M.B.; Camp, N.D.; Berndt, J.D.; Yi, X.; Goldenberg, S.J.; Hubbert, C.; Biechele, T.L.; Gingras, A.-C.; Zheng, N.; Maccoss, M.J.; et al. Wilms tumor suppressor WTX negatively regulates WNT/beta-catenin signaling. Science 2007, 316, 1043–1046. [Google Scholar] [CrossRef]
- Azzolin, L.; Panciera, T.; Soligo, S.; Enzo, E.; Bicciato, S.; Dupont, S.; Bresolin, S.; Frasson, C.; Basso, G.; Guzzardo, V.; et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell 2014, 158, 157–170. [Google Scholar] [CrossRef]
- Kim, S.-E.; Huang, H.; Zhao, M.; Zhang, X.; Zhang, A.; Semonov, M.V.; MacDonald, B.T.; Zhang, X.; Garcia Abreu, J.; Peng, L.; et al. Wnt stabilization of β-catenin reveals principles for morphogen receptor-scaffold assemblies. Science 2013, 340, 867–870. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Semenov, M.; Han, C.; Baeg, G.-H.; Tan, Y.; Zhang, Z.; Lin, X.; He, X. Control of β-Catenin Phosphorylation/Degradation by a Dual-Kinase Mechanism. Cell 2002, 108, 837–847. [Google Scholar] [CrossRef]
- Robertson, H.; Hayes, J.D.; Sutherland, C. A partnership with the proteasome; the destructive nature of GSK3. Biochem. Pharmacol. 2018, 147, 77–92. [Google Scholar] [CrossRef]
- Stamos, J.L.; Weis, W.I. The β-catenin destruction complex. Cold Spring Harb Perspect. Biol. 2013, 5, a007898. [Google Scholar] [CrossRef]
- Chodaparambil, J.V.; Pate, K.T.; Hepler, M.R.D.; Tsai, B.P.; Muthurajan, U.M.; Luger, K.; Waterman, M.L.; Weis, W.I. Molecular functions of the TLE tetramerization domain in Wnt target gene repression. EMBO J. 2014, 33, 719–731. [Google Scholar] [CrossRef] [Green Version]
- Li, V.S.W.; Ng, S.S.; Boersema, P.J.; Low, T.Y.; Karthaus, W.R.; Gerlach, J.P.; Mohammed, S.; Heck, A.J.R.; Maurice, M.M.; Mahmoudi, T.; et al. Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell 2012, 149, 1245–1256. [Google Scholar] [CrossRef]
- Lien, W.-H.; Fuchs, E. Wnt some lose some: Transcriptional governance of stem cells by Wnt/-catenin signaling. Genes Dev. 2014, 28, 1517–1532. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef]
- Baron, R.; Kneissel, M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat. Med. 2013, 19, 179–192. [Google Scholar] [CrossRef]
- Ghosh, N.; Hossain, U.; Mandal, A.; Sil, P.C. The Wnt signaling pathway: A potential therapeutic target against cancer. Ann. N. Y. Acad. Sci. 2019, 1443, 54–74. [Google Scholar] [CrossRef]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef]
- Ishibashi, M. Screening for natural products that affect Wnt signaling activity. J. Nat. Med. 2019, 1–9. [Google Scholar] [CrossRef]
- Le, P.N.; McDermott, J.D.; Jimeno, A. Targeting the Wnt pathway in human cancers: Therapeutic targeting with a focus on OMP-54F28. Pharmacol. Ther. 2015, 146, 1–11. [Google Scholar] [CrossRef]
- Tabatabai, R.; Linhares, Y.; Bolos, D.; Mita, M.; Mita, A. Targeting the Wnt Pathway in Cancer: A Review of Novel Therapeutics. Target Oncol. 2017, 12, 623–641. [Google Scholar] [CrossRef]
- Tran, F.H.; Zheng, J.J. Modulating the wnt signaling pathway with small molecules: Modulating the Wnt Signaling Pathway. Protein Sci. 2017, 26, 650–661. [Google Scholar] [CrossRef]
- Zhang, X.; Hao, J. Development of anticancer agents targeting the Wnt/β-catenin signaling. Am. J. Cancer Res. 2015, 5, 2344–2360. [Google Scholar]
- Harb, J.; Lin, P.-J.; Hao, J. Recent Development of Wnt Signaling Pathway Inhibitors for Cancer Therapeutics. Curr. Oncol. Rep. 2019, 21, 12. [Google Scholar] [CrossRef]
- Krishnamurthy, N.; Kurzrock, R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat. Rev. 2018, 62, 50–60. [Google Scholar] [CrossRef]
- Kahn, M. Can we safely target the WNT pathway? Nat. Rev. Drug Discov. 2014, 13, 513–532. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Zhao, M.; Tian, A.; Zhang, X.; Yao, Z.; Ma, X. Aberrant activation of Wnt/β-catenin signaling drives proliferation of bone sarcoma cells. Oncotarget 2015, 6, 17570–17583. [Google Scholar] [CrossRef]
- Iwaya, K.; Ogawa, H.; Kuroda, M.; Izumi, M.; Ishida, T.; Mukai, K. Cytoplasmic and/or nuclear staining of beta-catenin is associated with lung metastasis. Clin. Exp. Metastasis 2003, 20, 525–529. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, Z.; Wang, Y.; Li, W.; Su, Q.; Jia, Q.; Zhang, J.; Zhang, X.; Shen, J.; Yin, J. Dioscin inhibits stem-cell-like properties and tumor growth of osteosarcoma through Akt/GSK3/β-catenin signaling pathway. Cell Death Dis. 2018, 9, 343. [Google Scholar] [CrossRef]
- Lu, Y.; Guan, G.-F.; Chen, J.; Hu, B.; Sun, C.; Ma, Q.; Wen, Y.-H.; Qiu, X.-C.; Zhou, Y. Aberrant CXCR4 and β-catenin expression in osteosarcoma correlates with patient survival. Oncol. Lett. 2015, 10, 2123–2129. [Google Scholar] [CrossRef]
- Cai, Y.; Mohseny, A.B.; Karperien, M.; Hogendoorn, P.C.W.; Zhou, G.; Cleton-Jansen, A.-M. Inactive Wnt/beta-catenin pathway in conventional high-grade osteosarcoma. J. Pathol. 2010, 220, 24–33. [Google Scholar] [CrossRef]
- Cleton-Jansen, A.-M.; Anninga, J.K.; Briaire-de Bruijn, I.H.; Romeo, S.; Oosting, J.; Egeler, R.M.; Gelderblom, H.; Taminiau, A.H.M.; Hogendoorn, P.C.W. Profiling of high-grade central osteosarcoma and its putative progenitor cells identifies tumourigenic pathways. Br. J. Cancer 2009, 101, 1909–1918. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Yang, J.; Yang, D.; Tian, W.; Zhu, Z. The genetic basis for inactivation of Wnt pathway in human osteosarcoma. BMC Cancer 2014, 14, 450. [Google Scholar] [CrossRef]
- Shimozaki, S.; Yamamoto, N.; Domoto, T.; Nishida, H.; Hayashi, K.; Kimura, H.; Takeuchi, A.; Miwa, S.; Igarashi, K.; Kato, T.; et al. Efficacy of glycogen synthase kinase-3β targeting against osteosarcoma via activation of β-catenin. Oncotarget 2016, 7, 77038–77051. [Google Scholar] [CrossRef]
- Jie, X.-X.; Zhang, X.-Y.; Xu, C.-J. Epithelial-to-mesenchymal transition, circulating tumor cells and cancer metastasis: Mechanisms and clinical applications. Oncotarget 2017, 8, 81558–81571. [Google Scholar] [CrossRef]
- Lei, P.; Ding, D.; Xie, J.; Wang, L.; Liao, Q.; Hu, Y. Expression profile of Twist, vascular endothelial growth factor and CD34 in patients with different phases of osteosarcoma. Oncol. Lett. 2015, 10, 417–421. [Google Scholar] [CrossRef] [Green Version]
- Sharili, A.-S.; Allen, S.; Smith, K.; Hargreaves, J.; Price, J.; McGonnell, I. Expression of Snail2 in long bone osteosarcomas correlates with tumour malignancy. Tumour Biol. 2011, 32, 515–526. [Google Scholar] [CrossRef] [Green Version]
- Shen, A.; Zhang, Y.; Yang, H.; Xu, R.; Huang, G. Overexpression of ZEB1 relates to metastasis and invasion in osteosarcoma. J. Surg. Oncol. 2012, 105, 830–834. [Google Scholar] [CrossRef]
- Yang, G.; Yuan, J.; Li, K. EMT transcription factors: Implication in osteosarcoma. Med. Oncol. 2013, 30, 697. [Google Scholar] [CrossRef]
- Verrecchia, F.; Rédini, F. Transforming Growth Factor-β Signaling Plays a Pivotal Role in the Interplay Between Osteosarcoma Cells and Their Microenvironment. Front. Oncol. 2018, 8, 133. [Google Scholar] [CrossRef]
- Fuxe, J.; Vincent, T.; de Garcia Herreros, A. Transcriptional crosstalk between TGF-β and stem cell pathways in tumor cell invasion: Role of EMT promoting Smad complexes. Cell Cycle 2010, 9, 2363–2374. [Google Scholar] [CrossRef]
- Tian, H.; Zhou, T.; Chen, H.; Li, C.; Jiang, Z.; Lao, L.; Kahn, S.A.; Duarte, M.E.L.; Zhao, J.; Daubs, M.D.; et al. Bone morphogenetic protein-2 promotes osteosarcoma growth by promoting epithelial-mesenchymal transition (EMT) through the Wnt/β-catenin signaling pathway. J. Orthop. Res. 2019, 37, 1638–1648. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, D.; Han, S.; Gao, P.; Liu, C.; Li, J.; Pan, X. Fibulin-3 promotes osteosarcoma invasion and metastasis by inducing epithelial to mesenchymal transition and activating the Wnt/β-catenin signaling pathway. Sci. Rep. 2017, 7, 6215. [Google Scholar] [CrossRef]
- Fan, S.; Gao, X.; Chen, P.; Li, X. Carboxypeptidase E-ΔN promotes migration, invasiveness, and epithelial-mesenchymal transition of human osteosarcoma cells via the Wnt-β-catenin pathway. Biochem. Cell Biol. 2018, 1–8. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, Z.; Zhao, X.; Guo, L.; Yu, C.; Qin, J.; Zhang, S.; Zhang, Y.; Yang, X. Ubiquitin-specific protease 7 promotes osteosarcoma cell metastasis by inducing epithelial-mesenchymal transition. Oncol. Rep. 2019, 41, 543–551. [Google Scholar] [CrossRef]
- Cai, Z.; Cao, Y.; Luo, Y.; Hu, H.; Ling, H. Signalling mechanism(s) of epithelial-mesenchymal transition and cancer stem cells in tumour therapeutic resistance. Clin. Chim. Acta 2018, 483, 156–163. [Google Scholar] [CrossRef]
- Takebe, N.; Miele, L.; Harris, P.J.; Jeong, W.; Bando, H.; Kahn, M.; Yang, S.X.; Ivy, S.P. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: Clinical update. Nat. Rev. Clin. Oncol. 2015, 12, 445–464. [Google Scholar] [CrossRef]
- Adhikari, A.S.; Agarwal, N.; Wood, B.M.; Porretta, C.; Ruiz, B.; Pochampally, R.R.; Iwakuma, T. CD117 and Stro-1 identify osteosarcoma tumor-initiating cells associated with metastasis and drug resistance. Cancer Res. 2010, 70, 4602–4612. [Google Scholar] [CrossRef]
- Siclari, V.A.; Qin, L. Targeting the osteosarcoma cancer stem cell. J. Orthop. Surg. Res. 2010, 5, 78. [Google Scholar] [CrossRef]
- Martins-Neves, S.R.; Corver, W.E.; Paiva-Oliveira, D.I.; van den Akker, B.E.W.M.; Briaire-de-Bruijn, I.H.; Bovée, J.V.M.G.; Gomes, C.M.F.; Cleton-Jansen, A.-M. Osteosarcoma Stem Cells Have Active Wnt/β-catenin and Overexpress SOX2 and KLF4. J. Cell. Physiol. 2016, 231, 876–886. [Google Scholar] [CrossRef]
- Cai, W.; Xu, Y.; Yin, J.; Zuo, W.; Su, Z. miR-552-5p facilitates osteosarcoma cell proliferation and metastasis by targeting WIF1. Exp. Ther. Med. 2019, 17, 3781–3788. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Yang, H.; Zhao, L.; Song, R.; Tan, H.; Wang, L. MicroRNA-873 targets HOXA9 to inhibit the aggressive phenotype of osteosarcoma by deactivating the Wnt/β-catenin pathway. Int. J. Oncol. 2019, 54, 1809–1820. [Google Scholar] [CrossRef]
- Liu, Y.; Bao, Z.; Tian, W.; Huang, G. miR-885-5p suppresses osteosarcoma proliferation, migration and invasion through regulation of β-catenin. Oncol. Lett. 2019, 17, 1996–2004. [Google Scholar] [CrossRef]
- Ren, J.; Yang, M.; Xu, F.; Chen, J. microRNA-758 inhibits the malignant phenotype of osteosarcoma cells by directly targeting HMGA1 and deactivating the Wnt/β-catenin pathway. Am. J. Cancer Res. 2019, 9, 36–52. [Google Scholar]
- Xia, P.; Gu, R.; Zhang, W.; Shao, L.; Li, F.; Wu, C.; Sun, Y. MicroRNA-377 exerts a potent suppressive role in osteosarcoma through the involvement of the histone acetyltransferase 1-mediated Wnt axis. J. Cell. Physiol. 2019. [Google Scholar] [CrossRef]
- Li, C.; Wang, F.; Wei, B.; Wang, L.; Kong, D. LncRNA AWPPH promotes osteosarcoma progression via activation of Wnt/β-catenin pathway through modulating miR-93-3p/FZD7 axis. Biochem. Biophys. Res. Commun. 2019, 514, 1017–1022. [Google Scholar] [CrossRef]
- Lin, C.H.; Ji, T.; Chen, C.-F.; Hoang, B.H. Wnt signaling in osteosarcoma. Adv. Exp. Med. Biol. 2014, 804, 33–45. [Google Scholar]
- Pridgeon, M.G.; Grohar, P.J.; Steensma, M.R.; Williams, B.O. Wnt Signaling in Ewing Sarcoma, Osteosarcoma, and Malignant Peripheral Nerve Sheath Tumors. Curr. Osteoporos. Rep. 2017, 15, 239–246. [Google Scholar] [CrossRef]
- Pedersen, E.A.; Menon, R.; Bailey, K.M.; Thomas, D.G.; Van Noord, R.A.; Tran, J.; Wang, H.; Qu, P.P.; Hoering, A.; Fearon, E.R.; et al. Activation of Wnt/beta-catenin in Ewing sarcoma cells antagonizes EWS/ETS function and promotes phenotypic transition to more metastatic cell states. Cancer Res. 2016, 76, 5040–5053. [Google Scholar] [CrossRef]
- Scannell, C.A.; Pedersen, E.A.; Mosher, J.T.; Krook, M.A.; Nicholls, L.A.; Wilky, B.A.; Loeb, D.M.; Lawlor, E.R. LGR5 is Expressed by Ewing Sarcoma and Potentiates Wnt/β-Catenin Signaling. Front. Oncol. 2013, 3, 81. [Google Scholar] [CrossRef]
- Uren, A.; Wolf, V.; Sun, Y.-F.; Azari, A.; Rubin, J.S.; Toretsky, J.A. Wnt/Frizzled signaling in Ewing sarcoma. Pediatr. Blood Cancer 2004, 43, 243–249. [Google Scholar] [CrossRef]
- Endo, Y.; Beauchamp, E.; Woods, D.; Taylor, W.G.; Toretsky, J.A.; Uren, A.; Rubin, J.S. Wnt-3a and Dickkopf-1 stimulate neurite outgrowth in Ewing tumor cells via a Frizzled3- and c-Jun N-terminal kinase-dependent mechanism. Mol. Cell. Biol. 2008, 28, 2368–2379. [Google Scholar] [CrossRef]
- Navarro, D.; Agra, N.; Pestaña, A.; Alonso, J.; González-Sancho, J.M. The EWS/FLI1 oncogenic protein inhibits expression of the Wnt inhibitor DICKKOPF-1 gene and antagonizes beta-catenin/TCF-mediated transcription. Carcinogenesis 2010, 31, 394–401. [Google Scholar] [CrossRef]
- Hayashi, M.; Baker, A.; Goldstein, S.D.; Albert, C.M.; Jackson, K.W.; McCarty, G.; Kahlert, U.D.; Loeb, D.M. Inhibition of porcupine prolongs metastasis free survival in a mouse xenograft model of Ewing sarcoma. Oncotarget 2017, 8, 78265–78276. [Google Scholar] [CrossRef] [Green Version]
- Brown, H.K.; Schiavone, K.; Gouin, F.; Heymann, M.-F.; Heymann, D. Biology of Bone Sarcomas and New Therapeutic Developments. Calcif. Tissue Int. 2018, 102, 174–195. [Google Scholar] [CrossRef]
- Pederson, L.; Ruan, M.; Westendorf, J.J.; Khosla, S.; Oursler, M.J. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc. Natl. Acad. Sci. USA 2008, 105, 20764–20769. [Google Scholar] [CrossRef] [Green Version]
- Westendorf, J.J.; Kahler, R.A.; Schroeder, T.M. Wnt signaling in osteoblasts and bone diseases. Gene 2004, 341, 19–39. [Google Scholar] [CrossRef]
- Zhong, Z.; Zylstra-Diegel, C.R.; Schumacher, C.A.; Baker, J.J.; Carpenter, A.C.; Rao, S.; Yao, W.; Guan, M.; Helms, J.A.; Lane, N.E.; et al. Wntless functions in mature osteoblasts to regulate bone mass. Proc. Natl. Acad. Sci. USA 2012, 109, E2197–E2204. [Google Scholar] [CrossRef] [Green Version]
- Weivoda, M.M.; Ruan, M.; Hachfeld, C.M.; Pederson, L.; Howe, A.; Davey, R.A.; Zajac, J.D.; Kobayashi, Y.; Williams, B.O.; Westendorf, J.J.; et al. Wnt Signaling Inhibits Osteoclast Differentiation by Activating Canonical and Noncanonical cAMP/PKA Pathways. J. Bone Miner. Res. 2016, 31, 65–75. [Google Scholar] [CrossRef]
- Sadanandam, A.; Futakuchi, M.; Lyssiotis, C.A.; Gibb, W.J.; Singh, R.K. A cross-species analysis of a mouse model of breast cancer-specific osteolysis and human bone metastases using gene expression profiling. BMC Cancer 2011, 11, 304. [Google Scholar] [CrossRef]
- Bu, G.; Lu, W.; Liu, C.-C.; Selander, K.; Yoneda, T.; Hall, C.; Keller, E.T.; Li, Y. Breast cancer-derived Dickkopf1 inhibits osteoblast differentiation and osteoprotegerin expression: Implication for breast cancer osteolytic bone metastases. Int. J. Cancer 2008, 123, 1034–1042. [Google Scholar] [CrossRef]
- Bjørnland, K.; Flatmark, K.; Pettersen, S.; Aaasen, A.O.; Fodstad, O.; Maelandsmo, G.M. Matrix metalloproteinases participate in osteosarcoma invasion. J. Surg. Res. 2005, 127, 151–156. [Google Scholar] [CrossRef]
- Kunz, P.; Sähr, H.; Lehner, B.; Fischer, C.; Seebach, E.; Fellenberg, J. Elevated ratio of MMP2/MMP9 activity is associated with poor response to chemotherapy in osteosarcoma. BMC Cancer 2016, 16, 223. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X. Association of MMP-2 expression and prognosis in osteosarcoma patients. Int. J. Clin. Exp. Pathol. 2015, 8, 14965–14970. [Google Scholar]
- Zhou, J.; Liu, T.; Wang, W. Prognostic significance of matrix metalloproteinase 9 expression in osteosarcoma: A meta-analysis of 16 studies. Medicine 2018, 97, e13051. [Google Scholar] [CrossRef]
- Mateo, E.C.; Motta, F.J.N.; de Paula Queiroz, R.G.; Scrideli, C.A.; Tone, L.G. Protein expression of matrix metalloproteinase (MMP-1, -2, -3, -9 and -14) in Ewing family tumors and medulloblastomas of pediatric patients. J. Pediatr. Genet. 2012, 1, 181–187. [Google Scholar]
- Ye, C.; Yu, X.; Liu, X.; Zhan, P.; Nie, T.; Guo, R.; Liu, H.; Dai, M.; Zhang, B. Beclin-1 knockdown decreases proliferation, invasion and migration of Ewing sarcoma SK-ES-1 cells via inhibition of MMP-9. Oncol. Lett. 2018, 15, 3221–3225. [Google Scholar] [CrossRef]
- Guo, Y.; Zi, X.; Koontz, Z.; Kim, A.; Xie, J.; Gorlick, R.; Holcombe, R.F.; Hoang, B.H. Blocking Wnt/LRP5 signaling by a soluble receptor modulates the epithelial to mesenchymal transition and suppresses met and metalloproteinases in osteosarcoma Saos-2 cells. J. Orthop. Res. 2007, 25, 964–971. [Google Scholar] [CrossRef]
- Liu, B.; Li, G.; Wang, X.; Liu, Y. A furin inhibitor downregulates osteosarcoma cell migration by downregulating the expression levels of MT1-MMP via the Wnt signaling pathway. Oncol. Lett. 2014, 7, 1033–1038. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, A.; Chen, J.; Yu, T.; Guo, F. Influence of β-catenin small interfering RNA on human osteosarcoma cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2011, 31, 353–358. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, A.; Chen, J.; Yu, T.; Guo, F. SiRNA-mediated silencing of beta-catenin suppresses invasion and chemosensitivity to doxorubicin in MG-63 osteosarcoma cells. Asian Pac. J. Cancer Prev. 2011, 12, 239–245. [Google Scholar]
- Lowy, C.M.; Oskarsson, T. Tenascin C in metastasis: A view from the invasive front. Cell Adh. Migr. 2015, 9, 112–124. [Google Scholar] [CrossRef] [Green Version]
- Olsen, J.J.; Pohl, S.Ö.-G.; Deshmukh, A.; Visweswaran, M.; Ward, N.C.; Arfuso, F.; Agostino, M.; Dharmarajan, A. The Role of Wnt Signalling in Angiogenesis. Clin. Biochem. Rev. 2017, 38, 131–142. [Google Scholar]
- Carmeliet, P.; Jain, R.K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 2011, 10, 417–427. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Vottis, C.T.; Megaloikonomos, P.D.; Agrogiannis, G.D.; Theocharis, S. Neovascularization in Ewing’s sarcoma. Neoplasma 2018, 65, 317–325. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y.-J.; Zhu, K.; Wang, W.-C. A systematic review of vascular endothelial growth factor expression as a biomarker of prognosis in patients with osteosarcoma. Tumour Biol. 2013, 34, 1895–1899. [Google Scholar] [CrossRef]
- Yang, J.; Yang, D.; Sun, Y.; Sun, B.; Wang, G.; Trent, J.C.; Araujo, D.M.; Chen, K.; Zhang, W. Genetic amplification of the vascular endothelial growth factor (VEGF) pathway genes, including VEGFA, in human osteosarcoma. Cancer 2011, 117, 4925–4938. [Google Scholar] [CrossRef]
- Fuchs, B.; Inwards, C.Y.; Janknecht, R. Vascular endothelial growth factor expression is up-regulated by EWS-ETS oncoproteins and Sp1 and may represent an independent predictor of survival in Ewing’s sarcoma. Clin. Cancer Res. 2004, 10, 1344–1353. [Google Scholar] [CrossRef]
- Van der Schaft, D.W.J.; Hillen, F.; Pauwels, P.; Kirschmann, D.A.; Castermans, K.; Egbrink, M.G.A.O.; Tran, M.G.B.; Sciot, R.; Hauben, E.; Hogendoorn, P.C.W.; et al. Tumor cell plasticity in Ewing sarcoma, an alternative circulatory system stimulated by hypoxia. Cancer Res. 2005, 65, 11520–11528. [Google Scholar] [CrossRef]
- Reddy, K.; Zhou, Z.; Schadler, K.; Jia, S.-F.; Kleinerman, E.S. Bone marrow subsets differentiate into endothelial cells and pericytes contributing to Ewing’s tumor vessels. Mol. Cancer Res. 2008, 6, 929–936. [Google Scholar] [CrossRef]
- Reddy, K.; Cao, Y.; Zhou, Z.; Yu, L.; Jia, S.-F.; Kleinerman, E.S. VEGF165 expression in the tumor microenvironment influences the differentiation of bone marrow-derived pericytes that contribute to the Ewing’s sarcoma vasculature. Angiogenesis 2008, 11, 257–267. [Google Scholar] [CrossRef]
- Easwaran, V.; Lee, S.H.; Inge, L.; Guo, L.; Goldbeck, C.; Garrett, E.; Wiesmann, M.; Garcia, P.D.; Fuller, J.H.; Chan, V.; et al. beta-Catenin regulates vascular endothelial growth factor expression in colon cancer. Cancer Res. 2003, 63, 3145–3153. [Google Scholar]
- Zhang, X.; Gaspard, J.P.; Chung, D.C. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res. 2001, 61, 6050–6054. [Google Scholar]
- Kawano, Y.; Kypta, R. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 2003, 116, 2627–2634. [Google Scholar] [CrossRef] [Green Version]
- Dufourcq, P.; Leroux, L.; Ezan, J.; Descamps, B.; Lamazière, J.-M.D.; Costet, P.; Basoni, C.; Moreau, C.; Deutsch, U.; Couffinhal, T.; et al. Regulation of endothelial cell cytoskeletal reorganization by a secreted frizzled-related protein-1 and frizzled 4- and frizzled 7-dependent pathway: Role in neovessel formation. Am. J. Pathol. 2008, 172, 37–49. [Google Scholar] [CrossRef]
- Duplàa, C.; Jaspard, B.; Moreau, C.; D’Amore, P.A. Identification and cloning of a secreted protein related to the cysteine-rich domain of frizzled. Evidence for a role in endothelial cell growth control. Circ. Res. 1999, 84, 1433–1445. [Google Scholar] [CrossRef]
- Muley, A.; Majumder, S.; Kolluru, G.K.; Parkinson, S.; Viola, H.; Hool, L.; Arfuso, F.; Ganss, R.; Dharmarajan, A.; Chatterjee, S. Secreted frizzled-related protein 4: An angiogenesis inhibitor. Am. J. Pathol. 2010, 176, 1505–1516. [Google Scholar] [CrossRef]
- Zhao, S.; Kurenbekova, L.; Gao, Y.; Roos, A.; Creighton, C.J.; Rao, P.; Hicks, J.; Man, T.-K.; Lau, C.; Brown, A.M.C.; et al. NKD2, a negative regulator of Wnt signaling, suppresses tumor growth and metastasis in osteosarcoma. Oncogene 2015, 34, 5069. [Google Scholar] [CrossRef]
- Liao, D.; Johnson, R.S. Hypoxia: A key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007, 26, 281–290. [Google Scholar] [CrossRef]
- Forsythe, J.A.; Jiang, B.H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef] [Green Version]
- Krock, B.L.; Skuli, N.; Simon, M.C. Hypoxia-induced angiogenesis: Good and evil. Genes Cancer 2011, 2, 1117–1133. [Google Scholar] [CrossRef]
- Zeng, W.; Wan, R.; Zheng, Y.; Singh, S.R.; Wei, Y. Hypoxia, stem cells and bone tumor. Cancer Lett. 2011, 313, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-D.; Wu, Q.; Yang, S.-H. Effects of siRNA-mediated HIF-1α gene silencing on angiogenesis in osteosarcoma. Pak. J. Med. Sci. 2017, 33, 341–346. [Google Scholar] [CrossRef]
- Aryee, D.N.T.; Niedan, S.; Kauer, M.; Schwentner, R.; Bennani-Baiti, I.M.; Ban, J.; Muehlbacher, K.; Kreppel, M.; Walker, R.L.; Meltzer, P.; et al. Hypoxia modulates EWS-FLI1 transcriptional signature and enhances the malignant properties of Ewing’s sarcoma cells in vitro. Cancer Res. 2010, 70, 4015–4023. [Google Scholar] [CrossRef]
- Zhang, D.; Cui, G.; Sun, C.; Lei, L.; Lei, L.; Williamson, R.A.; Wang, Y.; Zhang, J.; Chen, P.; Wang, A.; et al. Hypoxia promotes osteosarcoma cell proliferation and migration through enhancing platelet-derived growth factor-BB/platelet-derived growth factor receptor-β axis. Biochem. Biophys. Res. Commun. 2019, 512, 360–366. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.-L.; Zhao, J.-L.; Zhen, O.; Yu, C.; Yang, B.-H.; Yu, X.-R. Hypoxia-inducible factor-1 promotes cancer progression through activating AKT/Cyclin D1 signaling pathway in osteosarcoma. Biomed. Pharmacother. 2018, 105, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Li, S.; Tu, C. Prognosis value of Hypoxia-inducible factor-1α expression in patients with bone and soft tissue sarcoma: A meta-analysis. Springerplus 2016, 5, 1370. [Google Scholar] [CrossRef]
- Wang, S.; Ren, T.; Huang, Y.; Bao, X.; Sun, K.; Shen, D.; Guo, W. BMPR2 and HIF1-α overexpression in resected osteosarcoma correlates with distant metastasis and patient survival. Chin. J. Cancer Res. 2017, 29, 447–454. [Google Scholar] [CrossRef]
- Ren, H.-Y.; Zhang, Y.-H.; Li, H.-Y.; Xie, T.; Sun, L.-L.; Zhu, T.; Wang, S.-D.; Ye, Z.-M. Prognostic role of hypoxia-inducible factor-1 alpha expression in osteosarcoma: A meta-analysis. Onco Targets Ther. 2016, 9, 1477–1487. [Google Scholar] [CrossRef]
- Demir, R.; Dimmler, A.; Naschberger, E.; Demir, I.; Papadopoulos, T.; Melling, N.; Sturzl, M.; Hohenberger, W. Malignant progression of invasive tumour cells seen in hypoxia present an accumulation of beta-catenin in the nucleus at the tumour front. Exp. Mol. Pathol. 2009, 87, 109–116. [Google Scholar] [CrossRef]
- Kaidi, A.; Williams, A.C.; Paraskeva, C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat. Cell Biol. 2007, 9, 210–217. [Google Scholar] [CrossRef]
- Scholten, D.J.; Timmer, C.M.; Peacock, J.D.; Pelle, D.W.; Williams, B.O.; Steensma, M.R. Down regulation of Wnt signaling mitigates hypoxia-induced chemoresistance in human osteosarcoma cells. PLoS ONE 2014, 9, e111431. [Google Scholar] [CrossRef]
- Casey, D.L.; Lin, T.-Y.; Cheung, N.-K.V. Exploiting Signaling Pathways and Immune Targets Beyond the Standard of Care for Ewing Sarcoma. Front. Oncol. 2019, 9, 537. [Google Scholar] [CrossRef]
- Qian, B.-Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, Y.-C.; Chen, Y.; Zhao, J.-L.; Gao, C.-C.; Han, H.; Liu, W.-C.; Qin, H.-Y. Crosstalk between hepatic tumor cells and macrophages via Wnt/β-catenin signaling promotes M2-like macrophage polarization and reinforces tumor malignant behaviors. Cell Death Dis. 2018, 9, 793. [Google Scholar] [CrossRef]
- Liu, T.; Fang, X.-C.; Ding, Z.; Sun, Z.-G.; Sun, L.-M.; Wang, Y.-L. Pre-operative lymphocyte-to-monocyte ratio as a predictor of overall survival in patients suffering from osteosarcoma. FEBS Open Bio 2015, 5, 682–687. [Google Scholar] [CrossRef]
- Assal, A.; Kaner, J.; Pendurti, G.; Zang, X. Emerging targets in cancer immunotherapy: Beyond CTLA-4 and PD-1. Immunotherapy 2015, 7, 1169–1186. [Google Scholar] [CrossRef]
- Koirala, P.; Roth, M.E.; Gill, J.; Chinai, J.M.; Ewart, M.R.; Piperdi, S.; Geller, D.S.; Hoang, B.H.; Fatakhova, Y.V.; Ghorpade, M.; et al. HHLA2, a member of the B7 family, is expressed in human osteosarcoma and is associated with metastases and worse survival. Sci. Rep. 2016, 6, 31154. [Google Scholar] [CrossRef]
- Koirala, P.; Roth, M.E.; Gill, J.; Piperdi, S.; Chinai, J.M.; Geller, D.S.; Hoang, B.H.; Park, A.; Fremed, M.A.; Zang, X.; et al. Immune infiltration and PD-L1 expression in the tumor microenvironment are prognostic in osteosarcoma. Sci. Rep. 2016, 6, 30093. [Google Scholar] [CrossRef]
- Spurny, C.; Kailayangiri, S.; Jamitzky, S.; Altvater, B.; Wardelmann, E.; Dirksen, U.; Hardes, J.; Hartmann, W.; Rossig, C. Programmed cell death ligand 1 (PD-L1) expression is not a predominant feature in Ewing sarcomas. Pediatr. Blood Cancer 2018, 65, e26719. [Google Scholar] [CrossRef]
- McCaughan, G.J.B.; Fulham, M.J.; Mahar, A.; Soper, J.; Hong, A.M.; Stalley, P.D.; Tattersall, M.H.N.; Bhadri, V.A. Programmed cell death-1 blockade in recurrent disseminated Ewing sarcoma. J. Hematol. Oncol. 2016, 9, 48. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Q.; Chen, W.; Shan, B.; Ding, Y.; Zhang, G.; Cao, N.; Liu, L.; Zhang, Y. B7-H3 is overexpressed in patients suffering osteosarcoma and associated with tumor aggressiveness and metastasis. PLoS ONE 2013, 8, e70689. [Google Scholar] [CrossRef]
- Yin, S.J.; Wang, W.J.; Zhang, J.Y. Expression of B7-H3 in cancer tissue during osteosarcoma progression in nude mice. Genet. Mol. Res. 2015, 14, 14253–14261. [Google Scholar] [CrossRef]
- Goldsberry, W.N.; Londoño, A.; Randall, T.D.; Norian, L.A.; Arend, R.C. A Review of the Role of Wnt in Cancer Immunomodulation. Cancers 2019, 11, 771. [Google Scholar] [CrossRef]
- Luke, J.J.; Bao, R.; Sweis, R.F.; Spranger, S.; Gajewski, T.F. WNT/β-catenin Pathway Activation Correlates with Immune Exclusion across Human Cancers. Clin. Cancer Res. 2019, 25, 3074–3083. [Google Scholar] [CrossRef]
- Spranger, S.; Bao, R.; Gajewski, T.F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 2015, 523, 231–235. [Google Scholar] [CrossRef]
- De Ruiz Galarreta, M.; Bresnahan, E.; Molina-Sanchez, P.; Lindblad, K.E.; Maier, B.; Sia, D.; Puigvehi, M.; Miguela, V.; Casanova-Acebes, M.; Dhainaut, M.; et al. β-catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discov. 2019. [Google Scholar] [CrossRef]
- Dhupkar, P.; Gordon, N.; Stewart, J.; Kleinerman, E.S. Anti-PD-1 therapy redirects macrophages from an M2 to an M1 phenotype inducing regression of OS lung metastases. Cancer Med. 2018, 7, 2654–2664. [Google Scholar] [CrossRef]
- Galluzzi, L.; Spranger, S.; Fuchs, E.; López-Soto, A. WNT Signaling in Cancer Immunosurveillance. Trends Cell Biol. 2019, 29, 44–65. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danieau, G.; Morice, S.; Rédini, F.; Verrecchia, F.; Brounais-Le Royer, B. New Insights about the Wnt/β-Catenin Signaling Pathway in Primary Bone Tumors and Their Microenvironment: A Promising Target to Develop Therapeutic Strategies? Int. J. Mol. Sci. 2019, 20, 3751. https://doi.org/10.3390/ijms20153751
Danieau G, Morice S, Rédini F, Verrecchia F, Brounais-Le Royer B. New Insights about the Wnt/β-Catenin Signaling Pathway in Primary Bone Tumors and Their Microenvironment: A Promising Target to Develop Therapeutic Strategies? International Journal of Molecular Sciences. 2019; 20(15):3751. https://doi.org/10.3390/ijms20153751
Chicago/Turabian StyleDanieau, Geoffroy, Sarah Morice, Françoise Rédini, Franck Verrecchia, and Bénédicte Brounais-Le Royer. 2019. "New Insights about the Wnt/β-Catenin Signaling Pathway in Primary Bone Tumors and Their Microenvironment: A Promising Target to Develop Therapeutic Strategies?" International Journal of Molecular Sciences 20, no. 15: 3751. https://doi.org/10.3390/ijms20153751
APA StyleDanieau, G., Morice, S., Rédini, F., Verrecchia, F., & Brounais-Le Royer, B. (2019). New Insights about the Wnt/β-Catenin Signaling Pathway in Primary Bone Tumors and Their Microenvironment: A Promising Target to Develop Therapeutic Strategies? International Journal of Molecular Sciences, 20(15), 3751. https://doi.org/10.3390/ijms20153751