An Update on Pharmacological Potential of Boswellic Acids against Chronic Diseases
Abstract
:1. Introduction
1.1. Sources and Chemical Analogues of Boswellic Acid
1.2. Pharmacological Activities of Boswellic Acid
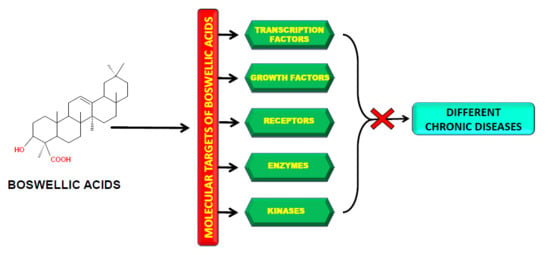
2. Molecular Targets of Boswellic Acids
3. Potential Role of BAs in the Treatment of Chronic Diseases
3.1. Arthritis
3.2. Asthma
3.3. Atherosclerosis
3.4. Cancer
3.4.1. Breast Cancer
3.4.2. Bladder Cancer
3.4.3. Brain Cancer
3.4.4. Cervical Cancer
3.4.5. Colon Cancer
3.4.6. Leukemia
3.4.7. Liver Cancer
3.4.8. Lung Cancer
3.4.9. Prostate Cancer
3.4.10. Pancreatic Cancer
3.4.11. Melanoma
3.5. Renal Intestinal Fibrosis
3.6. Inflammatory Bowel Diseases (IBDs)
3.7. Diabetes
3.8. Central Nervous System Disorders
3.9. Ischemia-Reperfusion Injury (IRI)
3.10. Psoriasis
3.11. Other Diseases
4. Boswellic Acid Implicated in Different Phases of Human Clinical Trials
5. Pharmacokinetic Properties of Boswellic Acids
6. Improvement in the Bioavailability of Boswellic Acids
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Raghupathi, W.; Raghupathi, V. An Empirical Study of Chronic Diseases in the United States: A Visual Analytics Approach. Int. J. Environ. Res. Public Health 2018, 15, E431. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Jachak, S.M. Recent developments in anti-inflammatory natural products. Med. Res. Rev. 2009, 29, 767–820. [Google Scholar] [CrossRef] [PubMed]
- Neeta; Dureja, H. Role of Boswellic Acids in Cancer Treatment. J. Med Sci. 2014, 14, 261–269. [Google Scholar] [Green Version]
- Behera, S.; Babu, S.M.; Ramani, Y.R.; Choudhury, P.K.; Panigrahi, R. Phytochemical investigation and study on antioxidant properties of Ocimum canum hydro-alcoholic leaf extracts. J. Drug Deliv. Ther. 2012, 2, 122–128. [Google Scholar] [CrossRef]
- Banik, K.; Harsha, C.; Bordoloi, D.; Lalduhsaki Sailo, B.; Sethi, G.; Leong, H.C.; Arfuso, F.; Mishra, S.; Wang, L.; Kumar, A.P.; et al. Therapeutic potential of gambogic acid, a caged xanthone, to target cancer. Cancer Lett. 2018, 416, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Harsha, C.; Banik, K.; Bordoloi, D.; Kunnumakkara, A.B. Antiulcer properties of fruits and vegetables: A mechanism based perspective. Food Chem. Toxicol. 2017, 108, 104–119. [Google Scholar] [CrossRef]
- Deorukhkar, A.; Krishnan, S.; Sethi, G.; Aggarwal, B.B. Back to basics: How natural products can provide the basis for new therapeutics. Expert Opin. Investig. Drugs 2007, 16, 1753–1773. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.F.; Weng, C.J.; Sethi, G.; Hu, D.N. Natural bioactives and phytochemicals serve in cancer treatment and prevention. Evid. -Based Complementary Altern. Med. 2013, 2013, 698190. [Google Scholar] [CrossRef]
- Tang, C.H.; Sethi, G.; Kuo, P.L. Novel medicines and strategies in cancer treatment and prevention. Biomed Res. Int. 2014, 2014, 474078. [Google Scholar] [CrossRef]
- Hsieh, Y.S.; Yang, S.F.; Sethi, G.; Hu, D.N. Natural bioactives in cancer treatment and prevention. Biomed Res. Int. 2015, 2015, 182835. [Google Scholar] [CrossRef]
- Yarla, N.S.; Bishayee, A.; Sethi, G.; Reddanna, P.; Kalle, A.M.; Dhananjaya, B.L.; Dowluru, K.S.; Chintala, R.; Duddukuri, G.R. Targeting arachidonic acid pathway by natural products for cancer prevention and therapy. Semin. Cancer Biol. 2016, 40–41, 48–81. [Google Scholar] [CrossRef] [PubMed]
- Hasanpourghadi, M.; Looi, C.Y.; Pandurangan, A.K.; Sethi, G.; Wong, W.F.; Mustafa, M.R. Phytometabolites Targeting the Warburg Effect in Cancer Cells: A Mechanistic Review. Curr. Drug Targets 2017, 18, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Warrier, S.; Kumar, A.P.; Sethi, G.; Arfuso, F. Potential Role of Natural Compounds as Anti-Angiogenic Agents in Cancer. Curr. Vasc. Pharmacol. 2017, 15, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Nabavi, S.F.; Nabavi, S.M.; Sureda, A.; Farooqi, A.A.; Atanasov, A.G.; Vacca, R.A.; Sethi, G.; Bishayee, A. Targeting activator protein 1 signaling pathway by bioactive natural agents: Possible therapeutic strategy for cancer prevention and intervention. Pharmacol. Res. 2018, 128, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Kannaiyan, R.; Sethi, G. Targeting cell signaling and apoptotic pathways by dietary agents: Role in the prevention and treatment of cancer. Nutr. Cancer 2011, 63, 161–173. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sethi, G.; Baladandayuthapani, V.; Krishnan, S.; Shishodia, S. Targeting cell signaling pathways for drug discovery: An old lock needs a new key. J. Cell. Biochem. 2007, 102, 580–592. [Google Scholar] [CrossRef]
- Parikh, N.R.; Mandal, A.; Bhatia, D.; Siveen, K.S.; Sethi, G.; Bishayee, A. Oleanane triterpenoids in the prevention and therapy of breast cancer: Current evidence and future perspectives. Phytochem. Rev.: Proc. Phytochem. Soc. Eur. 2014, 13, 793–810. [Google Scholar] [CrossRef]
- Sethi, G.; Shanmugam, M.K.; Warrier, S.; Merarchi, M.; Arfuso, F.; Kumar, A.P.; Bishayee, A. Pro-Apoptotic and Anti-Cancer Properties of Diosgenin: A Comprehensive and Critical Review. Nutrients 2018, 10, E645. [Google Scholar] [CrossRef]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, E2589. [Google Scholar] [CrossRef]
- Kanchi, M.M.; Shanmugam, M.K.; Rane, G.; Sethi, G.; Kumar, A.P. Tocotrienols: The unsaturated sidekick shifting new paradigms in vitamin E therapeutics. Drug Discov. Today 2017, 22, 1765–1781. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Wang, L.; Goh, B.C.; Ahn, K.S.; Bishayee, A.; Sethi, G. Modulation of diverse oncogenic transcription factors by thymoquinone, an essential oil compound isolated from the seeds of Nigella sativa Linn. Pharmacol. Res. 2018, 129, 357–364. [Google Scholar] [CrossRef]
- Ong, S.K.L.; Shanmugam, M.K.; Fan, L.; Fraser, S.E.; Arfuso, F.; Ahn, K.S.; Sethi, G.; Bishayee, A. Focus on Formononetin: Anticancer Potential and Molecular Targets. Cancers 2019, 11, E611. [Google Scholar] [CrossRef]
- Banik, K.; Ranaware, A.M.; Deshpande, V.; Nalawade, S.P.; Padmavathi, G.; Bordoloi, D.; Sailo, B.L.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; et al. Honokiol for cancer therapeutics: A traditional medicine that can modulate multiple oncogenic targets. Pharmacol. Res. 2019, 144, 192–209. [Google Scholar] [CrossRef]
- Varughese, R.S.; Lam, W.S.; Marican, A.; Viganeshwari, S.H.; Bhave, A.S.; Syn, N.L.; Wang, J.; Wong, A.L.; Kumar, A.P.; Lobie, P.E.; et al. Biopharmacological considerations for accelerating drug development of deguelin, a rotenoid with potent chemotherapeutic and chemopreventive potential. Cancer 2019, 125, 1789–1798. [Google Scholar] [CrossRef]
- Pathania, S.; Ramakrishnan, S.M.; Bagler, G. Phytochemica: A platform to explore phytochemicals of medicinal plants. Database 2015, 2015. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Singh, Y.P.; Girisa, S.; Banik, K.; Ghosh, S.; Swathi, P.; Deka, M.; Padmavathi, G.; Kotoky, J.; Sethi, G.; Fan, L.; et al. Potential application of zerumbone in the prevention and therapy of chronic human diseases. J. Funct. Foods 2019, 53, 248–258. [Google Scholar] [CrossRef]
- Merarchi, M.; Sethi, G.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; Ahn, K.S. Role of Natural Products in Modulating Histone Deacetylases in Cancer. Molecules 2019, 24, E1047. [Google Scholar] [CrossRef]
- Mishra, S.; Verma, S.S.; Rai, V.; Awasthee, N.; Chava, S.; Hui, K.M.; Kumar, A.P.; Challagundla, K.B.; Sethi, G.; Gupta, S.C. Long non-coding RNAs are emerging targets of phytochemicals for cancer and other chronic diseases. Cell. Mol. Life Sci. 2019, 76, 1947–1966. [Google Scholar] [CrossRef]
- Yang, M.H.; Jung, S.H.; Sethi, G.; Ahn, K.S. Pleiotropic Pharmacological Actions of Capsazepine, a Synthetic Analogue of Capsaicin, against Various Cancers and Inflammatory Diseases. Molecules 2019, 24, E995. [Google Scholar] [CrossRef]
- Deng, S.; Shanmugam, M.K.; Kumar, A.P.; Yap, C.T.; Sethi, G.; Bishayee, A. Targeting autophagy using natural compounds for cancer prevention and therapy. Cancer 2019, 125, 1228–1246. [Google Scholar] [CrossRef]
- Siddiqui, M.Z. Boswellia serrata, a potential antiinflammatory agent: An overview. Indian J. Pharm. Sci. 2011, 73, 255–261. [Google Scholar]
- Al-Yasiry, A.R.; Kiczorowska, B. Frankincense--therapeutic properties. Postepy Hig. I Med. Dosw. 2016, 70, 380–391. [Google Scholar] [CrossRef]
- Takahashi, M.; Sung, B.; Shen, Y.; Hur, K.; Link, A.; Boland, C.R.; Aggarwal, B.B.; Goel, A. Boswellic acid exerts antitumor effects in colorectal cancer cells by modulating expression of the let-7 and miR-200 microRNA family. Carcinogenesis 2012, 33, 2441–2449. [Google Scholar] [CrossRef] [Green Version]
- Hamidpour, R.; Hamidpour, S.; Hamidpour, M.; Shahlari, M. Frankincense (ru xiang; boswellia species): From the selection of traditional applications to the novel phytotherapy for the prevention and treatment of serious diseases. J. Tradit. Complementary Med. 2013, 3, 221–226. [Google Scholar] [CrossRef]
- Roy, N.K.; Deka, A.; Bordoloi, D.; Mishra, S.; Kumar, A.P.; Sethi, G.; Kunnumakkara, A.B. The potential role of boswellic acids in cancer prevention and treatment. Cancer Lett. 2016, 377, 74–86. [Google Scholar] [CrossRef]
- Buchele, B.; Zugmaier, W.; Simmet, T. Analysis of pentacyclic triterpenic acids from frankincense gum resins and related phytopharmaceuticals by high-performance liquid chromatography. Identification of lupeolic acid, a novel pentacyclic triterpene. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 791, 21–30. [Google Scholar] [CrossRef]
- Safayhi, H.; Mack, T.; Sabieraj, J.; Anazodo, M.I.; Subramanian, L.R.; Ammon, H.P. Boswellic acids: Novel, specific, nonredox inhibitors of 5-lipoxygenase. J. Pharmacol. Exp. Ther. 1992, 261, 1143–1146. [Google Scholar]
- Pawar, R.K.; Shivani, S.; Singh, K.C.; Sharma Rajeev, K.R. Physicochemical standardisation and development of HPTLC method for the determination of β Boswellic acid from Boswellia serrata Roxb (exudate). Int. J. Appl. Pharm. 2011, 3, 8–13. [Google Scholar]
- Ammon, H.P. Boswellic Acids and Their Role in Chronic Inflammatory Diseases. Adv. Exp. Med. Biol. 2016, 928, 291–327. [Google Scholar]
- Iram, F.; Khan, S.A.; Husain, A. Phytochemistry and potential therapeutic actions of Boswellic acids: A mini-review. Asian Pac. J. Trop. Biomed. 2017, 7, 513–523. [Google Scholar] [CrossRef]
- Wang, D.; Ge, S.; Bai, J.; Song, Y. Boswellic acid exerts potent anticancer effects in HCT-116 human colon cancer cells mediated via induction of apoptosis, cell cycle arrest, cell migration inhibition and inhibition of PI3K/AKT signalling pathway. J. BUON 2018, 23, 340–345. [Google Scholar]
- Akincilar, S.C.; Low, K.C.; Liu, C.Y.; Yan, T.D.; Oji, A.; Ikawa, M.; Li, S.; Tergaonkar, V. Quantitative assessment of telomerase components in cancer cell lines. FEBS Lett. 2015, 589, 974–984. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, H.S.; Chng, W.J.; Tergaonkar, V. Activation of mutant TERT promoter by RAS-ERK signaling is a key step in malignant progression of BRAF-mutant human melanomas. Proc. Natl. Acad. Sci. USA 2016, 113, 14402–14407. [Google Scholar] [CrossRef]
- Chakraborty, S.; Lakshmanan, M.; Swa, H.L.; Chen, J.; Zhang, X.; Ong, Y.S.; Loo, L.S.; Akincilar, S.C.; Gunaratne, J.; Tergaonkar, V.; et al. An oncogenic role of Agrin in regulating focal adhesion integrity in hepatocellular carcinoma. Nat. Commun. 2015, 6, 6184. [Google Scholar] [CrossRef]
- Bordoloi, D.; Banik, K.; Shabnam, B.; Padmavathi, G.; Monisha, J.; Arfuso, F.; Dharmarajan, A.; Mao, X.; Lim, L.H.K.; Wang, L.; et al. TIPE Family of Proteins and Its Implications in Different Chronic Diseases. Int. J. Mol. Sci. 2018, 19, E2974. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef]
- Sethi, G.; Sung, B.; Aggarwal, B.B. Nuclear factor-kappaB activation: From bench to bedside. Exp. Biol. Med. 2008, 233, 21–31. [Google Scholar] [CrossRef]
- Ahn, K.S.; Sethi, G.; Aggarwal, B.B. Nuclear factor-kappa B: From clone to clinic. Curr. Mol. Med. 2007, 7, 619–637. [Google Scholar] [CrossRef]
- Sethi, G.; Tergaonkar, V. Potential pharmacological control of the NF-kappaB pathway. Trends Pharmacol. Sci. 2009, 30, 313–321. [Google Scholar] [CrossRef]
- Li, F.; Sethi, G. Targeting transcription factor NF-kappaB to overcome chemoresistance and radioresistance in cancer therapy. Biochim. Et Biophys. Acta 2010, 1805, 167–180. [Google Scholar]
- Li, F.; Zhang, J.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Kumar, A.P.; Ahn, K.S.; Sethi, G. NF-kappaB in cancer therapy. Arch. Toxicol. 2015, 89, 711–731. [Google Scholar] [CrossRef]
- Chai, E.Z.; Siveen, K.S.; Shanmugam, M.K.; Arfuso, F.; Sethi, G. Analysis of the intricate relationship between chronic inflammation and cancer. Biochem. J. 2015, 468, 1–15. [Google Scholar] [CrossRef]
- Manu, K.A.; Shanmugam, M.K.; Ramachandran, L.; Li, F.; Fong, C.W.; Kumar, A.P.; Tan, P.; Sethi, G. First evidence that gamma-tocotrienol inhibits the growth of human gastric cancer and chemosensitizes it to capecitabine in a xenograft mouse model through the modulation of NF-kappaB pathway. Clin. Cancer Res. 2012, 18, 2220–2229. [Google Scholar] [CrossRef]
- Puar, Y.R.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; Sethi, G.; Tergaonkar, V. Evidence for the Involvement of the Master Transcription Factor NF-kappaB in Cancer Initiation and Progression. Biomedicines 2018, 6, E82. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Ahn, K.S.; Hsu, A.; Woo, C.C.; Yuan, Y.; Tan, K.H.B.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Koh, A.P.F.; et al. Thymoquinone Inhibits Bone Metastasis of Breast Cancer Cells Through Abrogation of the CXCR4 Signaling Axis. Front. Pharmacol. 2018, 9, 1294. [Google Scholar] [CrossRef]
- Liu, L.; Ahn, K.S.; Shanmugam, M.K.; Wang, H.; Shen, H.; Arfuso, F.; Chinnathambi, A.; Alharbi, S.A.; Chang, Y.; Sethi, G.; et al. Oleuropein induces apoptosis via abrogating NF-kappaB activation cascade in estrogen receptor-negative breast cancer cells. J. Cell. Biochem. 2019, 120, 4504–4513. [Google Scholar] [CrossRef]
- Mohan, C.D.; Bharathkumar, H.; Dukanya; Rangappa, S.; Shanmugam, M.K.; Chinnathambi, A.; Alharbi, S.A.; Alahmadi, T.A.; Bhattacharjee, A.; Lobie, P.E.; et al. N-Substituted Pyrido-1,4-Oxazin-3-Ones Induce Apoptosis of Hepatocellular Carcinoma Cells by Targeting NF-kappaB Signaling Pathway. Front. Pharmacol. 2018, 9, 1125. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Ahn, K.S.; Lee, J.H.; Kannaiyan, R.; Mustafa, N.; Manu, K.A.; Siveen, K.S.; Sethi, G.; Chng, W.J.; Kumar, A.P. Celastrol Attenuates the Invasion and Migration and Augments the Anticancer Effects of Bortezomib in a Xenograft Mouse Model of Multiple Myeloma. Front. Pharmacol. 2018, 9, 365. [Google Scholar] [CrossRef]
- Mohan, C.D.; Anilkumar, N.C.; Rangappa, S.; Shanmugam, M.K.; Mishra, S.; Chinnathambi, A.; Alharbi, S.A.; Bhattacharjee, A.; Sethi, G.; Kumar, A.P.; et al. Novel 1,3,4-Oxadiazole Induces Anticancer Activity by Targeting NF-kappaB in Hepatocellular Carcinoma Cells. Front. Oncol. 2018, 8, 42. [Google Scholar] [CrossRef]
- Chai, E.Z.; Shanmugam, M.K.; Arfuso, F.; Dharmarajan, A.; Wang, C.; Kumar, A.P.; Samy, R.P.; Lim, L.H.; Wang, L.; Goh, B.C.; et al. Targeting transcription factor STAT3 for cancer prevention and therapy. Pharmacol. Ther. 2016, 162, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.L.A.; Hirpara, J.L.; Pervaiz, S.; Eu, J.Q.; Sethi, G.; Goh, B.C. Do STAT3 inhibitors have potential in the future for cancer therapy? Expert Opin. Investig. Drugs 2017, 26, 883–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajendran, P.; Ong, T.H.; Chen, L.; Li, F.; Shanmugam, M.K.; Vali, S.; Abbasi, T.; Kapoor, S.; Sharma, A.; Kumar, A.P.; et al. Suppression of signal transducer and activator of transcription 3 activation by butein inhibits growth of human hepatocellular carcinoma in vivo. Clin. Cancer Res. 2011, 17, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Sikka, S.; Surana, R.; Dai, X.; Zhang, J.; Kumar, A.P.; Tan, B.K.; Sethi, G.; Bishayee, A. Targeting the STAT3 signaling pathway in cancer: Role of synthetic and natural inhibitors. Biochim. Et Biophys. Acta 2014, 1845, 136–154. [Google Scholar] [CrossRef] [Green Version]
- Subramaniam, A.; Shanmugam, M.K.; Perumal, E.; Li, F.; Nachiyappan, A.; Dai, X.; Swamy, S.N.; Ahn, K.S.; Kumar, A.P.; Tan, B.K.; et al. Potential role of signal transducer and activator of transcription (STAT)3 signaling pathway in inflammation, survival, proliferation and invasion of hepatocellular carcinoma. Biochim. Et Biophys. Acta 2013, 1835, 46–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, C.D.; Bharathkumar, H.; Bulusu, K.C.; Pandey, V.; Rangappa, S.; Fuchs, J.E.; Shanmugam, M.K.; Dai, X.; Li, F.; Deivasigamani, A.; et al. Development of a novel azaspirane that targets the Janus kinase-signal transducer and activator of transcription (STAT) pathway in hepatocellular carcinoma in vitro and in vivo. J. Biol. Chem. 2014, 289, 34296–34307. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Lee, S.G.; Yang, W.M.; Arfuso, F.; Um, J.Y.; Kumar, A.P.; Bian, J.; Sethi, G.; Ahn, K.S. Formononetin-induced oxidative stress abrogates the activation of STAT3/5 signaling axis and suppresses the tumor growth in multiple myeloma preclinical model. Cancer Lett. 2018, 431, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.Y.; Lee, J.H.; Nam, D.; Narula, A.S.; Namjoshi, O.A.; Blough, B.E.; Um, J.Y.; Sethi, G.; Ahn, K.S. Anti-myeloma Effects of Icariin Are Mediated Through the Attenuation of JAK/STAT3-Dependent Signaling Cascade. Front. Pharmacol. 2018, 9, 531. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Hirpara, J.L.; Eu, J.Q.; Sethi, G.; Wang, L.; Goh, B.C.; Wong, A.L. Targeting STAT3 and oxidative phosphorylation in oncogene-addicted tumors. Redox Biol. 2018, 101073. [Google Scholar] [CrossRef] [PubMed]
- Arora, L.; Kumar, A.P.; Arfuso, F.; Chng, W.J.; Sethi, G. The Role of Signal Transducer and Activator of Transcription 3 (STAT3) and Its Targeted Inhibition in Hematological Malignancies. Cancers 2018, 10, E327. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.Y.; Arya, A.; Naema, A.F.; Wong, W.F.; Sethi, G.; Looi, C.Y. Signal Transducer and Activator of Transcription (STATs) Proteins in Cancer and Inflammation: Functions and Therapeutic Implication. Front. Oncol. 2019, 9, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, S.M.; Li, F.; Rajendran, P.; Kumar, A.P.; Hui, K.M.; Sethi, G. Identification of beta-escin as a novel inhibitor of signal transducer and activator of transcription 3/Janus-activated kinase 2 signaling pathway that suppresses proliferation and induces apoptosis in human hepatocellular carcinoma cells. J. Pharmacol. Exp. Ther. 2010, 334, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Li, F.; Manu, K.A.; Shanmugam, M.K.; Loo, S.Y.; Kumar, A.P.; Sethi, G. gamma-Tocotrienol is a novel inhibitor of constitutive and inducible STAT3 signalling pathway in human hepatocellular carcinoma: Potential role as an antiproliferative, pro-apoptotic and chemosensitizing agent. Br. J. Pharmacol. 2011, 163, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Li, F.; Shanmugam, M.K.; Vali, S.; Abbasi, T.; Kapoor, S.; Ahn, K.S.; Kumar, A.P.; Sethi, G. Honokiol inhibits signal transducer and activator of transcription-3 signaling, proliferation, and survival of hepatocellular carcinoma cells via the protein tyrosine phosphatase SHP-1. J. Cell. Physiol. 2012, 227, 2184–2195. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Chatterjee, S.; Rajendran, P.; Li, F.; Shanmugam, M.K.; Wong, K.F.; Kumar, A.P.; Senapati, P.; Behera, A.K.; Hui, K.M.; et al. Inhibition of STAT3 dimerization and acetylation by garcinol suppresses the growth of human hepatocellular carcinoma in vitro and in vivo. Mol. Cancer 2014, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Shanmugam, M.K.; Chen, L.; Chatterjee, S.; Basha, J.; Kumar, A.P.; Kundu, T.K.; Sethi, G. Garcinol, a polyisoprenylated benzophenone modulates multiple proinflammatory signaling cascades leading to the suppression of growth and survival of head and neck carcinoma. Cancer Prev. Res. 2013, 6, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Khattar, E.; Kumar, P.; Liu, C.Y.; Akincilar, S.C.; Raju, A.; Lakshmanan, M.; Maury, J.J.; Qiang, Y.; Li, S.; Tan, E.Y.; et al. Telomerase reverse transcriptase promotes cancer cell proliferation by augmenting tRNA expression. J. Clin. Investig. 2016, 126, 4045–4060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akincilar, S.C.; Khattar, E.; Boon, P.L.; Unal, B.; Fullwood, M.J.; Tergaonkar, V. Long-Range Chromatin Interactions Drive Mutant TERT Promoter Activation. Cancer Discov. 2016, 6, 1276–1291. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Sailo, B.L.; Banik, K.; Harsha, C.; Prasad, S.; Gupta, S.C.; Bharti, A.C.; Aggarwal, B.B. Chronic diseases, inflammation, and spices: How are they linked? J. Transl. Med. 2018, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Poeckel, D.; Werz, O. Boswellic acids: Biological actions and molecular targets. Curr. Med. Chem. 2006, 13, 3359–3369. [Google Scholar] [CrossRef]
- Safayhi, H.; Rall, B.; Sailer, E.R.; Ammon, H.P. Inhibition by boswellic acids of human leukocyte elastase. J. Pharmacol. Exp. Ther. 1997, 281, 460–463. [Google Scholar] [PubMed]
- Sailer, E.R.; Subramanian, L.R.; Rall, B.; Hoernlein, R.F.; Ammon, H.P.; Safayhi, H. Acetyl-11-keto-beta-boswellic acid (AKBA): Structure requirements for binding and 5-lipoxygenase inhibitory activity. Br. J. Pharmacol. 1996, 117, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Ammon, H.P. Boswellic acids (components of frankincense) as the active principle in treatment of chronic inflammatory diseases. Wiener medizinische Wochenschrift 2002, 152, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Lee, J.H.; Harwalkar, J.A.; Bondar, J.; Safayhi, H.; Golubic, M. Acetyl-11-keto-beta-boswellic acid (AKBA) is cytotoxic for meningioma cells and inhibits phosphorylation of the extracellular-signal regulated kinase 1 and 2. Adv. Exp. Med. Biol. 2002, 507, 387–393. [Google Scholar] [PubMed]
- Syrovets, T.; Buchele, B.; Krauss, C.; Laumonnier, Y.; Simmet, T. Acetyl-boswellic acids inhibit lipopolysaccharide-mediated TNF-alpha induction in monocytes by direct interaction with IkappaB kinases. J. Immunol. 2005, 174, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Cuaz-Perolin, C.; Billiet, L.; Bauge, E.; Copin, C.; Scott-Algara, D.; Genze, F.; Buchele, B.; Syrovets, T.; Simmet, T.; Rouis, M. Antiinflammatory and antiatherogenic effects of the NF-kappaB inhibitor acetyl-11-keto-beta-boswellic acid in LPS-challenged ApoE-/- mice. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Huang, B.; Hooi, S.C. Acetyl-keto-beta-boswellic acid inhibits cellular proliferation through a p21-dependent pathway in colon cancer cells. Br. J. Pharmacol. 2006, 148, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Syrovets, T.; Gschwend, J.E.; Buchele, B.; Laumonnier, Y.; Zugmaier, W.; Genze, F.; Simmet, T. Inhibition of IkappaB kinase activity by acetyl-boswellic acids promotes apoptosis in androgen-independent PC-3 prostate cancer cells in vitro and in vivo. J. Biol. Chem. 2005, 280, 6170–6180. [Google Scholar] [CrossRef] [PubMed]
- Takada, Y.; Ichikawa, H.; Badmaev, V.; Aggarwal, B.B. Acetyl-11-keto-beta-boswellic acid potentiates apoptosis, inhibits invasion, and abolishes osteoclastogenesis by suppressing NF-kappa B and NF-kappa B-regulated gene expression. J. Immunol. 2006, 176, 3127–3140. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Prasad, S.; Yadav, V.; Sung, B.; Aggarwal, B.B. Boswellic acid suppresses growth and metastasis of human pancreatic tumors in an orthotopic nude mouse model through modulation of multiple targets. Plos One 2011, 6, e26943. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, Y.; Gao, Z.; Qu, X. The comparative study of acetyl-11-keto-beta-boswellic acid (AKBA) and aspirin in the prevention of intestinal adenomatous polyposis in APC(Min/+) mice. Drug Discov. Ther. 2014, 8, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wu, Q.; Chen, P.; Buchele, B.; Bian, M.; Dong, S.; Huang, D.; Ren, C.; Zhang, Y.; Hou, X.; et al. A boswellic acid-containing extract ameliorates schistosomiasis liver granuloma and fibrosis through regulating NF-kappaB signaling in mice. PLoS ONE 2014, 9, e100129. [Google Scholar]
- Qurishi, Y.; Hamid, A.; Sharma, P.R.; Wani, Z.A.; Mondhe, D.M.; Singh, S.K.; Zargar, M.A.; Andotra, S.S.; Shah, B.A.; Taneja, S.C.; et al. NF-kappaB down-regulation and PARP cleavage by novel 3-alpha-butyryloxy-beta-boswellic acid results in cancer cell specific apoptosis and in vivo tumor regression. Anti-Cancer Agents Med. Chem. 2013, 13, 777–790. [Google Scholar] [CrossRef]
- Kumar, A.; Shah, B.A.; Singh, S.; Hamid, A.; Singh, S.K.; Sethi, V.K.; Saxena, A.K.; Singh, J.; Taneja, S.C. Acyl derivatives of boswellic acids as inhibitors of NF-kappaB and STATs. Bioorganic Med. Chem. Lett. 2012, 22, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Hoernlein, R.F.; Orlikowsky, T.; Zehrer, C.; Niethammer, D.; Sailer, E.R.; Simmet, T.; Dannecker, G.E.; Ammon, H.P. Acetyl-11-keto-beta-boswellic acid induces apoptosis in HL-60 and CCRF-CEM cells and inhibits topoisomerase I. J. Pharmacol. Exp. Ther. 1999, 288, 613–619. [Google Scholar] [PubMed]
- Zhao, W.; Entschladen, F.; Liu, H.; Niggemann, B.; Fang, Q.; Zaenker, K.S.; Han, R. Boswellic acid acetate induces differentiation and apoptosis in highly metastatic melanoma and fibrosarcoma cells. Cancer Detect. Prev. 2003, 27, 67–75. [Google Scholar] [CrossRef]
- Chashoo, G.; Singh, S.K.; Sharma, P.R.; Mondhe, D.M.; Hamid, A.; Saxena, A.; Andotra, S.S.; Shah, B.A.; Qazi, N.A.; Taneja, S.C.; et al. A propionyloxy derivative of 11-keto-beta-boswellic acid induces apoptosis in HL-60 cells mediated through topoisomerase I & II inhibition. Chem.-Biol. Interact. 2011, 189, 60–71. [Google Scholar] [PubMed]
- Tibaldi, E.; Zonta, F.; Bordin, L.; Magrin, E.; Gringeri, E.; Cillo, U.; Idotta, G.; Pagano, M.A.; Brunati, A.M. The tyrosine phosphatase SHP-1 inhibits proliferation of activated hepatic stellate cells by impairing PDGF receptor signaling. Biochim. Et Biophys. Acta 2014, 1843, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Xia, L.; Hua, H.; Jing, Y. Acetyl-keto-beta-boswellic acid induces apoptosis through a death receptor 5-mediated pathway in prostate cancer cells. Cancer Res. 2008, 68, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.Q.; Kong, F.; Wang, X.L.; Young, C.Y.; Hu, X.Y.; Lou, H.X. Inhibitory effect of acetyl-11-keto-beta-boswellic acid on androgen receptor by interference of Sp1 binding activity in prostate cancer cells. Biochem. Pharmacol. 2008, 75, 2112–2121. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Yi, Z.; Zhang, X.; Sung, B.; Qu, W.; Lian, X.; Aggarwal, B.B.; Liu, M. Acetyl-11-keto-beta-boswellic acid inhibits prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis. Cancer Res. 2009, 69, 5893–5900. [Google Scholar] [CrossRef] [PubMed]
- LoPiccolo, J.; Granville, C.A.; Gills, J.J.; Dennis, P.A. Targeting Akt in cancer therapy. Anti-Cancer Drugs 2007, 18, 861–874. [Google Scholar] [PubMed]
- Roy, N.K.; Bordoloi, D.; Monisha, J.; Padmavathi, G.; Kotoky, J.; Golla, R.; Kunnumakkara, A.B. Specific Targeting of Akt Kinase Isoforms: Taking the Precise Path for Prevention and Treatment of Cancer. Curr. Drug Targets 2017, 18, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Banik, K.; Bordoloi, D.; Harsha, C.; Sailo, B.L.; Padmavathi, G.; Roy, N.K.; Gupta, S.C.; Aggarwal, B.B. Googling the Guggul (Commiphora and Boswellia) for Prevention of Chronic Diseases. Front. Pharmacol. 2018, 9, 686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, M.L.; Bani, S.; Singh, G.B. Anti-arthritic activity of boswellic acids in bovine serum albumin (BSA)-induced arthritis. Int. J. Immunopharmacol. 1989, 11, 647–652. [Google Scholar] [CrossRef]
- Dhaneshwar, S.; Dipmala, P.; Abhay, H.; Prashant, B. Disease-modifying effect of anthraquinone prodrug with boswellic acid on collagenase-induced osteoarthritis in Wistar rats. Inflamm. Allergy Drug Targets 2013, 12, 288–295. [Google Scholar]
- Wang, Q.; Pan, X.; Wong, H.H.; Wagner, C.A.; Lahey, L.J.; Robinson, W.H.; Sokolove, J. Oral and topical boswellic acid attenuates mouse osteoarthritis. Osteoarthr. Cartil. 2014, 22, 128–132. [Google Scholar] [CrossRef] [Green Version]
- Bai, F.; Chen, X.; Yang, H.; Xu, H.G. Acetyl-11-Keto-beta-Boswellic Acid Promotes Osteoblast Differentiation by Inhibiting Tumor Necrosis Factor-alpha and Nuclear Factor-kappaB Activity. J. Craniofacial Surg. 2018, 29, 1996–2002. [Google Scholar]
- Fathi, E.; Katouli, F.H.; Riazi, G.H.; Shasaltaneh, M.D.; Parandavar, E.; Bayati, S.; Afrasiabi, A.; Nazari, R. The Effects of Alpha Boswellic Acid on Reelin Expression and Tau Phosphorylation in Human Astrocytes. Neuromolecular Med. 2017, 19, 136–146. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Sang, L.; Liu, H.; Xu, Q.; Liu, Z. Boswellic acid attenuates asthma phenotypes by downregulation of GATA3 via pSTAT6 inhibition in a murine model of asthma. Int. J. Clin. Exp. Pathol. 2015, 8, 236–243. [Google Scholar]
- Zhou, X.; Cai, J.G.; Zhu, W.W.; Zhao, H.Y.; Wang, K.; Zhang, X.F. Boswellic acid attenuates asthma phenotype by downregulation of GATA3 via nhibition of PSTAT6. Genet. Mol. Res. 2015, 14, 7463–7468. [Google Scholar] [CrossRef] [PubMed]
- Mazzio, E.A.; Lewis, C.A.; Soliman, K.F.A. Transcriptomic Profiling of MDA-MB-231 Cells Exposed to Boswellia Serrata and 3-O-Acetyl-B-Boswellic Acid; ER/UPR Mediated Programmed Cell Death. Cancer Genom. Proteom. 2017, 14, 409–425. [Google Scholar]
- Frank, M.B.; Yang, Q.; Osban, J.; Azzarello, J.T.; Saban, M.R.; Saban, R.; Ashley, R.A.; Welter, J.C.; Fung, K.M.; Lin, H.K. Frankincense oil derived from Boswellia carteri induces tumor cell specific cytotoxicity. Bmc Complementary Altern. Med. 2009, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Hostanska, K.; Daum, G.; Saller, R. Cytostatic and apoptosis-inducing activity of boswellic acids toward malignant cell lines in vitro. Anticancer Res. 2002, 22, 2853–2862. [Google Scholar] [PubMed]
- Qurishi, Y.; Hamid, A.; Sharma, P.R.; Wani, Z.A.; Mondhe, D.M.; Singh, S.K.; Zargar, M.A.; Andotra, S.S.; Shah, B.A.; Taneja, S.C.; et al. PARP cleavage and perturbance in mitochondrial membrane potential by 3-alpha-propionyloxy-beta-boswellic acid results in cancer cell death and tumor regression in murine models. Future Oncol. 2012, 8, 867–881. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Nilsson, A.; Oredsson, S.; Badmaev, V.; Zhao, W.Z.; Duan, R.D. Boswellic acids trigger apoptosis via a pathway dependent on caspase-8 activation but independent on Fas/Fas ligand interaction in colon cancer HT-29 cells. Carcinogenesis 2002, 23, 2087–2093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Takahashi, M.; Byun, H.M.; Link, A.; Sharma, N.; Balaguer, F.; Leung, H.C.; Boland, C.R.; Goel, A. Boswellic acid induces epigenetic alterations by modulating DNA methylation in colorectal cancer cells. Cancer Biol. Ther. 2012, 13, 542–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girardi, B.; Principi, M.; Pricci, M.; Giorgio, F.; Iannone, A.; Losurdo, G.; Ierardi, E.; Di Leo, A.; Barone, M. Chemoprevention of inflammation-related colorectal cancer by silymarin-, acetyl-11-keto-beta-boswellic acid-, curcumin- and maltodextrin-enriched dietetic formulation in animal model. Carcinogenesis 2018, 39, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Sayed, A.S.; El Sayed, N.S. Co-administration of 3-Acetyl-11-Keto-Beta-Boswellic Acid Potentiates the Protective Effect of Celecoxib in Lipopolysaccharide-Induced Cognitive Impairment in Mice: Possible Implication of Anti-inflammatory and Antiglutamatergic Pathways. J. Mol. Neurosci. 2016, 59, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.S.; Saraswati, S.; Mathur, R.; Pandey, M. Antitumor properties of Boswellic acid against Ehrlich ascites cells bearing mouse. Food Chem. Toxicol. 2011, 49, 1924–1934. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, E.M.; Thabet, N.M.; Azab, K.S. Boswellic acid disables signal transduction of IL-6-STAT-3 in Ehrlich ascites tumor bearing irradiated mice. Biochem. Cell Biol. 2016, 94, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Glaser, T.; Winter, S.; Groscurth, P.; Safayhi, H.; Sailer, E.R.; Ammon, H.P.; Schabet, M.; Weller, M. Boswellic acids and malignant glioma: Induction of apoptosis but no modulation of drug sensitivity. Br. J. Cancer 1999, 80, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Ravanan, P.; Singh, S.K.; Rao, G.S.; Kondaiah, P. Growth inhibitory, apoptotic and anti-inflammatory activities displayed by a novel modified triterpenoid, cyano enone of methyl boswellates. J. Biosci. 2011, 36, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, J.; Fu, W.; Zheng, X.; Ren, L.; Liu, S.; Wang, J.; Ji, T.; Du, G. 3-O-acetyl-11-keto-beta-boswellic acid exerts anti-tumor effects in glioblastoma by arresting cell cycle at G2/M phase. J. Exp. Clin. Cancer Res. 2018, 37, 132. [Google Scholar] [CrossRef] [PubMed]
- Conti, S.; Vexler, A.; Edry-Botzer, L.; Kalich-Philosoph, L.; Corn, B.W.; Shtraus, N.; Meir, Y.; Hagoel, L.; Shtabsky, A.; Marmor, S.; et al. Combined acetyl-11-keto-beta-boswellic acid and radiation treatment inhibited glioblastoma tumor cells. PLoS ONE 2018, 13, e0198627. [Google Scholar] [CrossRef]
- Jing, Y.; Nakajo, S.; Xia, L.; Nakaya, K.; Fang, Q.; Waxman, S.; Han, R. Boswellic acid acetate induces differentiation and apoptosis in leukemia cell lines. Leuk. Res. 1999, 23, 43–50. [Google Scholar] [CrossRef]
- Xia, L.; Chen, D.; Han, R.; Fang, Q.; Waxman, S.; Jing, Y. Boswellic acid acetate induces apoptosis through caspase-mediated pathways in myeloid leukemia cells. Mol. Cancer Ther. 2005, 4, 381–388. [Google Scholar]
- Khan, S.; Kaur, R.; Shah, B.A.; Malik, F.; Kumar, A.; Bhushan, S.; Jain, S.K.; Taneja, S.C.; Singh, J. A novel cyano derivative of 11-keto-beta-boswellic acid causes apoptotic death by disrupting PI3K/AKT/Hsp-90 cascade, mitochondrial integrity, and other cell survival signaling events in HL-60 cells. Mol. Carcinog. 2012, 51, 679–695. [Google Scholar] [CrossRef]
- Huang, M.T.; Badmaev, V.; Ding, Y.; Liu, Y.; Xie, J.G.; Ho, C.T. Anti-tumor and anti-carcinogenic activities of triterpenoid, beta-boswellic acid. Biofactors 2000, 13, 225–230. [Google Scholar] [CrossRef]
- Liu, J.J.; Nilsson, A.; Oredsson, S.; Badmaev, V.; Duan, R.D. Keto- and acetyl-keto-boswellic acids inhibit proliferation and induce apoptosis in Hep G2 cells via a caspase-8 dependent pathway. Int. J. Mol. Med. 2002, 10, 501–505. [Google Scholar] [CrossRef]
- Huang, G.; Yang, J.; Zhang, L.; Cao, L.; Zhang, M.; Niu, X.; Zhou, Z.; Zhang, X.; Li, P.; Liu, J.F. Inhibitory effect of 11-carbonyl-beta-boswellic acid on non-small cell lung cancer H446 cells. Biochem. Biophys. Res. Commun. 2018, 503, 2202–2205. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, M.; Yang, Q.; Wang, M.; Wang, Z.; Zhu, Y.; Zhang, Y.; Wang, C.; Jia, Y.; Li, Y.; et al. Antioxidant effects of hydroxysafflor yellow A and acetyl-11-keto-beta-boswellic acid in combination on isoproterenol-induced myocardial injury in rats. Int. J. Mol. Med. 2016, 37, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Pathania, A.S.; Guru, S.K.; Kumar, S.; Kumar, A.; Ahmad, M.; Bhushan, S.; Sharma, P.R.; Mahajan, P.; Shah, B.A.; Sharma, S.; et al. Interplay between cell cycle and autophagy induced by boswellic acid analog. Sci. Rep. 2016, 6, 33146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ameen, A.M.; Elkazaz, A.Y.; Mohammad, H.M.F.; Barakat, B.M. Anti-inflammatory and neuroprotective activity of boswellic acids in rotenone parkinsonian rats. Can. J. Physiol. Pharmacol. 2017, 95, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Buchele, B.; Zugmaier, W.; Estrada, A.; Genze, F.; Syrovets, T.; Paetz, C.; Schneider, B.; Simmet, T. Characterization of 3alpha-acetyl-11-keto-alpha-boswellic acid, a pentacyclic triterpenoid inducing apoptosis in vitro and in vivo. Planta Med. 2006, 72, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- Morad, S.A.; Schmid, M.; Buchele, B.; Siehl, H.U.; El Gafaary, M.; Lunov, O.; Syrovets, T.; Simmet, T. A novel semisynthetic inhibitor of the FRB domain of mammalian target of rapamycin blocks proliferation and triggers apoptosis in chemoresistant prostate cancer cells. Mol. Pharmacol. 2013, 83, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Pathania, A.S.; Wani, Z.A.; Guru, S.K.; Kumar, S.; Bhushan, S.; Korkaya, H.; Seals, D.F.; Kumar, A.; Mondhe, D.M.; Ahmed, Z.; et al. The anti-angiogenic and cytotoxic effects of the boswellic acid analog BA145 are potentiated by autophagy inhibitors. Mol. Cancer 2015, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Wang, S.K.; Xu, Q.Q.; Yuan, H.Q.; Guo, Y.X.; Wang, Q.; Kong, F.; Lin, Z.M.; Sun, D.Q.; Wang, R.M.; et al. Acetyl-11-keto-beta-boswellic acid suppresses docetaxel-resistant prostate cancer cells in vitro and in vivo by blocking Akt and Stat3 signaling, thus suppressing chemoresistant stem cell-like properties. Acta Pharmacol. Sin. 2019, 40, 689–698. [Google Scholar] [CrossRef]
- Huang, M.; Li, A.; Zhao, F.; Xie, X.; Li, K.; Jing, Y.; Liu, D.; Zhao, L. Design, synthesis and biological evaluation of ring A modified 11-keto-boswellic acid derivatives as Pin1 inhibitors with remarkable anti-prostate cancer activity. Bioorganic Med. Chem. Lett. 2018, 28, 3187–3193. [Google Scholar] [CrossRef]
- Wang, M.X.; Zhao, J.X.; Meng, Y.J.; Di, T.T.; Xu, X.L.; Xie, X.J.; Lin, Y.; Zhang, L.; Wang, N.; Li, P.; et al. Acetyl-11-keto-beta-boswellic acid inhibits the secretion of cytokines by dendritic cells via the TLR7/8 pathway in an imiquimod-induced psoriasis mouse model and in vitro. Life Sci. 2018, 207, 90–104. [Google Scholar] [CrossRef]
- Bai, J.; Gao, Y.; Chen, L.; Yin, Q.; Lou, F.; Wang, Z.; Xu, Z.; Zhou, H.; Li, Q.; Cai, W.; et al. Identification of a natural inhibitor of methionine adenosyltransferase 2A regulating one-carbon metabolism in keratinocytes. EBioMedicine 2019, 39, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Zhang, G.; Ali Sheikh, M.S.; Shi, R. Protective Effects of alpha-Boswellic Acids in a Pulmonary Arterial Hypertensive Rat Model. Planta Med. 2017, 83, 78–86. [Google Scholar]
- von Rhein, C.; Weidner, T.; Henss, L.; Martin, J.; Weber, C.; Sliva, K.; Schnierle, B.S. Curcumin and Boswellia serrata gum resin extract inhibit chikungunya and vesicular stomatitis virus infections in vitro. Antivir. Res. 2016, 125, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, J.V.; Rosario, J.F.; Chandran, J.; Anbu, P.; Bakkiyanathan. Hypoglycemic and other related effects of Boswellia glabra in alloxan-induced diabetic rats. Indian J. Physiol. Pharmacol. 2007, 51, 29–39. [Google Scholar] [PubMed]
- Shehata, A.M.; Quintanilla-Fend, L.; Bettio, S.; Singh, C.B.; Ammon, H.P. Prevention of multiple low-dose streptozotocin (MLD-STZ) diabetes in mice by an extract from gum resin of Boswellia serrata (BE). Phytomedicine 2011, 18, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Azemi, M.E.; Namjoyan, F.; Khodayar, M.J.; Ahmadpour, F.; Darvish Padok, A.; Panahi, M. The Antioxidant Capacity and Anti-diabetic Effect of Boswellia serrata Triana and Planch Aqueous Extract in Fertile Female Diabetic Rats and the Possible Effects on Reproduction and Histological Changes in the Liver and Kidneys. Jundishapur J. Nat. Pharm. Prod. 2012, 7, 168–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shehata, A.M.; Quintanilla-Fend, L.; Bettio, S.; Jauch, J.; Scior, T.; Scherbaum, W.A.; Ammon, H.P. 11-Keto-beta-Boswellic Acids Prevent Development of Autoimmune Reactions, Insulitis and Reduce Hyperglycemia During Induction of Multiple Low-Dose Streptozotocin (MLD-STZ) Diabetes in Mice. Horm. Metab. Res. = Horm. - Und Stoffwechs. = Horm. Et Metab. 2015, 47, 463–469. [Google Scholar]
- Shehata, A.M.; Quintanilla-Fend, L.; Bettio, S.; Kamyabi-Moghaddam, Z.; Kohlhofer, U.A.; Scherbaum, W.A.; Ammon, H.P.T. 11-Keto-beta-Boswellic Acid Inhibits Lymphocyte (CD3) Infiltration Into Pancreatic Islets of Young None Obese Diabetic (NOD) Mice. Horm. Metab. Res. = Horm. - Und Stoffwechs. = Horm. Et Metab. 2017, 49, 693–700. [Google Scholar]
- Elshazly, S.M.; Abd El Motteleb, D.M.; Nassar, N.N. The selective 5-LOX inhibitor 11-keto-beta-boswellic acid protects against myocardial ischemia reperfusion injury in rats: Involvement of redox and inflammatory cascades. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2013, 386, 823–833. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, M.; Wang, M.; Wang, M.; Zhang, T.; Park, J.; Zhu, Y.; Guo, C.; Jia, Y.; Li, Y.; et al. Neuroprotection by acetyl-11-keto-beta-Boswellic acid, in ischemic brain injury involves the Nrf2/HO-1 defense pathway. Sci. Rep. 2014, 4, 7002. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, M.; Wang, M.; Li, Y.; Wen, A. Posttreatment with 11-Keto-beta-Boswellic Acid Ameliorates Cerebral Ischemia-Reperfusion Injury: Nrf2/HO-1 Pathway as a Potential Mechanism. Mol. Neurobiol. 2015, 52, 1430–1439. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, F.; Hosseinzadeh, H.; Ebrahimzadeh Bideskan, A.; Sadeghnia, H.R. Aqueous and Ethanolic Extracts of Boswellia serrata Protect Against Focal Cerebral Ischemia and Reperfusion Injury in Rats. Phytother. Res. 2016, 30, 1954–1967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jia, J.; Ding, Y.; Ma, Y.; Shang, P.; Liu, T.; Hui, G.; Wang, L.; Wang, M.; Zhu, Z.; et al. Alpha-boswellic acid protects against ethanol-induced gastric injury in rats: Involvement of nuclear factor erythroid-2-related factor 2/heme oxygenase-1 pathway. J. Pharm. Pharmacol. 2016, 68, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Khajuria, A.; Taneja, S.C.; Khajuria, R.K.; Singh, J.; Johri, R.K.; Qazi, G.N. The gastric ulcer protective effect of boswellic acids, a leukotriene inhibitor from Boswellia serrata, in rats. Phytomedicine: Int. J. Phytother. Phytopharm. 2008, 15, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.C.; Hu, L.H.; Yin, M.C. Alleviative effects from boswellic acid on acetaminophen-induced hepatic injury - Corrected and republished from: Biomedicine (Taipei). BioMedicine 2017, 7, 13. [Google Scholar] [CrossRef]
- Barakat, B.M.; Ahmed, H.I.; Bahr, H.I.; Elbahaie, A.M. Protective Effect of Boswellic Acids against Doxorubicin-Induced Hepatotoxicity: Impact on Nrf2/HO-1 Defense Pathway. Oxidative Med. Cell. Longev. 2018, 2018, 8296451. [Google Scholar] [CrossRef] [PubMed]
- Goswami, D.; Mahapatra, A.D.; Banerjee, S.; Kar, A.; Ojha, D.; Mukherjee, P.K.; Chattopadhyay, D. Boswellia serrata oleo-gum-resin and beta-boswellic acid inhibits HSV-1 infection in vitro through modulation of NF-small ka, CyrillicB and p38 MAP kinase signaling. Phytomedicine 2018, 51, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Chen, F.; Liu, A.; Sun, D.; Wu, J.; Kong, F.; Luan, Y.; Qu, X.; Wang, R. Reversal of the multidrug resistance of human ileocecal adenocarcinoma cells by acetyl-11-keto-beta-boswellic acid via downregulation of P-glycoprotein signals. Biosci. Trends 2016, 10, 392–399. [Google Scholar] [CrossRef]
- Liu, M.; Liu, T.; Shang, P.; Zhang, Y.; Liu, L.; Liu, T.; Sun, S. Acetyl-11-keto-beta-boswellic acid ameliorates renal interstitial fibrosis via Klotho/TGF-beta/Smad signalling pathway. J. Cell. Mol. Med. 2018, 22, 4997–5007. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Arvinda, S.; Singh, S.; Suri, J.; Koul, S.; Mondhe, D.M.; Singh, G.; Vishwakarma, R. IN0523 (Urs-12-ene-3alpha,24beta-diol) a plant based derivative of boswellic acid protect Cisplatin induced urogenital toxicity. Toxicol. Appl. Pharmacol. 2017, 318, 8–15. [Google Scholar] [CrossRef]
- Sayed, A.S.; Gomaa, I.E.O.; Bader, M.; El Sayed, N. Role of 3-Acetyl-11-Keto-Beta-Boswellic Acid in Counteracting LPS-Induced Neuroinflammation via Modulation of miRNA-155. Mol. Neurobiol. 2018, 55, 5798–5808. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. Ca: A Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girisa, S.; Shabnam, B.; Monisha, J.; Fan, L.; Halim, C.E.; Arfuso, F.; Ahn, K.S.; Sethi, G.; Kunnumakkara, A.B. Potential of Zerumbone as an Anti-Cancer Agent. Molecules 2019, 24, E734. [Google Scholar] [CrossRef] [PubMed]
- Sailo, B.L.; Banik, K.; Girisa, S.; Bordoloi, D.; Fan, L.; Halim, C.E.; Wang, H.; Kumar, A.P.; Zheng, D.; Mao, X.; et al. FBXW7 in Cancer: What Has Been Unraveled Thus Far? Cancers 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Shabnam, B.; Padmavathi, G.; Banik, K.; Girisa, S.; Monisha, J.; Sethi, G.; Fan, L.; Wang, L.; Mao, X.; Kunnumakkara, A.B. Sorcin a Potential Molecular Target for Cancer Therapy. Transl. Oncol. 2018, 11, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Monisha, J.; Jaiswal, A.; Banik, K.; Choudhary, H.; Singh, A.K.; Bordoloi, D.; Kunnumakkara, A.B. Cancer Cell Chemoresistance: A Prime Obstacle in Cancer Therapy. In Cancer Cell Chemoresistance and Chemosensitization; World Scientific: Singapore, 2018; pp. 15–49. [Google Scholar]
- Ranaware, A.M.; Banik, K.; Deshpande, V.; Padmavathi, G.; Roy, N.K.; Sethi, G.; Fan, L.; Kumar, A.P.; Kunnumakkara, A.B. Magnolol: A Neolignan from the Magnolia Family for the Prevention and Treatment of Cancer. Int. J. Mol. Sci. 2018, 19, E2362. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Bordoloi, D.; Sailo, B.L.; Roy, N.K.; Thakur, K.K.; Banik, K.; Shakibaei, M.; Gupta, S.C.; Aggarwal, B.B. Cancer drug development: The missing links. Exp. Biol. Med. 2019, 244, 663–689. [Google Scholar] [CrossRef] [PubMed]
- Sailo, B.L.; Banik, K.; Padmavathi, G.; Javadi, M.; Bordoloi, D.; Kunnumakkara, A.B. Tocotrienols: The promising analogues of vitamin E for cancer therapeutics. Pharmacol. Res. 2018, 130, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, G.; Rathnakaram, S.R.; Monisha, J.; Bordoloi, D.; Roy, N.K.; Kunnumakkara, A.B. Potential of butein, a tetrahydroxychalcone to obliterate cancer. Phytomedicine 2015, 22, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Bordoloi, D.; Roy, N.K.; Monisha, J.; Padmavathi, G.; Kunnumakkara, A.B. Multi-Targeted Agents in Cancer Cell Chemosensitization: What We Learnt from Curcumin Thus Far. Recent Pat. Anti-Cancer Drug Discov. 2016, 11, 67–97. [Google Scholar] [CrossRef]
- Varoni, E.M.; Lo Faro, A.F.; Sharifi-Rad, J.; Iriti, M. Anticancer Molecular Mechanisms of Resveratrol. Front. Nutr. 2016, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Shanmugam, M.K.; Lee, J.H.; Chai, E.Z.; Kanchi, M.M.; Kar, S.; Arfuso, F.; Dharmarajan, A.; Kumar, A.P.; Ramar, P.S.; Looi, C.Y.; et al. Cancer prevention and therapy through the modulation of transcription factors by bioactive natural compounds. Semin. Cancer Biol. 2016, 40–41, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.K.; Sharma, A.; Singh, A.K.; Bordoloi, D.; Sailo, B.L.; Monisha, J.; Kunnumakkara, A.B. Bladder Cancer: Chemoresistance and Chemosensitization. In Cancer Cell Chemoresistance and Chemosensitization; World Scientific: Singapore, 2018; pp. 51–80. [Google Scholar]
- Khwairakpam, A.D.; Monisha, J.; Banik, K.; Choudhary, H.; Sharma, A.; Bordoloi, D.; Kunnumakkara, A.B. Chemoresistance in Brain Cancer and Different Chemosensitization Approaches. In Cancer Cell Chemoresistance and Chemosensitization; World Scientific: Singapore, 2018; pp. 107–127. [Google Scholar]
- Banik, K.; Sailo, B.L.; Thakur, K.K.; Jaiswal, A.; Monisha, J.; Bordoloi, D.; Kunnumakkara, A.B. Potential of Different Chemosensitizers to Overcome Chemoresistance in Cervical Cancer. In Cancer Cell Chemoresistance and Chemosensitization; World Scientific: Singapore, 2018; pp. 163–179. [Google Scholar]
- Toden, S.; Okugawa, Y.; Buhrmann, C.; Nattamai, D.; Anguiano, E.; Baldwin, N.; Shakibaei, M.; Boland, C.R.; Goel, A. Novel Evidence for Curcumin and Boswellic Acid-Induced Chemoprevention through Regulation of miR-34a and miR-27a in Colorectal Cancer. Cancer Prev. Res. 2015, 8, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Roy, N.K.; Anip, A.; Banik, K.; Monisha, J.; Bordoloi, D.; Kunnumakkara, A.B. Different Methods to Inhibit Chemoresistance in Hepatocellular Carcinoma. In Cancer Cell Chemoresistance and Chemosensitization; World Scientific: Singapore, 2018; pp. 373–398. [Google Scholar]
- Siveen, K.S.; Ahn, K.S.; Ong, T.H.; Shanmugam, M.K.; Li, F.; Yap, W.N.; Kumar, A.P.; Fong, C.W.; Tergaonkar, V.; Hui, K.M.; et al. Y-tocotrienol inhibits angiogenesis-dependent growth of human hepatocellular carcinoma through abrogation of AKT/mTOR pathway in an orthotopic mouse model. Oncotarget 2014, 5, 1897–1911. [Google Scholar] [CrossRef] [PubMed]
- Swamy, S.G.; Kameshwar, V.H.; Shubha, P.B.; Looi, C.Y.; Shanmugam, M.K.; Arfuso, F.; Dharmarajan, A.; Sethi, G.; Shivananju, N.S.; Bishayee, A. Targeting multiple oncogenic pathways for the treatment of hepatocellular carcinoma. Target. Oncol. 2017, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Ahn, K.S.; Wang, L.Z.; Kim, C.; Deivasigamni, A.; Arfuso, F.; Um, J.Y.; Kumar, A.P.; Chang, Y.C.; Kumar, D.; et al. Ascochlorin Enhances the Sensitivity of Doxorubicin Leading to the Reversal of Epithelial-to-Mesenchymal Transition in Hepatocellular Carcinoma. Mol. Cancer Ther. 2016, 15, 2966–2976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, X.; Wang, L.; Deivasigamni, A.; Looi, C.Y.; Karthikeyan, C.; Trivedi, P.; Chinnathambi, A.; Alharbi, S.A.; Arfuso, F.; Dharmarajan, A.; et al. A novel benzimidazole derivative, MBIC inhibits tumor growth and promotes apoptosis via activation of ROS-dependent JNK signaling pathway in hepatocellular carcinoma. Oncotarget 2017, 8, 12831–12842. [Google Scholar] [CrossRef]
- Dai, X.; Ahn, K.S.; Kim, C.; Siveen, K.S.; Ong, T.H.; Shanmugam, M.K.; Li, F.; Shi, J.; Kumar, A.P.; Wang, L.Z.; et al. Ascochlorin, an isoprenoid antibiotic inhibits growth and invasion of hepatocellular carcinoma by targeting STAT3 signaling cascade through the induction of PIAS3. Mol. Oncol. 2015, 9, 818–833. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, A.; Shanmugam, M.K.; Ong, T.H.; Li, F.; Perumal, E.; Chen, L.; Vali, S.; Abbasi, T.; Kapoor, S.; Ahn, K.S.; et al. Emodin inhibits growth and induces apoptosis in an orthotopic hepatocellular carcinoma model by blocking activation of STAT3. Br. J. Pharmacol. 2013, 170, 807–821. [Google Scholar] [CrossRef] [Green Version]
- Manu, K.A.; Shanmugam, M.K.; Ong, T.H.; Subramaniam, A.; Siveen, K.S.; Perumal, E.; Samy, R.P.; Bist, P.; Lim, L.H.; Kumar, A.P.; et al. Emodin suppresses migration and invasion through the modulation of CXCR4 expression in an orthotopic model of human hepatocellular carcinoma. PLoS ONE 2013, 8, e57015. [Google Scholar] [CrossRef]
- Khan, M.A.; Singh, M.; Khan, M.S.; Najmi, A.K.; Ahmad, S. Caspase mediated synergistic effect of Boswellia serrata extract in combination with doxorubicin against human hepatocellular carcinoma. Biomed Res. Int. 2014, 2014, 294143. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, G.; Monisha, J.; Banik, K.; Thakur, K.K.; Choudhary, H.; Bordoloi, D.; Kunnumakkara, A.B. Different Chemosensitization Approaches to Overcome Chemoresistance in Prostate Cancer. In Cancer Cell Chemoresistance and Chemosensitization; World Scientific: Singapore, 2018; pp. 583–613. [Google Scholar]
- Sikka, S.; Chen, L.; Sethi, G.; Kumar, A.P. Targeting PPARgamma Signaling Cascade for the Prevention and Treatment of Prostate Cancer. Ppar Res. 2012, 2012, 968040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ahn, K.S.; Kim, C.; Shanmugam, M.K.; Siveen, K.S.; Arfuso, F.; Samym, R.P.; Deivasigamanim, A.; Lim, L.H.; Wang, L.; et al. Nimbolide-Induced Oxidative Stress Abrogates STAT3 Signaling Cascade and Inhibits Tumor Growth in Transgenic Adenocarcinoma of Mouse Prostate Model. Antioxid. Redox Signal. 2016, 24, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sikka, S.; Siveen, K.S.; Lee, J.H.; Um, J.Y.; Kumar, A.P.; Chinnathambi, A.; Alharbi, S.A.; Basappa; Rangappa, K.S.; et al. Cardamonin represses proliferation, invasion, and causes apoptosis through the modulation of signal transducer and activator of transcription 3 pathway in prostate cancer. Apoptosis 2017, 22, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, C.; Baek, S.H.; Ko, J.H.; Lee, S.G.; Yang, W.M.; Um, J.Y.; Sethi, G.; Ahn, K.S. Capsazepine inhibits JAK/STAT3 signaling, tumor growth, and cell survival in prostate cancer. Oncotarget 2017, 8, 17700–17711. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kim, S.M.; Bae, H.; Nam, D.; Lee, J.H.; Lee, S.G.; Shim, B.S.; Kim, S.H.; Ahn, K.S.; Choi, S.H.; et al. Embelin inhibits growth and induces apoptosis through the suppression of Akt/mTOR/S6K1 signaling cascades. Prostate 2013, 73, 296–305. [Google Scholar] [CrossRef]
- Sailo, B.L.; Monisha, J.; Jaiswal, A.; Prakash, J.; Roy, N.K.; Thakur, K.K.; Banik, K.; Bordoloi, D.; Kunnumakkara, A.B. Molecular Alterations Involved in Pancreatic Cancer Chemoresistance and Chemosensitization Strategies. In Cancer Cell Chemoresistance and Chemosensitization; World Scientific: Singapore, 2018; pp. 557–581. [Google Scholar]
- Yadav, V.R.; Prasad, S.; Sung, B.; Gelovani, J.G.; Guha, S.; Krishnan, S.; Aggarwal, B.B. Boswellic acid inhibits growth and metastasis of human colorectal cancer in orthotopic mouse model by downregulating inflammatory, proliferative, invasive and angiogenic biomarkers. Int. J. Cancer 2012, 130, 2176–2184. [Google Scholar] [CrossRef]
- Monisha, J.; Roy, N.K.; Sharma, A.; Banik, K.; Padmavathi, G.; Bordoloi, D.; Kunnumakkara, A.B. Chemoresistance and Chemosensitization in Melanoma. In Cancer Cell Chemoresistance and Chemosensitization; World Scientific: Singapore, 2018; pp. 479–527. [Google Scholar]
- Kolios, G. Animal models of inflammatory bowel disease: How useful are they really? Curr. Opin. Gastroenterol. 2016, 32, 251–257. [Google Scholar] [CrossRef]
- Gupta, I.; Parihar, A.; Malhotra, P.; Singh, G.B.; Ludtke, R.; Safayhi, H.; Ammon, H.P. Effects of Boswellia serrata gum resin in patients with ulcerative colitis. Eur. J. Med Res. 1997, 2, 37–43. [Google Scholar]
- Krieglstein, C.F.; Anthoni, C.; Rijcken, E.J.; Laukotter, M.; Spiegel, H.U.; Boden, S.E.; Schweizer, S.; Safayhi, H.; Senninger, N.; Schurmann, G. Acetyl-11-keto-beta-boswellic acid, a constituent of a herbal medicine from Boswellia serrata resin, attenuates experimental ileitis. Int. J. Colorectal Dis. 2001, 16, 88–95. [Google Scholar] [CrossRef]
- Kiela, P.R.; Midura, A.J.; Kuscuoglu, N.; Jolad, S.D.; Solyom, A.M.; Besselsen, D.G.; Timmermann, B.N.; Ghishan, F.K. Effects of Boswellia serrata in mouse models of chemically induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G798–G808. [Google Scholar] [CrossRef] [PubMed]
- Anthoni, C.; Laukoetter, M.G.; Rijcken, E.; Vowinkel, T.; Mennigen, R.; Muller, S.; Senninger, N.; Russell, J.; Jauch, J.; Bergmann, J.; et al. Mechanisms underlying the anti-inflammatory actions of boswellic acid derivatives in experimental colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G1131–G1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Haddad, R.; Karnib, N.; Assaad, R.A.; Bilen, Y.; Emmanuel, N.; Ghanem, A.; Younes, J.; Zibara, V.; Stephan, J.S.; Sleiman, S.F. Epigenetic changes in diabetes. Neurosci. Lett. 2016, 625, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Ahangarpour, A.; Heidari, H.; Fatemeh, R.A.; Pakmehr, M.; Shahbazian, H.; Ahmadi, I.; Mombeini, Z.; Mehrangiz, B.H. Effect of Boswellia serrata supplementation on blood lipid, hepatic enzymes and fructosamine levels in type2 diabetic patients. J. Diabetes Metab. Disord. 2014, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimpour, S.; Fazeli, M.; Mehri, S.; Taherianfard, M.; Hosseinzadeh, H. Boswellic Acid Improves Cognitive Function in a Rat Model Through Its Antioxidant Activity: - Neuroprotective effect of Boswellic acid. J. Pharmacopunct. 2017, 20, 10–17. [Google Scholar]
- Prieto-Moure, B.; Lloris-Carsi, J.M.; Barrios-Pitarque, C.; Toledo-Pereyra, L.H.; Lajara-Romance, J.M.; Berda-Antoli, M.; Lloris-Cejalvo, J.M.; Cejalvo-Lapena, D. Pharmacology of Ischemia-Reperfusion. Translational Research Considerations. J. Investig. Surg. 2016, 29, 234–249. [Google Scholar] [CrossRef]
- Wildfeuer, A.; Neu, I.S.; Safayhi, H.; Metzger, G.; Wehrmann, M.; Vogel, U.; Ammon, H.P. Effects of boswellic acids extracted from a herbal medicine on the biosynthesis of leukotrienes and the course of experimental autoimmune encephalomyelitis. Arzneim.-Forsch. 1998, 48, 668–674. [Google Scholar]
- Chen, L.C.; Hu, L.H.; Yin, M.C. Alleviative effects from boswellic acid on acetaminophen-induced hepatic injury. Biomedicine 2016, 6, 9. [Google Scholar] [CrossRef]
- Majeed, M.; Majeed, S.; Narayanan, N.K.; Nagabhushanam, K. A pilot, randomized, double-blind, placebo-controlled trial to assess the safety and efficacy of a novel Boswellia serrata extract in the management of osteoarthritis of the knee. Phytother. Res. PTR 2019, 33, 1457–1468. [Google Scholar] [CrossRef]
- Sengupta, K.; Alluri, K.V.; Satish, A.R.; Mishra, S.; Golakoti, T.; Sarma, K.V.; Dey, D.; Raychaudhuri, S.P. A double blind, randomized, placebo controlled study of the efficacy and safety of 5-Loxin for treatment of osteoarthritis of the knee. Arthritis Res. Ther. 2008, 10, R85. [Google Scholar] [CrossRef]
- Haroyan, A.; Mukuchyan, V.; Mkrtchyan, N.; Minasyan, N.; Gasparyan, S.; Sargsyan, A.; Narimanyan, M.; Hovhannisyan, A. Efficacy and safety of curcumin and its combination with boswellic acid in osteoarthritis: A comparative, randomized, double-blind, placebo-controlled study. BMC Complementary Altern. Med. 2018, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, A.; Maccagnano, G.; Moretti, L.; Pesce, V.; Tafuri, S.; Fiore, A.; Moretti, B. Methylsulfonylmethane and boswellic acids versus glucosamine sulfate in the treatment of knee arthritis: Randomized trial. Int. J. Immunopathol. Pharmacol. 2016, 29, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, A.; Tafuri, S.; Fusaro, L.; Moretti, L.; Pesce, V.; Moretti, B. The "MESACA" study: Methylsulfonylmethane and boswellic acids in the treatment of gonarthrosis. Adv. Ther. 2011, 28, 894–906. [Google Scholar] [CrossRef]
- Riva, A.; Giacomelli, L.; Togni, S.; Franceschi, F.; Eggenhoffner, R.; Zuccarini, M.C.; Belcaro, G. Oral administration of a lecithin-based delivery form of boswellic acids (Casperome(R)) for the prevention of symptoms of irritable bowel syndrome: A randomized clinical study. Minerva Gastroenterol. E Dietol. 2019, 65, 30–35. [Google Scholar] [CrossRef]
- Gupta, I.; Gupta, V.; Parihar, A.; Gupta, S.; Ludtke, R.; Safayhi, H.; Ammon, H.P. Effects of Boswellia serrata gum resin in patients with bronchial asthma: results of a double-blind, placebo-controlled, 6-week clinical study. Eur. J. Med. Res. 1998, 3, 511–514. [Google Scholar] [PubMed]
- Kirste, S.; Treier, M.; Wehrle, S.J.; Becker, G.; Abdel-Tawab, M.; Gerbeth, K.; Hug, M.J.; Lubrich, B.; Grosu, A.L.; Momm, F. Boswellia serrata acts on cerebral edema in patients irradiated for brain tumors: A prospective, randomized, placebo-controlled, double-blind pilot trial. Cancer 2011, 117, 3788–3795. [Google Scholar] [CrossRef]
- Gerhardt, H.; Seifert, F.; Buvari, P.; Vogelsang, H.; Repges, R. Therapy of active Crohn disease with Boswellia serrata extract H 15. Z. Fur Gastroenterol. 2001, 39, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Togni, S.; Maramaldi, G.; Di Pierro, F.; Biondi, M. A cosmeceutical formulation based on boswellic acids for the treatment of erythematous eczema and psoriasis. Clin. Cosmet. Investig. Derm. 2014, 7, 321–327. [Google Scholar]
- Calzavara-Pinton, P.; Zane, C.; Facchinetti, E.; Capezzera, R.; Pedretti, A. Topical Boswellic acids for treatment of photoaged skin. Dermatol. Ther. 2010, 23 (Suppl 1), S28–S32. [Google Scholar] [CrossRef]
- Pedretti, A.; Capezzera, R.; Zane, C.; Facchinetti, E.; Calzavara-Pinton, P. Effects of topical boswellic acid on photo and age-damaged skin: Clinical, biophysical, and echographic evaluations in a double-blind, randomized, split-face study. Planta Med. 2010, 76, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Sterk, V.; Buchele, B.; Simmet, T. Effect of food intake on the bioavailability of boswellic acids from a herbal preparation in healthy volunteers. Planta Med. 2004, 70, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Tawab, M.A.; Kaunzinger, A.; Bahr, U.; Karas, M.; Wurglics, M.; Schubert-Zsilavecz, M. Development of a high-performance liquid chromatographic method for the determination of 11-keto-beta-boswellic acid in human plasma. J. Chromatogr. BBiomed. Sci. Appl. 2001, 761, 221–227. [Google Scholar] [CrossRef]
- Buchele, B.; Simmet, T. Analysis of 12 different pentacyclic triterpenic acids from frankincense in human plasma by high-performance liquid chromatography and photodiode array detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 795, 355–362. [Google Scholar] [CrossRef]
- Sharma, S.; Thawani, V.; Hingorani, L.; Shrivastava, M.; Bhate, V.R.; Khiyani, R. Pharmacokinetic study of 11-Keto beta-Boswellic acid. Phytomedicine 2004, 11, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Reising, K.; Meins, J.; Bastian, B.; Eckert, G.; Mueller, W.E.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Determination of boswellic acids in brain and plasma by high-performance liquid chromatography/tandem mass spectrometry. Anal. Chem. 2005, 77, 6640–6645. [Google Scholar] [CrossRef] [PubMed]
- Kruger, P.; Daneshfar, R.; Eckert, G.P.; Klein, J.; Volmer, D.A.; Bahr, U.; Muller, W.E.; Karas, M.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Metabolism of boswellic acids in vitro and in vivo. Drug Metab. Dispos. 2008, 36, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Bagul, P.; Khomane, K.S.; Bansal, A.K. Investigating permeability related hurdles in oral delivery of 11-keto-beta-boswellic acid. Int. J. Pharm. 2014, 464, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Gerbeth, K.; Husch, J.; Fricker, G.; Werz, O.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. In vitro metabolism, permeation, and brain availability of six major boswellic acids from Boswellia serrata gum resins. Fitoterapia 2013, 84, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, Y.; Wang, C.; Huo, X.; Liu, P.; Wang, C.; Zhang, B.; Zhan, L.; Zhang, H.; Deng, S.; et al. Biotransformation of 11-keto-beta-boswellic acid by Cunninghamella blakesleana. Phytochemistry 2013, 96, 330–336. [Google Scholar] [CrossRef]
- Du, Z.; Liu, Z.; Ning, Z.; Liu, Y.; Song, Z.; Wang, C.; Lu, A. Prospects of boswellic acids as potential pharmaceutics. Planta Med. 2015, 81, 259–271. [Google Scholar] [CrossRef]
- Skarke, C.; Kuczka, K.; Tausch, L.; Werz, O.; Rossmanith, T.; Barrett, J.S.; Harder, S.; Holtmeier, W.; Schwarz, J.A. Increased bioavailability of 11-keto-beta-boswellic acid following single oral dose frankincense extract administration after a standardized meal in healthy male volunteers: Modeling and simulation considerations for evaluating drug exposures. J. Clin. Pharmacol. 2012, 52, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Kruger, P.; Kanzer, J.; Hummel, J.; Fricker, G.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Permeation of Boswellia extract in the Caco-2 model and possible interactions of its constituents KBA and AKBA with OATP1B3 and MRP2. Eur. J. Pharm. Sci. 2009, 36, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Husch, J.; Bohnet, J.; Fricker, G.; Skarke, C.; Artaria, C.; Appendino, G.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Enhanced absorption of boswellic acids by a lecithin delivery form (Phytosome((R))) of Boswellia extract. Fitoterapia 2013, 84, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013, 334, 133–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Su, R.; Nie, S.; Sun, M.; Zhang, J.; Wu, D.; Moustaid-Moussa, N. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J. Nutr. Biochem. 2014, 25, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Riva, A.; Morazzoni, P.; Artaria, C.; Allegrini, P.; Meins, J.; Savio, D.; Appendino, G.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. A single-dose, randomized, cross-over, two-way, open-label study for comparing the absorption of boswellic acids and its lecithin formulation. Phytomedicine 2016, 23, 1375–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Diseases | Mechanism/Outcome | References |
|---|---|---|
| Arthritis | ↓ Infiltration of leucocytes | [105] |
| ↓ Knee diameter | [106] | |
| ↓ IL-1β and TLR4, ↑ Synovial activation | [107] | |
| RA-derived bone loss disease | ↓TNF-α and NF-κB activity | [108] |
| Alzheimer’s disease | ↑ Reeling expression, ↓ ROS generation | [109] |
| Asthma | ↓ Expression of pSTAT6 and GATA3 | [110] |
| ↓ Expression of pSTAT6 and GATA3 | [111] | |
| Atherosclerosis | ↓ NF-κB activity | [86] |
| Breast cancer | ↑ ER/UPR response | [112] |
| Bladder cancer | ↑ Tumor cell specific cytotoxicity | [113] |
| Brain cancer | ↓ Phosphorylation of Erk-1 and Erk-2 | [84] |
| ↑ Apoptosis | [114] | |
| Cervical cancer | ↑ PARP cleavage | [115] |
| Colon cancer | ↑ let-7, CDK6, vimentin, and E-cadherin | [34] |
| ↓ 4E and cyclin D1, ↓ G2/M cell cycle | [42] | |
| ↓ Intestinal tumorigenesis | [91] | |
| ↓ Cyclin D1 and E, CDK 2 and 4 | [87] | |
| ↑ PARP cleavage | [115] | |
| ↓ Caspase-3 or caspase-8 | [116] | |
| ↑ Expression of SAMD14 and SMPD3 | [117] | |
| ↑ Apoptosis | [118] | |
| Cognitive impairment | ↓ Glutamate level | [119] |
| Ehrlich tumor | ↓ NF-κB and tumor growth,↑ PARP cleavage | [93] |
| ↑ PARP cleavage and apoptosis | [115] | |
| ↑ Tumor cell apoptosis | [120] | |
| ↑ Caspase-3, and apoptosis | [121] | |
| Glioma | ↑ p21 via p53-independent pathway | [122] |
| ↓ Growth of C6 glioma | [123] | |
| ↓ Topoisomerase I | [95] | |
| ↑ Apoptosis | [114] | |
| ↓ Topoisomerases I and II | [97] | |
| Glioblastoma | ↓ G2/M phase, p21/FOXM1/cyclin B1 | [124] |
| ↓ p53 and Bcl-2, ↓ IĸB-α | [125] | |
| Myeloid Leukemia | ↑ Apoptosis | [126] |
| ↑ Caspase-3 and -8, and DR4 and DR5 | [127] | |
| ↓ PI3K/Akt/Hsp-90 cascade | [128] | |
| ↓ DNA synthesis | [129] | |
| Liver cancer | ↑ Caspase-3 and -8 dependent apoptotic pathway | [130] |
| Lung cancer | ↓ NF-κB signaling | [89] |
| ↑ Apoptosis | [123] | |
| ↑ PARP cleavage, apoptosis | [115] | |
| ↑ PARP cleavage, JNK pathway | [131] | |
| Melanoma | ↓ Topoisomerase II, and MMPs | [96] |
| Meningioma | ↓ Phosphorylation of Erk-1 and Erk-2 | [84] |
| Myocardial injury | ↓ CK-MB and LDH | [132] |
| Neuroblastoma | ↑ PARP cleavage, ↑ Apoptosis | [115] |
| Pancreatic cancer | ↓ COX-2, MMP-9, CXCR4, and VEGF | [90] |
| ↓ p-mTOR, p-p70S6K (T389), p-4EBP and p-S6 | [133] | |
| Parkinson’s disease | ↓ Inflammatory markers | [134] |
| Prostate cancer | ↓ NF-κB signaling, Bcl-2, and Bcl-x(L) | [88] |
| ↑ DR5-mediated pathway | [99] | |
| ↓ AR signaling, ↑ p21(WAF1/CIP1) | [100] | |
| ↓ Tumor growth and angiogenesis | [101] | |
| ↑ Caspase 3 and apoptosis | [135] | |
| ↓ mTOR signaling | [136] | |
| ↑ PARP-1 cleavage, ↓ tumor growth | [137] | |
| ↓ Akt and STAT3 signaling | [138] | |
| ↓ Cyclin D1, and Pin1 | [139] | |
| Psoriasis | ↓ IL-12, IL-23, TLR7/8, and IRF | [140] |
| ↓ SAM/SAH ratio | [141] | |
| Pulmonary arterial hypertension | ↓ Apoptosis and proliferation | [142] |
| Chikungunya | ↓ Entry of CHIKV Env-pseudotyped lentiviral vectors | [143] |
| Diabetes | ↑ Synthesis of secretory granules | [144] |
| ↓ Islet destruction and consequent hyperglycemia | [145] | |
| ↑ Blood glucose and HbA1c | [146] | |
| ↓ Cytokine burst, and blood glucose | [147] | |
| ↓ Infiltration of lymphocytes into pancreatic islets | [148] | |
| Ischemia-reperfusion | ↑ Antioxidant capacity, ↓ inflammatory cascades | [149] |
| ↑ Nrf2 and HO-1 | [150] | |
| ↑ Nrf2 and HO-1 | [151] | |
| ↓ Brain infarction, neuronal cell loss, and apoptosis | [152] | |
| Gastric injury | ↑ Nrf2 and HO-1 | [153] |
| Gastric ulcer | ↓ Biosynthesis of leukotrienes | [154] |
| Hepatic injury | ↓ Glutathione, and ROS | [155] |
| Hepatotoxicity | ↑ Nrf2 and HO-1 | [156] |
| HSV-1 infection | ↓ NF-κB, p38 MAP-kinase, TNF-α,IL-1β, and IL-6 | [157] |
| Ileocecal adenocarcinoma | ↑ Rhodamine (Rh123), ↓P-gp, andMDR gene1 | [158] |
| Renal intestinal fibrosis | ↓ TGFβ-RI, TGFβ-RII, p-Smad2/3, and Smad4 | [159] |
| Urogenital toxicity | ↓ Glutathione peroxidase, catalase, and SOD | [160] |
| Neuroinflammation | ↓ P-IκB-α, miRNA-155 expression level | [161] |
| Disease | Dosage/Clinical Outcomes | References |
|---|---|---|
| Osteoarthritisa,B | (500 mg)/↓pain-related symptoms* | [209] |
| Osteoarthritisb,C | (100, 250 mg)/↓pain and ↑ physical functioning* | [208] |
| OsteoarthritisC | (300-500 mg)/↓pain and stiffness* | [207] |
| Knee arthritisc,C | (7.2 mg)/good and satisfactoryeffect* | [210] |
| Gonarthrosisc,C | (7.2 mg)/highly effective* | [211] |
| Brain tumorsA | (4200 mg)/↓ cerebral edema* | [214] |
| Photoaged skinC | (0.5 %)/well-tolerated withoutadverse effects* | [217] |
| Crohn diseased,C | (NIL)/well tolerated* | [215] |
| DiabetesC | (NIL)/↑ blood HDL levels, and ↓cholesterol* | [202] |
| Erythematous eczemaC | (NIL)/improvement in symptoms* | [216] |
| AsthmaC | (300 mg)/↓eosinophilic count and ESR* | [213] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, N.K.; Parama, D.; Banik, K.; Bordoloi, D.; Devi, A.K.; Thakur, K.K.; Padmavathi, G.; Shakibaei, M.; Fan, L.; Sethi, G.; et al. An Update on Pharmacological Potential of Boswellic Acids against Chronic Diseases. Int. J. Mol. Sci. 2019, 20, 4101. https://doi.org/10.3390/ijms20174101
Roy NK, Parama D, Banik K, Bordoloi D, Devi AK, Thakur KK, Padmavathi G, Shakibaei M, Fan L, Sethi G, et al. An Update on Pharmacological Potential of Boswellic Acids against Chronic Diseases. International Journal of Molecular Sciences. 2019; 20(17):4101. https://doi.org/10.3390/ijms20174101
Chicago/Turabian StyleRoy, Nand Kishor, Dey Parama, Kishore Banik, Devivasha Bordoloi, Amrita Khwairakpam Devi, Krishan Kumar Thakur, Ganesan Padmavathi, Mehdi Shakibaei, Lu Fan, Gautam Sethi, and et al. 2019. "An Update on Pharmacological Potential of Boswellic Acids against Chronic Diseases" International Journal of Molecular Sciences 20, no. 17: 4101. https://doi.org/10.3390/ijms20174101
APA StyleRoy, N. K., Parama, D., Banik, K., Bordoloi, D., Devi, A. K., Thakur, K. K., Padmavathi, G., Shakibaei, M., Fan, L., Sethi, G., & Kunnumakkara, A. B. (2019). An Update on Pharmacological Potential of Boswellic Acids against Chronic Diseases. International Journal of Molecular Sciences, 20(17), 4101. https://doi.org/10.3390/ijms20174101