Altered Lipid Metabolism in Blood Mononuclear Cells of Psoriatic Patients Indicates Differential Changes in Psoriasis Vulgaris and Psoriatic Arthritis
Abstract
:1. Introduction
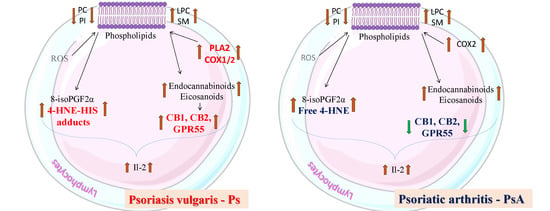
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Phospholipid Profile Estimation
4.2.2. Determination of the Activity of Lipid Metabolizing Enzymes
4.2.3. 4-HNE Determination
4.2.4. 4-HNE-Protein Adducts Determination
4.2.5. 8-isoPGF2α Determination
4.2.6. Determination of Endocannabinoids and Enzymes Them Degradation
4.2.7. Lipid Mediators Determination
4.2.8. Proteins Examination
4.3. Statistical analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 2-AG | 2-Arachidonylglycerol |
| 2-LG | 2-Linoleoylglycerol |
| 4-HNE | 4-Hydroxynonenal |
| 4-ONE | 4-Oxynonenal |
| 8-isoPGF2α | 8-iso prostaglandin F2α |
| 13-HODE | 13-hydroxyoctadecadienoic acid |
| 15-HETE | 15-Hydroxyeicosatetraenoic acid |
| 15d-PGJ2 | 15-deoxy-Δ12,14-prostaglandin J2 |
| AEA | Anandamide |
| AIDS | Acquired immunodeficiency syndrome |
| CB | Cannabinoid receptor |
| COX | Cyclooxygenase |
| EDTA | Ethylenediaminetetraacetic acid |
| ELISA | Enzyme-linked immunosorbent assay |
| ERK5 | Extracellular-signal-regulated kinase 5 |
| FAAH | Fatty acid amide hydrolase |
| GC | Gas chromatography |
| GPR55 | G protein-coupled receptor 55 |
| HILIC | Hydrophilic interaction chromatography |
| HPLC | High-performance liquid chromatography |
| IFNγ | Interferon γ |
| IKK | IκB kinase |
| IL | Interleukin |
| IMQ | Imiquimod |
| LC | Liquid chromatography |
| LEA | Dihomo-γ-linolenoylethanolamine |
| LOX | Lipoxygenases |
| LPC | Lysophosphatidylcholine |
| LTB4 | Leukotriene B4 |
| MAGL | Monoacylglycerol lipase |
| MS | Mass spectrometry |
| NFκB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NOX | NADPH oxidase |
| Ns | Not significant |
| OEA | Oleoylethanolamide |
| PAF-AH | Platelet-activating factor acetylhydrolase |
| PBS | Phosphate-buffered saline |
| PC | Phosphatidylcholine |
| PCA | Principal component analysis |
| PGE1 | Prostaglandin E1 |
| PI | Phosphatidylinositol |
| PLA2 | Phospholipase A2 |
| PLS-DA | partial least squares-discriminate analysis |
| PPAR | Peroxisome proliferator-activated receptor |
| Ps | Psoriasis vulgaris |
| PsA | Psoriatic arthritis |
| PUFAs | Polyunsaturated fatty acids |
| ROS | Reactive oxygen species |
| RT | Retention time |
| SIM | Selected ion monitoring |
| SLE | Systemic lupus erythematosus |
| SM | Sphingomyelin |
| Th | T helper lymphocytes |
| TNFα | Tumor necrosis factor α |
| TNFR | Tumor necrosis factor receptor |
| TRPV1 | The transient receptor potential cation channel subfamily V member 1 |
| TxA2 | Thromboxane A2 |
| TxB2 | Thromboxane B2 |
| UPLC | Ultra-performance liquid chromatography |
| VIP | variable importance in projection |
References
- Mease, P.J.; Armstrong, A.W. Managing Patients with Psoriatic Disease: The Diagnosis and Pharmacologic Treatment of Psoriatic Arthritis in Patients with Psoriasis. Drugs 2014, 74, 423–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkes, J.E.; Chan, T.C.; Krueger, J.G. Psoriasis pathogenesis and the development of novel targeted immune therapies. J. Allergy Clin. Immunol. 2017, 140, 645–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, N.; Perl, A. Metabolism as a Target for Modulation in Autoimmune Diseases. Trends Immunol. 2018, 39, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Ambrożewicz, E.; Wójcik, P.; Wroński, A.; Łuczaj, W.; Jastrząb, A.; Žarković, N.; Skrzydlewska, E. Pathophysiological Alterations of Redox Signaling and Endocannabinoid System in Granulocytes and Plasma of Psoriatic Patients. Cells 2018, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Belikov, A.V.; Schraven, B.; Simeoni, L. T cells and reactive oxygen species. J. Biomed. Sci. 2015, 22. [Google Scholar] [CrossRef] [PubMed]
- Łuczaj, W.; Gęgotek, A.; Skrzydlewska, E. Antioxidants and HNE in redox homeostasis. Free Radic. Biol. Med. 2017, 111, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Phillis, J.W.; O’Regan, M.H. The role of phospholipases, cyclooxygenases, and lipoxygenases in cerebral ischemic/traumatic injuries. Crit. Rev. Neurobiol. 2003, 15, 61–90. [Google Scholar] [CrossRef]
- Barrie, N.; Manolios, N. The endocannabinoid system in pain and inflammation: Its relevance to rheumatic disease. Eur. J. Rheumatol. 2017, 4, 210–218. [Google Scholar] [CrossRef]
- Navarini, L.; Bisogno, T.; Mozetic, P.; Piscitelli, F.; Margiotta, D.P.E.; Basta, F.; Afeltra, A.; Maccarrone, M. Endocannabinoid system in systemic lupus erythematosus: First evidence for a deranged 2-arachidonoylglycerol metabolism. Int. J. Biochem. Cell Biol. 2018, 99, 161–168. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Rajesh, M.; Bátkai, S.; Pan, H.; Mukhopadhyay, B.; Haskó, G.; Mackie, K.; Pacher, P. Opposing effects of cb1 and cb2 receptors on inflammation, oxidative stress, and cell death in nepropathy. Faseb. J. 2011, 25, 1087. [Google Scholar]
- Siegmund, S.V.; Wojtalla, A.; Schlosser, M.; Schildberg, F.A.; Knolle, P.A.; Nüsing, R.M.; Zimmer, A.; Strassburg, C.P.; Singer, M.V. Cyclooxygenase-2 contributes to the selective induction of cell death by the endocannabinoid 2-arachidonoyl glycerol in hepatic stellate cells. Biochem. Biophys. Res. Commun. 2016, 470, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Meirer, K.; Steinhilber, D.; Proschak, E. Inhibitors of the Arachidonic Acid Cascade: Interfering with Multiple Pathways. Basic Clin. Pharmacol. Toxicol. 2014, 114, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Gachet, M.S.; Rhyn, P.; Bosch, O.G.; Quednow, B.B.; Gertsch, J. A quantitiative LC-MS/MS method for the measurement of arachidonic acid, prostanoids, endocannabinoids, N-acylethanolamines and steroids in human plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 976–977, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Diani, M.; Altomare, G.; Reali, E. T cell responses in psoriasis and psoriatic arthritis. Autoimmun. Rev. 2015, 14, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Nunn, A.; Guy, G.; Bell, J.D. Endocannabinoids in neuroendopsychology: Multiphasic control of mitochondrial function. Philos Trans. R Soc Lond B Biol Sci 2012, 367, 3342–3352. [Google Scholar] [CrossRef] [PubMed]
- Carney, S.T.; Lloyd, M.L.; MacKinnon, S.E.; Newton, D.C.; Jones, J.D.; Howlett, A.C.; Norford, D.C. Cannabinoid Regulation of Nitric Oxide Synthase I (nNOS) in Neuronal Cells. J. Neuroimmune Pharm. 2009, 4, 338–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csala, M.; Kardon, T.; Legeza, B.; Lizák, B.; Mandl, J.; Margittai, É.; Puskás, F.; Száraz, P.; Szelényi, P.; Bánhegyi, G. On the role of 4-hydroxynonenal in health and disease. Biochim. Et Biophys. Acta (Bba)-Mol. Basis Dis. 2015, 1852, 826–838. [Google Scholar] [CrossRef] [Green Version]
- Timmermann, M.; Högger, P. Oxidative stress and 8-iso-prostaglandin F(2alpha) induce ectodomain shedding of CD163 and release of tumor necrosis factor-alpha from human monocytes. Free Radic. Biol. Med. 2005, 39, 98–107. [Google Scholar] [CrossRef]
- Jiao, X.; Guo, Z.; Chen, T.; Zhang, Y.; Li, M. Determination of antioxidant capacity and 8-iso-prostaglandin F2α levels in patients with psoriasis and their significance. Chin. J. Dermatol. 2012, 45, 388–391. [Google Scholar]
- Basu, S.; Whiteman, M.; Mattey, D.; Halliwell, B. Raised levels of F2-isoprostanes and prostaglandin F2α in different rheumatic diseases. Ann. Rheum. Dis. 2001, 60, 627–631. [Google Scholar] [CrossRef]
- Man, X.-Y.; Zheng, M. Role of Angiogenic and Inflammatory Signal Pathways in Psoriasis. J. Investig. Derm. Symp. Proc. 2015, 17, 43–45. [Google Scholar] [CrossRef] [Green Version]
- Miki, H.; Funato, Y. Regulation of intracellular signalling through cysteine oxidation by reactive oxygen species. J. Biochem. 2012, 151, 255–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, J.P.; Jung, T.; Grune, T.; Siems, W. 4-Hydroxynonenal (HNE) modified proteins in metabolic diseases. Free Radic. Biol. Med. 2017, 111, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Grabarczyk, D.B.; Chappell, P.E.; Eisel, B.; Johnson, S.; Lea, S.M.; Berks, B.C. Mechanism of Thiosulfate Oxidation in the SoxA Family of Cysteine-ligated Cytochromes. J. Biol. Chem. 2015, 290, 9209–9221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, R.; MacMillan-Crow, L.A.; Rafferty, T.M.; Saba, H.; Roberts, D.W.; Fifer, E.K.; James, L.P.; Hinson, J.A. Acetaminophen-Induced Hepatotoxicity in Mice Occurs with Inhibition of Activity and Nitration of Mitochondrial Manganese Superoxide Dismutase. J. Pharm. Exp. 2011, 337, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Westcott, P.M.K.; To, M.D. The genetics and biology of KRAS in lung cancer. Chin. J. Cancer 2013, 32, 63–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turcotte, C.; Chouinard, F.; Lefebvre, J.S.; Flamand, N. Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites. J. Leukoc. Biol. 2015, 97, 1049–1070. [Google Scholar] [CrossRef] [PubMed]
- Jean-Gilles, L.; Braitch, M.; Latif, M.L.; Aram, J.; Fahey, A.J.; Edwards, L.J.; Robins, R.A.; Tanasescu, R.; Tighe, P.J.; Gran, B.; et al. Effects of pro-inflammatory cytokines on cannabinoid CB1 and CB2 receptors in immune cells. Acta Physiol. (Oxf) 2015, 214, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk, M.; Sobolewska-Włodarczyk, A.; Cygankiewicz, A.I.; Jacenik, D.; Krajewska, W.M.; Stec-Michalska, K.; Piechota-Polańczyk, A.; Wiśniewska-Jarosińska, M.; Fichna, J. G protein-coupled receptor 55 (GPR55) expresses differently in patients with Crohn’s disease and ulcerative colitis. Scand. J. Gastroenterol. 2017, 52, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Gouin, O.; L’Herondelle, K.; Lebonvallet, N.; Le Gall-Ianotto, C.; Sakka, M.; Buhé, V.; Plée-Gautier, E.; Carré, J.-L.; Lefeuvre, L.; Misery, L.; et al. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: Pro-inflammatory response induced by their activation and their sensitization. Protein Cell 2017, 8, 644–661. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.W.; Newton, C.; Larsen, K.; Chou, J.; Perkins, I.; Lu, L.; Nong, L.; Friedman, H. Cannabinoid receptors and T helper cells. J. Neuroimmunol. 2004, 147, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Camara-Lemarroy, C.R.; Gonzalez-Moreno, E.I.; Guzman-de la Garza, F.J.; Fernandez-Garza, N.E. Arachidonic Acid Derivatives and Their Role in Peripheral Nerve Degeneration and Regeneration. Sci. World J. 2012, 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Tanada-Ueharaguchi, Y.; Honda, T.; Murata, T.; Arita, M.; Miyachi, Y.; Kabashima, K. Thromboxane A2 promotes the development of imiquimod-induced mouse psoriasis model via TP receptor. J. Dermatol. Sci. 2016, 84, e5. [Google Scholar] [CrossRef]
- Del Prete, A.; Shao, W.-H.; Mitola, S.; Santoro, G.; Sozzani, S.; Haribabu, B. Regulation of dendritic cell migration and adaptive immune response by leukotriene B4 receptors: A role for LTB4 in up-regulation of CCR7 expression and function. Blood 2007, 109, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Traver, M.K.; Kashyap, A.K.; Washington, M.A.; Latoche, J.R.; Schaefer, B.C. T cell receptor signals to NF-κB are transmitted by a cytosolic p62-Bcl10-Malt1-IKK signalosome. Sci. Signal. 2014, 7, ra45. [Google Scholar] [CrossRef] [PubMed]
- Ament, Z.; Masoodi, M.; Griffin, J.L. Applications of metabolomics for understanding the action of peroxisome proliferator-activated receptors (PPARs) in diabetes, obesity and cancer. Genome Med. 2012, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E. Cannabinoids go nuclear: Evidence for activation of peroxisome proliferator-activated receptors. Br. J. Pharm. 2007, 152, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, G.; Zhu, Y.; Liu, R.; Song, J.; Ni, Y.; Sun, H.; Yang, B.; Hou, M.; Chen, L.; et al. PPAR-γ agonist ameliorates liver pathology accompanied by increasing regulatory B and T cells in high-fat-diet mice. Obesity 2017, 25, 581–590. [Google Scholar] [CrossRef]
- Aleshin, S.; Reiser, G. Role of the peroxisome proliferator-activated receptors (PPAR)-α, β/δ and γ triad in regulation of reactive oxygen species signaling in brain. Biol. Chem. 2013, 394, 1553–1570. [Google Scholar] [CrossRef]
- Contreras, A.V.; Torres, N.; Tovar, A.R. PPAR-α as a Key Nutritional and Environmental Sensor for Metabolic Adaptation. Adv. Nutr. 2013, 4, 439–452. [Google Scholar] [CrossRef]
- Kim, M.-S.; Pyun, H.-B.; Hwang, J.-K. Panduratin A, an activator of PPAR-α/δ, suppresses the development of oxazolone-induced atopic dermatitis-like symptoms in hairless mice. Life Sci. 2014, 100, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Ham, S.A.; Paek, K.S.; Hwang, J.S.; Jung, S.Y.; Kim, M.Y.; Jin, H.; Kang, E.S.; Woo, I.S.; Kim, H.J.; et al. Transcriptional up-regulation of antioxidant genes by PPARδ inhibits angiotensin II-induced premature senescence in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2011, 406, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Marx, N.; Kehrle, B.; Kohlhammer, K.; Grüb, M.; Koenig, W.; Hombach, V.; Libby, P.; Plutzky, J. PPAR Activators as Antiinflammatory Mediators in Human T Lymphocytes. Circ. Res. 2002, 90, 703–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, E.M.; Lewis, D.H. Spectrophotometric determination of phosphate esters in the presence and absence of orthophosphate. Anal. Biochem. 1970, 36, 159–167. [Google Scholar] [CrossRef]
- Łuczaj, W.; Domingues, P.; Domingues, M.R.; Pancewicz, S.; Skrzydlewska, E. Phospholipidomic Analysis Reveals Changes in Sphingomyelin and Lysophosphatidylcholine Profiles in Plasma from Patients with Neuroborreliosis. Lipids 2017, 52, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Pulfer, M.; Murphy, R.C. Electrospray mass spectrometry of phospholipids. Mass Spectrom. Rev. 2003, 22, 332–364. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.J.; Hughes, L.L.; Yu, L.; Dennis, E.A. 1-Hexadecyl-2-arachidonoylthio-2-deoxy-sn-glycero-3-phosphorylcholine as a substrate for the microtiterplate assay of human cytosolic phospholipase A2. Anal. Biochem. 1994, 217, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Aarsman, A.J.; Neys, F.W.; Van den Bosch, H. Catabolism of platelet-activating factor and its acyl analog. Differentiation of the activities of lysophospholipase and platelet-activating-factor acetylhydrolase. Eur. J. Biochem. 1991, 200, 187–193. [Google Scholar] [CrossRef]
- Kulmacz, R.J.; Lands, W.E. Requirements for hydroperoxide by the cyclooxygenase and peroxidase activities of prostaglandin H synthase. Prostaglandins 1983, 25, 531–540. [Google Scholar] [CrossRef]
- Smith, C.J.; Zhang, Y.; Koboldt, C.M.; Muhammad, J.; Zweifel, B.S.; Shaffer, A.; Talley, J.J.; Masferrer, J.L.; Seibert, K.; Isakson, P.C. Pharmacological analysis of cyclooxygenase-1 in inflammation. PNAS 1998, 95, 13313–13318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.P.; Yazdanpanah, M.; Bhooi, N.; Lehotay, D.C. Determination of aldehydes and other lipid peroxidation products in biological samples by gas chromatography-mass spectrometry. Anal. Biochem. 1995, 228, 294–298. [Google Scholar] [CrossRef]
- Weber, D.; Milkovic, L.; Bennett, S.J.; Griffiths, H.R.; Zarkovic, N.; Grune, T. Measurement of HNE-protein adducts in human plasma and serum by ELISA—Comparison of two primary antibodies. Redox Biol. 2013, 1, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Coolen, S.A.J.; van Buuren, B.; Duchateau, G.; Upritchard, J.; Verhagen, H. Kinetics of biomarkers: Biological and technical validity of isoprostanes in plasma. Amino Acids 2005, 29, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Gouveia-Figueira, S.; Nording, M.L. Development and validation of a sensitive UPLC-ESI-MS/MS method for the simultaneous quantification of 15 endocannabinoids and related compounds in milk and other biofluids. Anal. Chem. 2014, 86, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Siegmund, S.V.; Seki, E.; Osawa, Y.; Uchinami, H.; Cravatt, B.F.; Schwabe, R.F. Fatty acid amide hydrolase determines anandamide-induced cell death in the liver. J. Biol. Chem. 2006, 281, 10431–10438. [Google Scholar] [CrossRef]
- Ulloa, N.M.; Deutsch, D.G. Assessment of a Spectrophotometric Assay for Monoacylglycerol Lipase Activity. AAPS J. 2010, 12, 197–201. [Google Scholar] [CrossRef]
- Watkins, B.A.; Kim, J.; Kenny, A.; Pedersen, T.L.; Pappan, K.L.; Newman, J.W. Circulating levels of endocannabinoids and oxylipins altered by dietary lipids in older women are likely associated with previously identified gene targets. Biochim. et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2016, 1861, 1693–1704. [Google Scholar] [CrossRef] [Green Version]
- Eissa, S.; Seada, L.S. Quantitation of bcl-2 protein in bladder cancer tissue by enzyme immunoassay: Comparison with Western blot and immunohistochemistry. Clin. Chem. 1998, 44, 1423–1429. [Google Scholar]






| m/z | RT | ID | Composition | VIP | Log2 (Fold-Change) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control vs. Psoriasis Vulgaris | p Value | Control vs. Psoriatic Arthritis | p Value | Psoriasis Vulgaris vs. Psoriatic Arthritis | p Value | |||||
| 928.6940 | 18.31 | PC(42:2) | PC(20:0/22:2) | 1.97 | 0.63 | 2.34 × 10−5 | 0.34 | 1.17 × 10−3 | 0.29 | ns |
| 954.7113 | 18.12 | PC(44:3) | PC(20:3/24:0) | 1.89 | 0.54 | 3.76 × 10−5 | 0.26 | 2.84 × 10−3 | 0.29 | ns |
| 917.6085 | 3.47 | PI(40:2) | PI(18:2/22:0) | 1.57 | 0.68 | 1.13 × 10−5 | 0.90 | 1.81 × 10−8 | −0.21 | ns |
| 915.5954 | 3.47 | PI(40:3) | PI(18:0/22:3) | 1.57 | 0.75 | 1.13 × 10−5 | 0.97 | 3.91 × 10−8 | −0.21 | ns |
| 1033.7629 | 3.90 | PI(48:0) | PI(24:0/24:0) | 1.52 | −1.06 | 5.81 × 10−4 | −0.85 | 2.06 × 10−3 | −0.21 | ns |
| 857.6755 | 18.16 | SM(d41:2) | SM(d17:0/24:2) | 1.51 | −0.50 | 5.81 × 10−4 | −0.26 | 3.36 × 10−2 | −0.23 | ns |
| 868.6052 | 16.49 | PC(38:4) | PC(18:0/20:4) | 1.47 | −0.181 | 2.00 × 10−3 | 0.31 | 1.66 × 10−2 | 0.26 | ns |
| 1031.7489 | 3.90 | PI(48:1) | PI(24:0/24:1) | 1.44 | −1.64 | 1.03 × 10−3 | −1.43 | 1.93 × 10−3 | −0.2109 | ns |
| 857.5159 | 3.85 | PI(36:4) | PI(16:0/20:4) | 1.39 | 0.68 | 5.81 × 10−4 | 0.89 | 1.26 × 10−5 | −0.21 | ns |
| 840.5732 | 16.71 | PC(36:4) | PC(16:0/20:4) | 1.39 | 0.56 | 3.51 × 10−3 | 0.31 | 3.00 × 10−2 | 0.25 | ns |
| 896.6372 | 16.37 | PC(40:4) | PC(18:0/22:4) | 1.38 | 0.42 | 4.04 × 10−3 | 0.14 | ns | 0.27 | ns |
| 909.5490 | 3.84 | PI(40:6) | PI(18:2/22:4) | 1.38 | 0.61 | 3.33 × 10−4 | 0.82 | 2.76 × 10−6 | −0.21 | ns |
| 792.5746 | 17.39 | PC(32:0) | PC(16:0/16:0) | 1.37 | 0.54 | 4.04 × 10−3 | 0.28 | ns | 0.25 | ns |
| 921.6399 | 3.99 | PI(40:0) | PI(18:0/22:0) | 1.36 | −1.89 | 1.63 × 10−3 | −1.68 | 3.02 × 10−3 | −0.21 | ns |
| 885.5472 | 3.83 | PI(38:4) | PI(18:0/20:4) | 1.35 | 0.57 | 2.61 × 10−4 | −0.26 | ns | −0.21 | ns |
| 554.3463 | 20.37 | LPC(16:0) | 1.33 | −1.77 | 5.81 × 10−4 | −2.6 | 3.53 × 10−6 | 0.83 | ns | |
| 582.3771 | 20.10 | LPC(18:0) | 1.31 | −1.50 | 3.32 × 10−5 | −2.3 | 7.98 × 10−8 | 0.83 | ns | |
| 894.6222 | 16.40 | PC(40:5) | PC(18:1/22:4) | 1.30 | 0.50 | 4.72 × 10−3 | 0.80 | ns | 0.26 | ns |
| 843.6591 | 18.25 | SM(d40:2) | SM(d18:1/22:1) | 1.26 | −0.50 | 4.70 × 10−3 | −0.20 | ns | −0.29 | ns |
| 911.5644 | 3.84 | PI(40:5) | PI(18:1/20:4) | 1.26 | 0.59 | 1.63 × 10−3 | 0.24 | 4.78 × 10−5 | −0.21 | ns |
| Analyzed Parameters | Healthy Subjects | Psoriasis Vulgaris | Psoriatic Arthritis |
|---|---|---|---|
| AEA (pmol/mg protein) | 0.17 ± 0.02 | 0.19 ± 0.03a | 0.23 ± 0.04ax |
| 2-AG (pmol/mg protein) | 1.87 ± 0.18 | 3.24 ± 0.45a | 2.78 ± 0.37ax |
| 2-LG (pmol/mg protein) | 5.59 ± 0.87 | 7.92 ± 0.99a | 7.41 ± 0.93a |
| LEA (pmol/mg protein) | 0.79 ± 0.08 | 0.94 ± 0.15a | 1.17 ± 0.15ax |
| OEA (pmol/mg protein) | 0.33 ± 0.05 | 0.47 ± 0.07a | 0.36 ± 0.05a |
| FAAH (pmol/min/mg protein) | 170 ± 16 | 219 ± 28a | 242 ± 31ax |
| MAGL (pmol/min/mg protein) | 55 ± 6 | 75 ± 10a | 83 ± 14ax |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik, P.; Biernacki, M.; Wroński, A.; Łuczaj, W.; Waeg, G.; Žarković, N.; Skrzydlewska, E. Altered Lipid Metabolism in Blood Mononuclear Cells of Psoriatic Patients Indicates Differential Changes in Psoriasis Vulgaris and Psoriatic Arthritis. Int. J. Mol. Sci. 2019, 20, 4249. https://doi.org/10.3390/ijms20174249
Wójcik P, Biernacki M, Wroński A, Łuczaj W, Waeg G, Žarković N, Skrzydlewska E. Altered Lipid Metabolism in Blood Mononuclear Cells of Psoriatic Patients Indicates Differential Changes in Psoriasis Vulgaris and Psoriatic Arthritis. International Journal of Molecular Sciences. 2019; 20(17):4249. https://doi.org/10.3390/ijms20174249
Chicago/Turabian StyleWójcik, Piotr, Michał Biernacki, Adam Wroński, Wojciech Łuczaj, Georg Waeg, Neven Žarković, and Elżbieta Skrzydlewska. 2019. "Altered Lipid Metabolism in Blood Mononuclear Cells of Psoriatic Patients Indicates Differential Changes in Psoriasis Vulgaris and Psoriatic Arthritis" International Journal of Molecular Sciences 20, no. 17: 4249. https://doi.org/10.3390/ijms20174249
APA StyleWójcik, P., Biernacki, M., Wroński, A., Łuczaj, W., Waeg, G., Žarković, N., & Skrzydlewska, E. (2019). Altered Lipid Metabolism in Blood Mononuclear Cells of Psoriatic Patients Indicates Differential Changes in Psoriasis Vulgaris and Psoriatic Arthritis. International Journal of Molecular Sciences, 20(17), 4249. https://doi.org/10.3390/ijms20174249