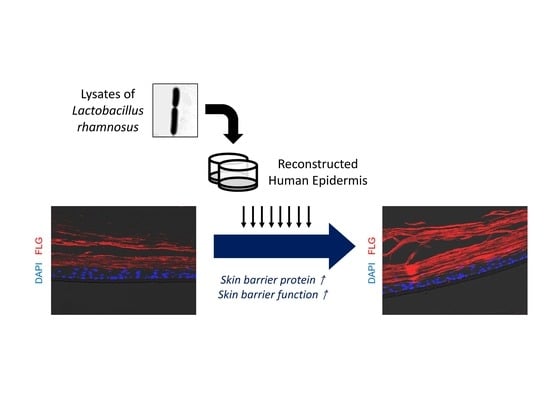
Lysates of a Probiotic, Lactobacillus rhamnosus, Can Improve Skin Barrier Function in a Reconstructed Human Epidermis Model
Abstract
:1. Introduction
2. Results
2.1. Topical Treatment of LR Lysate Increases Epidermal Differentiation Markers of a Reconstructed Human Epidermis, Keraskin™
2.2. LR Lysate Increases the Expression of Skin Barrier Proteins, Loricrin and Filaggrin in KeraskinTM
2.3. Topical Treatment of LR Lysate Attenuated Irritant-Induced Cytotoxicity in Keraskin™
2.4. Topical Treatment of LR Lysate Reduced the Skin Penetration of Rhodamine B
2.5. LR Lysate Protects the Desmosome Degradation in Keraskin™
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. A Reconstructed Human Epidermis Model (Keraskin™)
4.3. WST-1 Assay
4.4. Histological Analysis
4.5. Immunohistochemistry (IHC) and Immunofluorescence Staining (IF)
4.6. RNA Preparation and Quantitative Real-Time PCR (qRT-PCR) for the Determination of mRNA Expression
4.7. Skin Penetration Study
4.8. Transmission Electron Microscopy (TEM)
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LR | Lactobacillus rhamnosus |
| SLS | Sodium lauryl sulfate |
| ETEC | Enterotoxigenic Escherichia coli |
| K5 | Cytokeratin 5 |
| K1 | Cytokeratin 1 |
| K10 | Cytokeratin 10 |
| LOR | Loricrin |
| FLG | Filaggrin |
| BL | Basal layer |
| SL | Spinous layer |
| GL | Granular layer |
| CL | Cornified layer |
| WST-1 | 4-[3-(indophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate |
| PBS | Phosphate-buffered saline |
| PFA | Phosphate-buffered formalin |
| IHC | Immunohistochemistry |
| IF | Immunofluorescence staining |
| PB | Phosphate buffer |
| TEM | Transmission electron microscopy |
References
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Derm. 2008, 17, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, A.; Marekov, L.N.; Steinert, P.M. Assembly of the epidermal cornified cell envelope. J. Cell Sci. 2001, 114, 3069–3070. [Google Scholar] [PubMed]
- Draelos, Z.D. New treatments for restoring impaired epidermal barrier permeability: Skin barrier repair creams. Clin. Derm. 2012, 30, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Segre, J.A. Epidermal barrier formation and recovery in skin disorders. J. Clin. Investig. 2006, 116, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Hudson, T.J. Skin barrier function and allergic risk. Nat. Genet. 2006, 38, 399–400. [Google Scholar] [CrossRef] [PubMed]
- Hengge, U.R.; Ruzicka, T.; Schwartz, R.A.; Cork, M.J. Adverse effects of topical glucocorticosteroids. J. Am. Acad. Derm. 2006, 54, 1–15. [Google Scholar] [CrossRef]
- Voegeli, D.D. Topical steroids and emollients in atopic eczema—Which should be applied first? Pract. Nurs. 2017, 28, 14–20. [Google Scholar] [CrossRef]
- Joo, K.M.; Han, J.Y.; Son, E.D.; Nam, G.W.; Chung, H.Y.; Jeong, H.J.; Cho, J.C.; Lim, K.M. Rapid, simultaneous and nanomolar determination of pyroglutamic acid and cis-/trans-urocanic acid in human stratum corneum by hydrophilic interaction liquid chromatography (HILIC)-electrospray ionization tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 897, 55–63. [Google Scholar] [CrossRef]
- Joo, K.M.; Hwang, J.H.; Bae, S.; Nahm, D.H.; Park, H.S.; Ye, Y.M.; Lim, K.M. Relationship of ceramide-, and free fatty acid-cholesterol ratios in the stratum corneum with skin barrier function of normal, atopic dermatitis lesional and non-lesional skins. J. Derm. Sci. 2015, 77, 71–74. [Google Scholar] [CrossRef]
- Joo, K.M.; Nam, G.W.; Park, S.Y.; Han, J.Y.; Jeong, H.J.; Lee, S.Y.; Kim, H.K.; Lim, K.M. Relationship between cutaneous barrier function and ceramide species in human stratum corneum. J. Derm. Sci. 2010, 60, 47–50. [Google Scholar] [CrossRef]
- Gupta, M.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S.; Rawat, R. Peroxisome proliferator-activated receptors (PPARs) and PPAR agonists: The ‘future’ in dermatology therapeutics? Arch. Derm. Res. 2015, 307, 767–780. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation; World Health Organization (WHO): Rome, Italy, 2006. [Google Scholar]
- Simmering, R.; Blaut, M. Pro- and prebiotics—The tasty guardian angels? Appl. Microbiol. Biotechnol. 2001, 55, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Roudsari, M.R.; Karimi, R.; Sohrabvandi, S.; Mortazavian, A.M. Health Effects of Probiotics on the Skin. Crit. Rev. Food Sci. Nutr. 2015, 55, 1219–1240. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; Batsman, A.; Salminen, S. Probiotics for the skin: A new area of potential application? Lett. Appl. Microbiol. 2003, 36, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, Y.H.; Yang, J.C.; Yang, G.Y.; Zhou, D.; Wang, J.F. A Selected Lactobacillus rhamnosus Strain Promotes EGFR-Independent Akt Activation in an Enterotoxigenic Escherichia coli K88-Infected IPEC-J2 Cell Model. PLoS ONE 2015, 10, e0125717. [Google Scholar] [CrossRef] [PubMed]
- Mohammedsaeed, W.; Cruickshank, S.; McBain, A.J.; O’Neill, C.A. Lactobacillus rhamnosus GG Lysate Increases Re-Epithelialization of Keratinocyte Scratch Assays by Promoting Migration. Sci. Rep. 2015, 5, 16147. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R.; McBain, A.J.; O’Neill, C.A. Strain-Dependent Augmentation of Tight-Junction Barrier Function in Human Primary Epidermal Keratinocytes by Lactobacillus and Bifidobacterium Lysates. Appl. Env. Microbiol. 2013, 79, 4887–4894. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.M.; Lee, S.H.; Jang, W.H.; Jung, H.S.; Heo, Y.; Park, Y.H.; Bae, S.; Lim, K.M.; Seok, S.H. KeraSkin-VM: A novel reconstructed human epidermis model for skin irritation tests. Toxicol. Vitr. 2014, 28, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Park, H.; Choi, D.W.; Nam, K.T.; Lim, K.M. Investigation of dermal toxicity of ionic liquids in monolayer-cultured skin cells and 3D reconstructed human skin models. Toxicol. Vitr. 2018, 46, 194–202. [Google Scholar] [CrossRef]
- zur Muhlen, A.; Klotz, A.; Weimans, S.; Veeger, M.; Thorner, B.; Diener, B.; Hermann, M. Using skin models to assess the effects of a protection cream on skin barrier function. Skin Pharm. Physiol. 2004, 17, 167–175. [Google Scholar] [CrossRef]
- Darlenski, R.; Kazandjieva, J.; Tsankov, N. Skin barrier function: Morphological basis and regulatory mechanisms. J. Clin. Med. 2011, 4, 36–45. [Google Scholar]
- Sandilands, A.; Sutherland, C.; Irvine, A.D.; McLean, W.H. Filaggrin in the frontline: Role in skin barrier function and disease. J. Cell Sci. 2009, 122, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Sandilands, A.; Terron-Kwiatkowski, A.; Hull, P.R.; O’Regan, G.M.; Clayton, T.H.; Watson, R.M.; Carrick, T.; Evans, A.T.; Liao, H.; Zhao, Y.; et al. Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat. Genet. 2007, 39, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Torma, H.; Lindberg, M.; Berne, B. Skin barrier disruption by sodium lauryl sulfate-exposure alters the expressions of involucrin, transglutaminase 1, profilaggrin, and kallikreins during the repair phase in human skin in vivo. J. Investig. Derm. 2008, 128, 1212–1219. [Google Scholar] [CrossRef]
- Calautti, E.; Li, J.; Saoncella, S.; Brissette, J.L.; Goetinck, P.F. Phosphoinositide 3-Kinase Signaling to Akt Promotes Keratinocyte Differentiation Versus Death. J. Biol. Chem. 2005, 280, 32856–32865. [Google Scholar] [CrossRef] [Green Version]
- Rogerson, C.; O’Shaughnessy, R. Protein kinases involved in epidermal barrier formation: The AKT family and other animals. Exp. Derm. 2018, 27. [Google Scholar] [CrossRef]
- Yuki, T.; Yoshida, H.; Akazawa, Y.; Komiya, A.; Sugiyama, Y.; Inoue, S. Activation of TLR2 Enhances Tight Junction Barrier in Epidermal Keratinocytes. J. Immunol. 2011, 187, 3230–3237. [Google Scholar] [CrossRef] [Green Version]
- Herrera, A.G. Microbiological analysis of cosmetics. Methods Mol. Biol. 2004, 268, 293–295. [Google Scholar]
- Lebeer, S.; Oerlemans, E.; Claes, I.; Wuyts, S.; Henkens, T.; Spacova, I.; van den Broek, M.; Tuyaerts, I.; Wittouck, S.; De Boeck, I. Topical cream with live lactobacilli modulates the skin microbiome and reduce acne symptoms. bioRxiv 2018, 463307. [Google Scholar] [CrossRef]
- Lee, M.; Hwang, J.H.; Lim, K.M. Alternatives to In Vivo Draize Rabbit Eye and Skin Irritation Tests with a Focus on 3D Reconstructed Human Cornea-Like Epithelium and Epidermis Models. Toxicol Res. 2017, 33, 191–203. [Google Scholar] [CrossRef]
- Joo, K.M.; Kim, S.; Koo, Y.J.; Lee, M.; Lee, S.H.; Choi, D.; Lim, K.M. Development and validation of UPLC method for WST-1 cell viability assay and its application to MCTT HCE eye irritation test for colorful substances. Toxicol. Vitr. 2019, 60, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Park, H.; Jeon, S.W.; Bang, J.; Chung, K.Y.; Choi, D.W.; Kim, E.; Lim, K.M. Novel anti-melanogenic hexapeptoids, PAL-10 and PAL-12. Arch. Derm. Res. 2015, 307, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Park, H.; Lee, S.H.; Kim, M.J.; Kim, E.J.; Lim, K.M. PAL-12, a new anti-aging hexa-peptoid, inhibits UVB-induced photoaging in human dermal fibroblasts and 3D reconstructed human full skin model, Keraskin-FT. Arch. Derm. Res. 2017, 309, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Nounou, M.I.; Zaghloul, T.I.; Ahmed, N.A.; Eid, A.A.; El-Khordagui, L.K. Skin permeability enhancement by Bacillus subtilis alkaline protease: Application to transdermal drug delivery. Int. J. Pharm. 2017, 529, 423–432. [Google Scholar] [CrossRef] [PubMed]







| Target Protein | Test Chemical | Localization | Score (1–3) |
|---|---|---|---|
| K5 (Cytokeratin5) | Control | BL, SL | 1 |
| LR | BL, SL, GL, CL | 3 | |
| K1 (Cytokeratin1) | Control | BL, SL | 2 |
| LR | BL, SL, GL, CL | 3 | |
| K10 (Cytokeratin10) | Control | SL, GL | 3 |
| LR | SL, GL | 3 | |
| LOR (Loricrin) | Control | SL, GL, CL | 2 |
| LR | SL, GL, CL | 3 | |
| FLG (Filaggrin) | Control | GL, CL | 2 |
| LR | BL, GL, CL | 3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, Y.-O.; Jeong, H.; Cho, Y.; Lee, E.-O.; Jang, H.-W.; Kim, J.; Nam, K.T.; Lim, K.-M. Lysates of a Probiotic, Lactobacillus rhamnosus, Can Improve Skin Barrier Function in a Reconstructed Human Epidermis Model. Int. J. Mol. Sci. 2019, 20, 4289. https://doi.org/10.3390/ijms20174289
Jung Y-O, Jeong H, Cho Y, Lee E-O, Jang H-W, Kim J, Nam KT, Lim K-M. Lysates of a Probiotic, Lactobacillus rhamnosus, Can Improve Skin Barrier Function in a Reconstructed Human Epidermis Model. International Journal of Molecular Sciences. 2019; 20(17):4289. https://doi.org/10.3390/ijms20174289
Chicago/Turabian StyleJung, Ye-On, Haengdueng Jeong, Yejin Cho, Eun-Ok Lee, Hye-Won Jang, Jinwook Kim, Ki Taek Nam, and Kyung-Min Lim. 2019. "Lysates of a Probiotic, Lactobacillus rhamnosus, Can Improve Skin Barrier Function in a Reconstructed Human Epidermis Model" International Journal of Molecular Sciences 20, no. 17: 4289. https://doi.org/10.3390/ijms20174289
APA StyleJung, Y. -O., Jeong, H., Cho, Y., Lee, E. -O., Jang, H. -W., Kim, J., Nam, K. T., & Lim, K. -M. (2019). Lysates of a Probiotic, Lactobacillus rhamnosus, Can Improve Skin Barrier Function in a Reconstructed Human Epidermis Model. International Journal of Molecular Sciences, 20(17), 4289. https://doi.org/10.3390/ijms20174289