A Novel Tanshinone Analog Exerts Anti-Cancer Effects in Prostate Cancer by Inducing Cell Apoptosis, Arresting Cell Cycle at G2 Phase and Blocking Metastatic Ability
Abstract
:1. Introduction
2. Results
2.1. TC7 Inhibited the Proliferation of PCa Cells and Induced Apoptosis
2.2. TC7 Arrested the Cell Cycle at G2/M Phase in PCa Cells
2.3. TC7 Inhibited Migration and Invasion Ability of PCa Cells
2.4. TC7 Inhibited the Proliferation of Human PCa Cells in Vivo
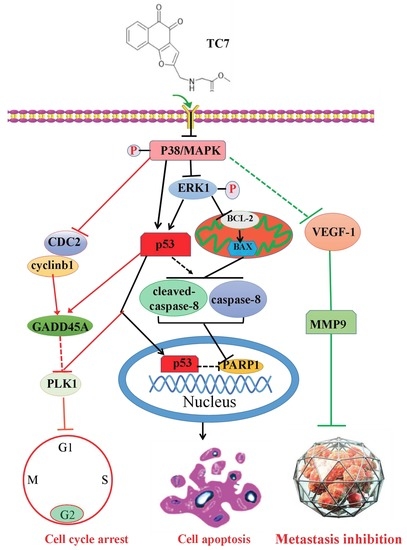
3. Discussion
4. Materials and Methods
4.1. Ethical Issue
4.2. Cell Culture
4.3. TC7 Compound
4.4. Antibodies
4.5. MTT Assay
4.6. EDU-DNA Synthesis Assay
4.7. Invasion and Migration Assay
4.8. Cell Cycle Assay
4.9. Cell Apoptosis Assay
4.10. Western Blot Assay
4.11. Mouse Xenograft Tumor Model and TC7Antitumor Effect
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TC7 | 2-((Glycine methyl ester)methyl)-naphtho |
| LNCAP | Human prostate cancer cell |
| PCa | Prostate cancer |
| DMEM | Dulbecco’s Modified Eagle Medium |
| MTT | 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide, |
| DMSO | Dimethyl sulfoxide |
| DNA | Deoxyribonucleic acid |
| PBS | Phosphate buffer saline |
| PI | Propidium Iodide |
| FITC | Fluoresceine isothiocyanate |
| ERK1/2 | Extracellular regulated protein kinases 1 |
| P-p38 | Phospho-mitogen-activated protein kinases |
| p-38 | Mitogen-activated protein kinases |
| p53 | Phosphoprotein p53 |
| Bcl-2 | B-cell lymphoma-2 |
| MMP-1 | Matrix metalloproteinase 1 |
| MMP-9 | Matrix metalloproteinase 9 |
| VEGF1 | Vascular endothelial growth factor |
| BAX | Bcl-2 associated X protein |
| PARP1 | Poly ADP-ribose polymerase-1 |
| Caspase8 | Cystine containing asparate specific protease |
| Cleaved-Caspase8 | Cleaved-Cystine containing asparate specific protease |
| CDC2 | Cell division cycle gene |
| GADD45A | Growth arrest DNA damage |
| PLK1 | Polo-like Kinase 1 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| Cyclinb1 | Cyclin box-1 |
| EDU-DNA | 5-ethynyl-2′-deoxyuridine-DNA |
| BSA | Bovine serum albumin |
| EDTA | Ethylene Diamine Tetraacetic Acid |
| BCA | Bicinchoninic acid |
| PVDF | PolyVinylideneFluoride |
References
- Wang, G.; Zhao, D.; Spring, D.J.; Depinho, R.A. Genetics and biology of prostate cancer. Genome Res. 2018, 32, 1105–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanan, S.; Srinivas, S.; Feldman, D. Androgen–glucocorticoid interactions in the era of novel prostate cancer therapy. Nat. Rev. Urol. 2015, 13, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Castanares, M.A.; Copeland, B.T.; Chowdhury, W.H.; Liu, M.M.; Rodriguez, R.; Pomper, M.G.; Lupold, S.E.; Foss, C.A. Characterization of a novel metastatic prostate cancer cell line of LNCaP origin. Prostate 2016, 76, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Ishikura, N.; Kawata, H.; Nishimoto, A.; Nakamura, R.; Ishii, N.; Aoki, Y. Establishment and characterization of an androgen receptor-dependent, androgen-independent human prostate cancer cell line, LNCaP-CS10. Prostate 2010, 70, 457–466. [Google Scholar] [CrossRef]
- Lee, S.O.; Dutt, S.S.; Nadiminty, N.; Pinder, E.; Liao, H.; Gao, A.C. Development of an androgen-deprivation induced and androgen suppressed human prostate cancer cell line. Prostate 2007, 67, 1293–1300. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, C.; Yao, S.; Wang, Z.; Xu, L.; Yang, R.; Meng, X.; Wu, J.; Zhou, L.; Sun, Z. Proteomic analysis of human prostate cancer PC-3M-1E8 cells and PC-3M-2B4 cells of same origin but with different metastatic potential. PLoS ONE 2018, 13, e0206139. [Google Scholar] [CrossRef]
- Crawford, E.D.; Higano, C.S.; Shore, N.D.; Hussain, M.; Petrylak, D.P. Treating Patients with Metastatic Castration Resistant Prostate Cancer: A Comprehensive Review of Available Therapies. J. Urol. 2015, 194, 1537–1547. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Zhu, X.; Albertsen, P.C.; Andriole, G.L.; Roobol, M.J.; Schroder, F.H.; Vickers, A.J. Risk-based prostate cancer screening. Eur. Urol. 2012, 61, 652–661. [Google Scholar] [CrossRef]
- Attard, G.; Parker, C.; Eeles, R.A.; Schroder, F.; Tomlins, S.A.; Tannock, I.; Drake, C.G.; de Bono, J.S. Prostate cancer. Lancet (Lond. Engl.) 2016, 387, 70–82. [Google Scholar] [CrossRef]
- Wilt, T.J.; Brawer, M.K.; Jones, K.M.; Barry, M.J.; Aronson, W.J.; Fox, S.; Gingrich, J.R.; Wei, J.T.; Gilhooly, P.; Grob, B.M.; et al. Radical Prostatectomy versus Observation for Localized Prostate Cancer. N. Engl. J. Med. 2012, 367, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Mullins, J.K.; Feng, Z.; Trock, B.J.; Epstein, J.I.; Walsh, P.C.; Loeb, S. The Impact of Anatomical Radical Retropubic Prostatectomy on Cancer Control: The 30-Year Anniversary. J. Urol. 2012, 188, 2219–2224. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Kim, B. Anti-Cancer Natural Products and Their Bioactive Compounds Inducing ER Stress-Mediated Apoptosis: A Review. Nutrients 2018, 10, 1021. [Google Scholar] [CrossRef] [PubMed]
- David, B.; Wolfender, J.-L.; Dias, D.A. The pharmaceutical industry and natural products: Historical status and new trends. Phytochem. Rev. 2014, 14, 299–315. [Google Scholar] [CrossRef]
- Bernardini, S.; Tiezzi, A.; Laghezza Masci, V.; Ovidi, E. Natural products for human health: An historical overview of the drug discovery approaches. Nat. Prod. Res. 2018, 32, 1926–1950. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [Green Version]
- Shakeri, A.; Cicero, A.F.G.; Panahi, Y.; Mohajeri, M.; Sahebkar, A. Curcumin: A naturally occurring autophagy modulator. J. Cell Physiol. 2019, 234, 5643–5654. [Google Scholar] [CrossRef]
- Zou, K.; Li, Z.; Zhang, Y.; Zhang, H.Y.; Li, B.; Zhu, W.L.; Shi, J.Y.; Jia, Q.; Li, Y.M. Advances in the study of berberine and its derivatives: A focus on anti-inflammatory and anti-tumor effects in the digestive system. Acta Pharmacol. Sin. 2017, 38, 157–167. [Google Scholar] [CrossRef]
- Millimouno, F.M.; Dong, J.; Yang, L.; Li, J.; Li, X. Targeting Apoptosis Pathways in Cancer and Perspectives with Natural Compounds from Mother Nature. Cancer Prev. Res. 2014, 7, 1081–1107. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.M.; Shim, J.S.; Jung, H.J.; Kwon, H.J. Cryptotanshinone but not tanshinone IIA inhibits angiogenesis in vitro. Exp. Mol. Med. 2005, 37, 133–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, B.; You, M.G.; Ling, J.J.; Wei, L.L.; Wang, K.; Li, W.W.; Chen, T.; Du, Q.M.; Ji, H. Regulation of antioxidant system, lipids and fatty acid beta-oxidation contributes to the cardioprotective effect of sodium tanshinone IIA sulphonate in isoproterenol-induced myocardial infarction in rats. Atherosclerosis 2013, 230, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zuo, Z.; Chow, M.S.S. Danshen: An Overview of Its Chemistry, Pharmacology, Pharmacokinetics, and Clinical Use. J. Clin. Pharmacol. 2005, 45, 1345–1359. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Little, P.J.; Xu, S. Atheroprotective Effects and Molecular Targets of Tanshinones Derived from Herbal Medicine Danshen. Med. Res. Rev. 2018, 38, 201–228. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hu, H.; Guan, J.; Da, J.; Xie, Y.; Liu, Y.; Kong, R.; Song, G.; Zhou, H. Synthesis of novel tanshinone derivatives for treatment of castration-resistant prostate cancer. Chem. Biol. Drug Des. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, S.; Fang, J.; Peng, B.; Zhang, Y.; Cao, T. Tanshinone IIA inhibits cell proliferation and tumor growth by downregulating STAT3 in human gastric cancer. Exp. Ther. Med. 2018, 16, 2931–2937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.Y.; Hsu, P.I.; Wu, D.C.; Chen, T.C.; Jarman, A.P.; Powell, L.M.; Chen, A. SUMOs Mediate the Nuclear Transfer of p38 and p-p38 during Helicobacter Pylori Infection. Int. J. Mol. Sci. 2018, 19, 2482. [Google Scholar] [CrossRef]
- Wu, Y.J.; Su, T.R.; Dai, G.F.; Su, J.H.; Liu, C.I. Flaccidoxide-13-Acetate-Induced Apoptosis in Human Bladder Cancer Cells is through Activation of p38/JNK, Mitochondrial Dysfunction, and Endoplasmic Reticulum Stress Regulated Pathway. Mar. Drugs 2019, 17, 287. [Google Scholar] [CrossRef]
- Schonrogge, M.; Kerndl, H.; Zhang, X.; Kumstel, S.; Vollmar, B.; Zechner, D. alpha-cyano-4-hydroxycinnamate impairs pancreatic cancer cells by stimulating the p38 signaling pathway. Cell. Signal. 2018, 47, 101–108. [Google Scholar] [CrossRef]
- Chen, S.; Ren, Z.; Yu, D.; Ning, B.; Guo, L. DNA damage-induced apoptosis and mitogen-activated protein kinase pathway contribute to the toxicity of dronedarone in hepatic cells. Environ. Mol. Mutagen. 2018, 59, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, G.P. The TGFbeta-induced phosphorylation and activation of p38 mitogen-activated protein kinase is mediated by MAP3K4 and MAP3K10 but not TAK1. Open Biol. 2013, 3, 130067. [Google Scholar] [CrossRef] [PubMed]
- Bakin, A.V.; Rinehart, C.; Tomlinson, A.K.; Arteaga, C.L. p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. J. Cell Sci. 2002, 115, 3193–3206. [Google Scholar] [PubMed]
- Yu, L.; Hebert, M.C.; Zhang, Y.E. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J. 2002, 21, 3749–3759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aasrum, M.; Thoresen, G.H.; Christoffersen, T.; Brusevold, I.J. p38 differentially regulates ERK, p21, and mitogenic signalling in two pancreatic carcinoma cell lines. J. Cell Commun. Signal. 2018, 12, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Vougiouklakis, T.; Sone, K.; Saloura, V.; Cho, H.-S.; Suzuki, T.; Dohmae, N.; Alachkar, H.; Nakamura, Y.; Hamamoto, R. SUV420H1 enhances the phosphorylation and transcription of ERK1 in cancer cells. Oncotarget 2015, 6, 43162–43171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen-Armon, M.; Visochek, L.; Rozensal, D.; Kalal, A.; Geistrikh, I.; Klein, R.; Bendetz-Nezer, S.; Yao, Z.; Seger, R. DNA-Independent PARP-1 Activation by Phosphorylated ERK2 Increases Elk1 Activity: A Link to Histone Acetylation. Mol. Cell 2007, 25, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-X.; Li, Y.; Sun, C.; Jiang, D.; Lin, Y.-J.; Jin, F.-X.; Lee, S.-K.; Jin, Y.-H. p53-dependent Fas expression is critical for Ginsenoside Rh2 triggered caspase-8 activation in HeLa cells. Protein Cell 2014, 5, 224–234. [Google Scholar] [CrossRef]
- Ahn, C.-H.; Hong, K.-O.; Jin, B.; Lee, W.; Jung, Y.C.; Lee, H.; Shin, J.-A.; Cho, S.-D.; Hong, S.D. Contribution of p38 MAPK Pathway to Norcantharidin-Induced Programmed Cell Death in Human Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 3487. [Google Scholar] [CrossRef]
- Saggioro, F.P.; Neder, L.; Stávale, J.N.; Paixão-Becker, A.N.P.; Malheiros, S.M.; Soares, F.A.; Pittella, J.E.H.; Matias, C.C.M.; Colli, B.O.; Carlotti, C.G.; et al. Fas, FasL, and cleaved caspases 8 and 3 in glioblastomas: A tissue microarray-based study. Pathol. Res. Pract. 2014, 210, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Sobhan, P.K.; Seervi, M.; Deb, L.; Varghese, S.; Soman, A.; Joseph, J.; Mathew, K.A.; Raghu, G.; Thomas, G.; E, S.; et al. Calpain and Reactive Oxygen Species Targets Bax for Mitochondrial Permeabilisation and Caspase Activation in Zerumbone Induced Apoptosis. PLoS ONE 2013, 8, e59350. [Google Scholar] [CrossRef] [PubMed]
- Bulavin, D.V.; Saito, S.; Hollander, M.C.; Sakaguchi, K.; Anderson, C.W.; Appella, E.; Fornace, A.J. Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 1999, 18, 6845–6854. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Mao, X.O.; Sun, Y.; Xia, Z.; Greenberg, D.A. p38 Mitogen-activated Protein Kinase Mediates Hypoxic Regulation of Mdm2 and p53 in Neurons. J. Biol. Chem. 2002, 277, 22909–22914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Héron-Milhavet, L.; Leroith, D. Insulin-like Growth Factor I Induces MDM2-dependent Degradation of p53 via the p38 MAPK Pathway in Response to DNA Damage. J. Biol. Chem. 2002, 277, 15600–15606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alarcon-Vargas, D.; Ronai, Z. p53-Mdm2--the affair that never ends. Carcinogenesis 2002, 23, 541–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elias, B.; Laine, A.; Ronai, Z. Phosphorylation of MdmX by CDK2/Cdc2p34 is required for nuclear export of Mdm2. Oncogene 2005, 24, 2574–2579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-H.; Seong, M.-A.; Lee, H.-Y. p38 MAPK-induced MDM2 degradation confers paclitaxel resistance through p53-mediated regulation of EGFR in human lung cancer cells. Oncotarget 2016, 7, 8184–8199. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.L.; Swisher, E.M.; Kaufmann, S.H. Poly (ADP-Ribose) Polymerase Inhibitors: Recent Advances and Future Development. J. Clin. Oncol. 2015, 33, 1397–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulavin, D.V.; Fornace, A.J., Jr. p38 MAP kinase’s emerging role as a tumor suppressor. Adv. Cancer Res. 2004, 92, 95–118. [Google Scholar] [PubMed]
- Xu, Y.; Li, N.; Xiang, R.; Sun, P. Emerging roles of the p38 MAPK and PI3K/AKT/mTOR pathways in oncogene-induced senescence. Trends Biochem. Sci. 2014, 39, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Hou, G.; Chen, B.; Xu, W.; Zhao, H.; Liu, K.; Yao, H. Expression level of CDC2 gene in osteosarcoma and its clinical significance. Oncol. Lett. 2018, 15, 7884–7888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.-C.; Hung, C.-M.; Yang, Y.-R.; Lee, M.-J.; Hsu, Y.-C. Sulforaphane induced cell cycle arrest in the G2/M phase via the blockade of cyclin B1/CDC2 in human ovarian cancer cells. J. Ovarian Res. 2013, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.C.; Su, N.; Shi, X.J.; Zhao, W.; Ke, Y.; Zi, X.; Zhao, N.M.; Qin, Y.H.; Zhao, H.W.; Liu, H.M. Jaridonin-induced G2/M phase arrest in human esophageal cancer cells is caused by reactive oxygen species-dependent Cdc2-tyr15 phosphorylation via ATM-Chk1/2-Cdc25C pathway. Toxicol. Appl. Pharmacol. 2015, 282, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liao, Y.; Long, D.; Yu, T.; Shen, F.; Lin, X. The Cdc2/Cdk1 inhibitor, purvalanol A, enhances the cytotoxic effects of taxol through Op18/stathmin in non-small cell lung cancer cells in vitro. Int. J. Mol. Med. 2017, 40, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, K.; Bai, Y.; Zhu, X.; Chen, Z.; Wang, C.; Zhao, Y.; Li, M. Screening of diagnostic markers for osteosarcoma. Mol. Med. Rep. 2014, 10, 2415–2420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Leng, C.; Wu, C.; Zhang, Z.; Dou, L.; Luo, X.; Zhang, B.; Chen, X. Smad4 sensitizes colorectal cancer to 5-fluorouracil through cell cycle arrest by inhibiting the PI3K/Akt/CDC2/survivin cascade. Oncol. Rep. 2016, 35, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Moskalev, A.A.; Smit-McBride, Z.; Shaposhnikov, M.V.; Plyusnina, E.N.; Zhavoronkov, A.; Budovsky, A.; Tacutu, R.; Fraifeld, V.E. Gadd45 proteins: Relevance to aging, longevity and age-related pathologies. Ageing Res. Rev. 2012, 11, 51–66. [Google Scholar] [CrossRef] [Green Version]
- Wingert, S.; Thalheimer, F.B.; Haetscher, N.; Rehage, M.; Schroeder, T.; Rieger, M.A. DNA-damage response gene GADD45A induces differentiation in hematopoietic stem cells without inhibiting cell cycle or survival. Stem Cells (Dayt. Ohio) 2016, 34, 699–710. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.; Farzan, R.; Ali, S.; Buluwela, L.; Saurin, A.T.; Meek, D.W. Author Correction: The responses of cancer cells to PLK1 inhibitors reveal a novel protective role for p53 in maintaining centrosome separation. Sci. Rep. 2018, 8, 5237. [Google Scholar] [CrossRef] [Green Version]
- Taylor, W.R.; Stark, G.R. Regulation of the G2/M transition by p53. Oncogene 2001, 20, 1803–1815. [Google Scholar] [CrossRef] [Green Version]
- Zachary, I. Neuroprotective Role of Vascular Endothelial Growth Factor: Signalling Mechanisms, Biological Function, and Therapeutic Potential. Neurosignals 2005, 14, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Peach, C.J.; Mignone, V.W.; Arruda, M.A.; Alcobia, D.C.; Hill, S.J.; Kilpatrick, L.E.; Woolard, J. Molecular Pharmacology of VEGF-A Isoforms: Binding and Signalling at VEGFR2. Int. J. Mol. Sci. 2018, 19, 1264. [Google Scholar] [CrossRef] [PubMed]
- Engsig, M.T.; Chen, Q.-J.; Vu, T.H.; Pedersen, A.-C.; Therkidsen, B.; Lund, L.R.; Henriksen, K.; Lenhard, T.; Foged, N.T.; Werb, Z.; et al. Matrix Metalloproteinase 9 and Vascular Endothelial Growth Factor Are Essential for Osteoclast Recruitment into Developing Long Bones. J. Cell Biol. 2000, 151, 879–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.H.; Kwon, H.J.; Kim, D.S. Matrix metalloproteinase 9 (MMP-9)-dependent processing of betaig-h3 protein regulates cell migration, invasion, and adhesion. J. Biol. Chem. 2012, 287, 38957–38969. [Google Scholar] [CrossRef] [PubMed]
- Foxton, R.; Osborne, A.; Martin, K.R.; Ng, Y.S.; Shima, D.T. Distal retinal ganglion cell axon transport loss and activation of p38 MAPK stress pathway following VEGF-A antagonism. Cell Death Dis. 2016, 7, e2212. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.L.; Sun, B.F.; Huang, L.R.; Yuan, H.B.; Zhang, S.; Chen, J.; Yu, Z.J.; Luo, H. Potent Inhibition of miR-34b on Migration and Invasion in Metastatic Prostate Cancer Cells by Regulating the TGF-beta Pathway. Int. J. Mol. Sci. 2017, 18, 2762. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Wen, Z.-H.; Wan, K.; Yuan, D.; Zeng, X.; Liang, G.; Zhu, J.; Xu, B.; Luo, H. A novel synthesized 3′, 5′-diprenylated chalcone mediates the proliferation of human leukemia cells by regulating apoptosis and autophagy pathways. Biomed. Pharmacother. 2018, 106, 794–804. [Google Scholar] [CrossRef]
- Kongkathip, N.; Kongkathip, B.; Siripong, P.; Sangma, C.; Luangkamin, S.; Niyomdecha, M.; Pattanapa, S.; Piyaviriyagul, S.; Kongsaeree, P. Potent antitumor activity of synthetic 1,2-Naphthoquinones and 1,4-Naphthoquinones. Bioorganic Med. Chem. 2003, 11, 3179–3191. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Fu, W.; Zheng, X.; Ren, L.; Liu, S.; Wang, J.; Ji, T.; Du, G. 3-O-acetyl-11-keto-beta-boswellic acid exerts anti-tumor effects in glioblastoma by arresting cell cycle at G2/M phase. J. Exp. Clin. Cancer Res. CR 2018, 37, 132. [Google Scholar] [CrossRef]














© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zeng, X.; Li, S.; Sun, Z.; Yu, J.; Chen, C.; Shen, X.; Pan, W.; Luo, H. A Novel Tanshinone Analog Exerts Anti-Cancer Effects in Prostate Cancer by Inducing Cell Apoptosis, Arresting Cell Cycle at G2 Phase and Blocking Metastatic Ability. Int. J. Mol. Sci. 2019, 20, 4459. https://doi.org/10.3390/ijms20184459
Wang M, Zeng X, Li S, Sun Z, Yu J, Chen C, Shen X, Pan W, Luo H. A Novel Tanshinone Analog Exerts Anti-Cancer Effects in Prostate Cancer by Inducing Cell Apoptosis, Arresting Cell Cycle at G2 Phase and Blocking Metastatic Ability. International Journal of Molecular Sciences. 2019; 20(18):4459. https://doi.org/10.3390/ijms20184459
Chicago/Turabian StyleWang, Mengling, Xueyi Zeng, Shengyou Li, Zekun Sun, Jia Yu, Chao Chen, Xiangchun Shen, Weidong Pan, and Heng Luo. 2019. "A Novel Tanshinone Analog Exerts Anti-Cancer Effects in Prostate Cancer by Inducing Cell Apoptosis, Arresting Cell Cycle at G2 Phase and Blocking Metastatic Ability" International Journal of Molecular Sciences 20, no. 18: 4459. https://doi.org/10.3390/ijms20184459
APA StyleWang, M., Zeng, X., Li, S., Sun, Z., Yu, J., Chen, C., Shen, X., Pan, W., & Luo, H. (2019). A Novel Tanshinone Analog Exerts Anti-Cancer Effects in Prostate Cancer by Inducing Cell Apoptosis, Arresting Cell Cycle at G2 Phase and Blocking Metastatic Ability. International Journal of Molecular Sciences, 20(18), 4459. https://doi.org/10.3390/ijms20184459