Mechanisms of Stress-Induced Spermatogenesis Impairment in Male Rats Following Unpredictable Chronic Mild Stress (uCMS)
Abstract
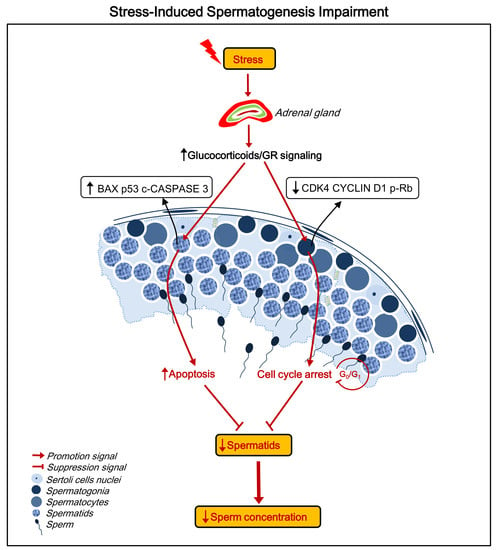
:1. Introduction
2. Results
2.1. The Procedure and Verification of the uCMS Model
2.2. Effects of Stress on Body Weight, Testicular Structure, and Semen Parameters
2.3. Stress Decreases the Number of Spermatids
2.4. Stress Induces Apoptosis of Spermatids
2.5. Stress Induces Cell Cycle Arrest at the G0/G1 Phase in Spermatogonia
2.6. Stress Activates the Hypothalamic-Pituitary-Adrenal (HPA) Axis and Elevates GR Levels in Testes
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Ethics Statement
4.3. Drug Solutions and Administrations
4.4. Experimental Design, Stress Model, and Behavioral Assessment
4.5. Sperm Concentration and Sperm Motility Assessments
4.6. Serum Corticosterone and Testosterone Levels
4.7. Tissue Histology and Morphometry
4.8. Western Blot Analysis
4.9. Terminal Deoxynucleotidyl Transferase-Mediated dUTP-Biotin Nick End Labeling (TUNEL) Assay
4.10. Cellular DNA Content Determination
4.11. Immunofluorescence Staining
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Amelar, R.D.; Dubin, L.; Lawrence, J. Other Factors Affecting Male Fertility; Amelar, R., Dubin, L., Walsh, P.C., Eds.; WB Saunders: Philadelphia, PA, USA, 1977; Chapter 4; pp. 69–101. [Google Scholar]
- Nordkap, L.; Jensen, T.K.; Hansen, A.M.; Lassen, T.H.; Bang, A.K.; Joensen, U.N.; Blomberg Jensen, M.; Skakkebaek, N.E.; Jorgensen, N. Psychological stress and testicular function: A cross-sectional study of 1215 Danish men. Fertil. Steril. 2016, 105, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Zorn, B.; Sucur, V.; Stare, J.; Meden-Vrtovec, H. Decline in sex ratio at birth after 10-day war in Slovenia: Brief communication. Hum. Reprod. 2002, 17, 3173–3177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorn, B.; Auger, J.; Velikonja, V.; Kolbezen, M.; Meden-Vrtovec, H. Psychological factors in male partners of infertile couples: Relationship with semen quality and early miscarriage. Int. J. Androl. 2008, 31, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Yuan, K.M.; Zhou, H.Y.; Bu, T.; Su, H.; Liu, S.; Zhu, Q.; Wang, Y.; Hu, Y.; Shan, Y.; et al. Time-course changes of steroidogenic gene expression and steroidogenesis of rat Leydig cells after acute immobilization stress. Int. J. Mol. Sci. 2014, 15, 21028–21044. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Rojas, A.L.; Garcia-Lorenzana, M.; Aragon-Martinez, A.; Gomez-Quiroz, L.E.; Retana-Marquez Mdel, S. Intrinsic and extrinsic apoptotic pathways are involved in rat testis by cold water immersion-induced acute and chronic stress. Syst. Biol. Reprod. Med. 2015, 61, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Yazawa, H.; Sasagawa, I.; Nakada, T. Apoptosis of testicular germ cells induced by exogenous glucocorticoid in rats. Hum. Reprod. 2000, 15, 1917–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, H.B.; Tong, M.H.; Hu, Y.Q.; You, H.Y.; Guo, Q.S.; Ge, R.S.; Hardy, M.P. Mechanisms of glucocorticoid-induced Leydig cell apoptosis. Mol. Cell Endocrinol. 2003, 199, 153–163. [Google Scholar] [CrossRef]
- Orr, T.E.; Mann, D.R. Role of glucocorticoids in the stress-induced suppression of testicular steroidogenesis in adult male rats. Horm. Behav. 1992, 26, 350–363. [Google Scholar] [CrossRef]
- Smith, L.B.; Walker, W.H. The regulation of spermatogenesis by androgens. Semin. Cell Dev. Biol. 2014, 30, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Hazra, R.; Upton, D.; Jimenez, M.; Desai, R.; Handelsman, D.J.; Allan, C.M. In vivo actions of the Sertoli cell glucocorticoid receptor. Endocrinology 2014, 155, 1120–1130. [Google Scholar] [CrossRef]
- Motta, S.C.; Canteras, N.S. Restraint stress and social defeat: What they have in common. Physiol. Behav. 2015, 146, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Orazizadeh, M.; Khorsandi, L.S.; Hashemitabar, M. Toxic effects of dexamethasone on mouse testicular germ cells. Andrologia 2010, 42, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Antoniuk, S.; Bijata, M.; Ponimaskin, E.; Wlodarczyk, J. Chronic Unpredictable Mild Stress for Modeling Depression in Rodents:Meta-analysis of Model Reliability. Neurosci. Biobehav. Rev. 2018, 99, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Novati, A.; Yu-Taeger, L.; Gonzalez Menendez, I.; Quintanilla Martinez, L.; Nguyen, H.P. Sexual behavior and testis morphology in the BACHD rat model. PLoS ONE 2018, 13, e0198338. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Sun, J.; Li, T.; Zhang, Q.; Bu, S.; Wang, Q.; Lai, D. Melatonin ameliorates restraint stress-induced oxidative stress and apoptosis in testicular cells via NF-κB/iNOS and Nrf2/ HO-1 signaling pathway. Sci. Rep. 2017, 7, 9599. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.S.; Shakes, D.C. Spermatogenesis. Adv. Exp. Med. Biol. 2013, 757, 171–203. [Google Scholar] [PubMed]
- Carlsen, E.; Giwercman, A.; Keiding, N.; Skakkebaek, N.E. Evidence for decreasing quality of semen during past 50 years. Bmj 1992, 305, 609–613. [Google Scholar] [CrossRef]
- Zou, P.; Wang, X.; Sun, L.; Chen, Q.; Yang, H.; Zhou, N.; Chen, H.; Zhang, G.; Ling, X.; Wang, Z. Semen Quality in Chinese College Students: Associations With Depression and Physical Activity in a Cross-Sectional Study. Psychosom. Med. 2018, 80, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Sun, L.; Chen, Q.; Zhang, G.; Yang, W.; Zeng, Y.; Zhou, N.; Li, Y.; Liu, J.; Ao, L.; et al. Social support modifies an association between work stress and semen quality: Results from 384 Chinese male workers. J. Psychosom. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Neto, F.T.; Bach, P.V.; Najari, B.B.; Li, P.S.; Goldstein, M. Spermatogenesis in humans and its affecting factors. Semin. Cell Dev. Biol. 2016, 59, 10–26. [Google Scholar] [CrossRef]
- Flak, J.N.; Myers, B.; Solomon, M.B.; McKlveen, J.M.; Krause, E.G.; Herman, J.P. Role of paraventricular nucleus-projecting norepinephrine/epinephrine neurons in acute and chronic stress. Eur. J. Neurosci. 2014, 39, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Kim, E.; Choi, M.H. Technical and clinical aspects of cortisol as a biochemical marker of chronic stress. Bmb. Rep. 2015, 48, 209–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalantaridou, S.N.; Zoumakis, E.; Makrigiannakis, A.; Lavasidis, L.G.; Vrekoussis, T.; Chrousos, G.P. Corticotropin-releasing hormone, stress and human reproduction: An update. J. Reprod. Immunol. 2010, 85, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Gilbeau, P.M.; Smith, C.G. Naloxone reversal of stress-induced reproductive effects in the male rhesus monkey. Neuropeptides 1985, 5, 335–338. [Google Scholar] [CrossRef]
- Wang, O. Glucocorticoids inhibit kisspeptin neurons during stress-induced reproductive inhibition. Endocr. Rev. 2011, 32, P3–P141. [Google Scholar]
- Hautanen, A. Synthesis and regulation of sex hormone-binding globulin in obesity. Int. J. Obes. Relat. Metab. Disord. 2000, 24, S64–S70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Wang, Q.; Wang, F.F.; Gao, H.B.; Zhang, P. Stress induces glucocorticoid-mediated apoptosis of rat Leydig cells in vivo. Stress 2012, 15, 74–84. [Google Scholar] [CrossRef]
- Hu, G.X.; Lian, Q.Q.; Lin, H.; Latif, S.A.; Morris, D.J.; Hardy, M.P.; Ge, R.S. Rapid mechanisms of glucocorticoid signaling in the Leydig cell. Steroids 2008, 73, 1018–1024. [Google Scholar] [CrossRef] [Green Version]
- Whirledge, S.; Cidlowski, J.A. Glucocorticoids, stress, and fertility. Minerva Endocrinol. 2010, 35, 109–125. [Google Scholar]
- Peck, W.A. The effects of glucocorticoids on bone cell metabolism and function. Adv. Exp. Med. Biol. 1984, 171, 111–119. [Google Scholar]
- Pozzesi, N.; Fierabracci, A.; Liberati, A.M.; Martelli, M.P.; Ayroldi, E.; Riccardi, C.; Delfino, D.V. Role of caspase-8 in thymus function. Cell Death Differ. 2014, 21, 226–233. [Google Scholar] [CrossRef]
- Carson, R.; Monaghan-Nichols, A.P.; DeFranco, D.B.; Rudine, A.C. Effects of antenatal glucocorticoids on the developing brain. Steroids 2016, 114, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultz, R.; Isola, J.; Parvinen, M.; Honkaniemi, J.; Wikstrom, A.C.; Gustafsson, J.A.; Pelto-Huikko, M. Localization of the glucocorticoid receptor in testis and accessory sexual organs of male rat. Mol. Cell. Endocrinol. 1993, 95, 115–120. [Google Scholar] [CrossRef]
- Wagner, A.; Claus, R. Involvement of glucocorticoids in testicular involution after active immunization of boars against GnRH. Reproduction 2004, 127, 275–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadepond, F.; Ulmann, A.; Baulieu, E.E. RU486 (mifepristone): Mechanisms of action and clinical uses. Annu. Rev. Med. 1997, 48, 129–156. [Google Scholar] [CrossRef] [PubMed]
- Lippman, M.; Bolan, G.; Huff, K. The effects of glucocorticoids and progesterone on hormone-responsive human breast cancer in long-term tissue culture. Cancer Res. 1976, 36, 4602–4609. [Google Scholar] [PubMed]
- Goya, L.; Maiyar, A.C.; Ge, Y.; Firestone, G.L. Glucocorticoids induce a G1/G0 cell cycle arrest of Con8 rat mammary tumor cells that is synchronously reversed by steroid withdrawal or addition of transforming growth factor-alpha. Mol. Endocrinol. 1993, 7, 1121–1132. [Google Scholar] [PubMed]
- Jeje, S.O.; Raji, Y. Effects of Maternal Dexamethasone Exposure During Lactation on Metabolic Imbalance and Oxidative Stress in the Liver of Male Offsprings of Wistar Rats. Niger. J. Physiol. Sci. Off. Publ. Physiol. Soc. Niger. 2015, 30, 131–137. [Google Scholar]
- Nassar, G.N.; Leslie, S.W. Physiology, Testosterone. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2019. [Google Scholar]
- Evain, D.; Morera, A.M.; Saez, J.M. [Glucocorticoids receptors in rat testis: Their role in Leydig cells specific function and DNA synthesis (author′s transl)]. Ann. D’endocrinologie 1976, 37, 101–102. [Google Scholar]
- Perfalk, E.; Cunha-Bang, S.D.; Holst, K.K.; Keller, S.; Svarer, C.; Knudsen, G.M.; Frokjaer, V.G. Testosterone levels in healthy men correlate negatively with serotonin 4 receptor binding. Psychoneuroendocrinology 2017, 81, 22–28. [Google Scholar] [CrossRef]
- Vadakkadath Meethal, S.; Atwood, C.S. The role of hypothalamic-pituitary-gonadal hormones in the normal structure and functioning of the brain. Cell. Mol. Life Sci. Cmls 2005, 62, 257–270. [Google Scholar] [CrossRef]
- Barreiro, M.L.; Gaytan, F.; Castellano, J.M.; Suominen, J.S.; Roa, J.; Gaytan, M.; Aguilar, E.; Dieguez, C.; Toppari, J.; Tena-Sempere, M. Ghrelin inhibits the proliferative activity of immature Leydig cells in vivo and regulates stem cell factor messenger ribonucleic acid expression in rat testis. Endocrinology 2004, 145, 4825–4834. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ma, H.; Li, Z.; Gao, Y.; Cao, X.; Jiang, Y.; Zhou, Y.; Liu, S. Chronic unpredictable stress exacerbates surgery-induced sickness behavior and neuroinflammatory responses via glucocorticoids secretion in adult rats. PLoS ONE 2017, 12, e0183077. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, K.; Matsuura, N.; Takeshita, Y.; Ito, S.; Sano, Y.; Yamada, Y.; Uchinaka, A.; Murohara, T.; Nagata, K. Attenuation of cold stress-induced exacerbation of cardiac and adipose tissue pathology and metabolic disorders in a rat model of metabolic syndrome by the glucocorticoid receptor antagonist RU486. Nutr. Diabetes 2016, 6, e207. [Google Scholar] [CrossRef] [PubMed]
- Filho, C.B.; Jesse, C.R.; Donato, F.; Giacomeli, R.; Del Fabbro, L.; da Silva Antunes, M.; de Gomes, M.G.; Goes, A.T.; Boeira, S.P.; Prigol, M.; et al. Chronic unpredictable mild stress decreases BDNF and NGF levels and Na+,K+-ATPase activity in the hippocampus and prefrontal cortex of mice: Antidepressant effect of chrysin. Neuroscience 2015, 289, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Willner, P. Validity, reliability and utility of the chronic mild stress model of depression: A 10-year review and evaluation. Psychopharmacology 1997, 134, 319–329. [Google Scholar] [CrossRef]
- Avolio, E.; Fazzari, G.; Zizza, M.; De Lorenzo, A.; Di Renzo, L.; Alo, R.; Facciolo, R.M.; Canonaco, M. Probiotics modify body weight together with anxiety states via pro-inflammatory factors in HFD-treated Syrian golden hamster. Behav. Brain Res. 2018. [Google Scholar] [CrossRef]
- Jia, K.K.; Zheng, Y.J.; Zhang, Y.X.; Liu, J.H.; Jiao, R.Q.; Pan, Y.; Kong, L.D. Banxia-houpu decoction restores glucose intolerance in CUMS rats through improvement of insulin signaling and suppression of NLRP3 inflammasome activation in liver and brain. J. Ethnopharmacol. 2017, 209, 219–229. [Google Scholar] [CrossRef]
- Luo, Y.W.; Xu, Y.; Cao, W.Y.; Zhong, X.L.; Duan, J.; Wang, X.Q.; Hu, Z.L.; Li, F.; Zhang, J.Y.; Zhou, M.; et al. Insulin-like growth factor 2 mitigates depressive behavior in a rat model of chronic stress. Neuropharmacology 2015, 89, 318–324. [Google Scholar] [CrossRef]
- Ma, L.H.; Ding, Q.; Wang, X.; Wang, Y.; Zhang, Y.F. [Isolation, purification and immunochemical characteristics of spermatogonia of rat]. Zhonghua Yi Xue Za Zhi 2006, 86, 1371–1375. [Google Scholar]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, P.; Wang, X.; Yang, W.; Liu, C.; Chen, Q.; Yang, H.; Zhou, N.; Zeng, Y.; Chen, H.; Zhang, G.; et al. Mechanisms of Stress-Induced Spermatogenesis Impairment in Male Rats Following Unpredictable Chronic Mild Stress (uCMS). Int. J. Mol. Sci. 2019, 20, 4470. https://doi.org/10.3390/ijms20184470
Zou P, Wang X, Yang W, Liu C, Chen Q, Yang H, Zhou N, Zeng Y, Chen H, Zhang G, et al. Mechanisms of Stress-Induced Spermatogenesis Impairment in Male Rats Following Unpredictable Chronic Mild Stress (uCMS). International Journal of Molecular Sciences. 2019; 20(18):4470. https://doi.org/10.3390/ijms20184470
Chicago/Turabian StyleZou, Peng, Xiaogang Wang, Wang Yang, Chang Liu, Qing Chen, Huan Yang, Niya Zhou, Yingfei Zeng, Hongqiang Chen, Guowei Zhang, and et al. 2019. "Mechanisms of Stress-Induced Spermatogenesis Impairment in Male Rats Following Unpredictable Chronic Mild Stress (uCMS)" International Journal of Molecular Sciences 20, no. 18: 4470. https://doi.org/10.3390/ijms20184470
APA StyleZou, P., Wang, X., Yang, W., Liu, C., Chen, Q., Yang, H., Zhou, N., Zeng, Y., Chen, H., Zhang, G., Liu, J., Cao, J., Ao, L., & Sun, L. (2019). Mechanisms of Stress-Induced Spermatogenesis Impairment in Male Rats Following Unpredictable Chronic Mild Stress (uCMS). International Journal of Molecular Sciences, 20(18), 4470. https://doi.org/10.3390/ijms20184470