Non-Coding RNAs in Cartilage Development: An Updated Review
Abstract
:1. Introduction
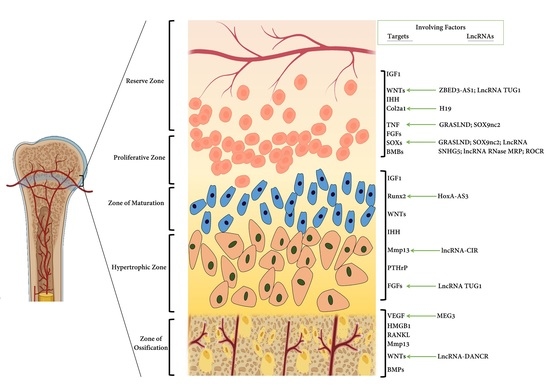
2. Cartilage Development
3. miRNA and Chondrocyte Differentiation
4. The Role of miRNAs in Cartilage Homeostasis
5. miRNAs Contribute to Chondrocyte Differentiation
6. miRNAs, Environmental Factors, and Cartilage Development
7. Smad Family and Cartilage Development
8. Cartilage Development and Epigenetic Modulations
9. miRNAs and Their Protective Roles in Cartilage
10. Role of lncRNAs in Chondrogenesis
11. Other ncRNAs and Cartilage Development
12. ncRNAs and Cartilage Pertinent Diseases
13. Therapeutics of ncRNAs on the Cartilage Diseases
14. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aubin, J.; Liu, F.; Malaval, L.; Gupta, A. Osteoblast and chondroblast differentiation. Bone 1995, 17, S77–S83. [Google Scholar] [CrossRef]
- Goldring, M.B.; Tsuchimochi, K.; Ijiri, K. The control of chondrogenesis. J. Cell. Biochem. 2006, 97, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Cordero, D.R.; Brugmann, S.; Chu, Y.; Bajpai, R.; Jame, M.; Helms, J.A. Cranial neural crest cells on the move: Their roles in craniofacial development. Am. J. Med Genet. Part A 2011, 155, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Dockter, J.L. 3 Sclerotome Induction and Differentiation. In Current topics in developmental biology; Elsevier: Amsterdam, The Netherland, 1999; Volume 48, pp. 77–127. [Google Scholar]
- Taft, R.J.; Pang, K.C.; Mercer, T.R.; Dinger, M.; Mattick, J.S. Non-coding RNAs: Regulators of disease. J. Pathol. 2010, 220, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Cai, Z.; Cheung, W.K.; Yang, K.; Xu, L.; Lu, W.W.; Yang, H.; Chiu, K.-Y. Multichromatic TTF staining characterizes cartilage matrix in osteoarthritis and bone development. Histol. Histopathol. 2019, 34, 275–286. [Google Scholar] [PubMed]
- Lamandé, S.R.; Bateman, J.F. Genetic disorders of the extracellular matrix. Anat. Rec. 2019, 28. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, G.; Li, W.; Wu, X. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells following transfection with Indian hedgehog and sonic hedgehog using a rotary cell culture system. Cell. Mol. Biol. Lett. 2019, 24, 16. [Google Scholar] [CrossRef]
- Edith, C.; Céline, D.; Federica, C.; Olivier, M.; Sophie, N.; Zelda, P.; Michel, M. Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem. Pharmacol. 2019. [Google Scholar]
- Foyt, D.A.; Taheem, D.K.; Ferreira, S.A.; Norman, M.D.; Petzold, J.; Jell, G.; Grigoriadis, A.E.; Gentleman, E. Hypoxia impacts human MSC response to substrate stiffness during chondrogenic differentiation. Acta Biomater. 2019, 89, 73–83. [Google Scholar] [CrossRef]
- Ko, J.-Y.; Lee, J.; Lee, J.; Ryu, Y.H.; Im, G.-I. SOX-6, 9-transfected adipose stem cells to treat surgically-induced osteoarthritis in goats. Tissue Eng. Part A 2019. [Google Scholar] [CrossRef]
- Takai, H.; van Wijnen, A.J.; Ogata, Y. Induction of chondrogenic or mesenchymal stem cells from human periodontal ligament cells through inhibition of Twist2 or Klf12. J. Oral Sci. 2019, 61, 313–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, S.; Alkhatib, B.; Serra, R. Development of the axial skeleton and intervertebral disc. Curr. Top. Dev. Biol. 2019, 133, 49–90. [Google Scholar] [PubMed]
- Yoshida, C.A.; Yamamoto, H.; Fujita, T.; Furuichi, T.; Ito, K.; Inoue, K.-i.; Yamana, K.; Zanma, A.; Takada, K.; Ito, Y. Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev. 2004, 18, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.A.; Kim, Y.; Czubryt, M.P.; Phan, D.; McAnally, J.; Qi, X.; Shelton, J.M.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Dev. Cell 2007, 12, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.; Kozhemyakina, E.; Nicolae, C.; Kaestner, K.H.; Olsen, B.R.; Lassar, A.B. FoxA family members are crucial regulators of the hypertrophic chondrocyte differentiation program. Dev. Cell 2012, 22, 927–939. [Google Scholar] [CrossRef]
- Cucchiarini, M.; Thurn, T.; Weimer, A.; Kohn, D.; Terwilliger, E.F.; Madry, H. Restoration of the extracellular matrix in human osteoarthritic articular cartilage by overexpression of the transcription factor SOX9. Arthritis Rheum. 2007, 56, 158–167. [Google Scholar] [CrossRef]
- Dong, Y.F.; Soung, D.Y.; Schwarz, E.M.; O’Keefe, R.J.; Drissi, H. Wnt induction of chondrocyte hypertrophy through the Runx2 transcription factor. J. Cell. Physiol. 2006, 208, 77–86. [Google Scholar] [CrossRef]
- Long, F. Building strong bones: Molecular regulation of the osteoblast lineage. Nat. Rev. Mol. Cell Biol. 2012, 13, 27. [Google Scholar] [CrossRef]
- Zhang, X.; Siclari, V.A.; Lan, S.; Zhu, J.; Koyama, E.; Dupuis, H.L.; Enomoto-Iwamoto, M.; Beier, F.; Qin, L. The critical role of the epidermal growth factor receptor in endochondral ossification. J. Bone Miner. Res. 2011, 26, 2622–2633. [Google Scholar] [CrossRef]
- Goldring, M.B. Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2012, 4, 269–285. [Google Scholar] [CrossRef]
- Somoza, R.A.; Welter, J.F.; Correa, D.; Caplan, A.I. Chondrogenic differentiation of mesenchymal stem cells: Challenges and unfulfilled expectations. Tissue Eng. Part B Rev. 2014, 20, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, I.; Colter, D.C.; Prockop, D.J. BMP-6 enhances chondrogenesis in a subpopulation of human marrow stromal cells. Biochem. Biophys. Res. Commun. 2001, 284, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, M.K.; Wang, E.; Morris, E.A. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J. Cell. Physiol. 2001, 189, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Usami, Y.; Gunawardena, A.T.; Iwamoto, M.; Enomoto-Iwamoto, M. Wnt signaling in cartilage development and diseases: Lessons from animal studies. Lab. Investig. 2016, 96, 186. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, D.M.; Marie, P.J. Fibroblast growth factor signaling in skeletal development and disease. Genes Dev. 2015, 29, 1463–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, U.-i.; Schipani, E.; McMahon, A.P.; Kronenberg, H.M. Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development. J. Clin. Investig. 2001, 107, 295–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amizuka, N.; Davidson, D.; Liu, H.; Valverde-Franco, G.; Chai, S.; Maeda, T.; Ozawa, H.; Hammond, V.; Ornitz, D.M.; Goltzman, D. Signalling by fibroblast growth factor receptor 3 and parathyroid hormone-related peptide coordinate cartilage and bone development. Bone 2004, 34, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Gerstenfeld, L.C.; Cruceta, J.; Shea, C.M.; Sampath, K.; Barnes, G.L.; Einhorn, T.A. Chondrocytes provide morphogenic signals that selectively induce osteogenic differentiation of mesenchymal stem cells. J. Bone Miner. Res. 2002, 17, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Bobis, S.; Jarocha, D.; Majka, M. Mesenchymal stem cells: Characteristics and clinical applications. Folia Histochem. Et Cytobiol. 2006, 44, 215–230. [Google Scholar]
- Onyekwelu, I.; Goldring, M.B.; Hidaka, C. Chondrogenesis, joint formation, and articular cartilage regeneration. J. Cell. Biochem. 2009, 107, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Quan, Z.; Zheng, D.; Qing, H. Regulatory roles of long non-coding RNAs in the central nervous system and associated neurodegenerative diseases. Front. Cell. Neurosci. 2017, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Stefani, G.; Slack, F.J. Small non-coding RNAs in animal development. Nat. Rev. Mol. Cell Biol. 2008, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Rajewsky, K. MicroRNA control in the immune system: Basic principles. Cell 2009, 136, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Kobayashi, T.; Lu, J.; Cobb, B.S.; Rodda, S.J.; McMahon, A.P.; Schipani, E.; Merkenschlager, M.; Kronenberg, H.M. Dicer-dependent pathways regulate chondrocyte proliferation and differentiation. Proc. Natl. Acad. Sci. USA 2008, 105, 1949–1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y.; Inloes, J.B.; Katagiri, T.; Kobayashi, T. Chondrocyte-specific microRNA-140 regulates endochondral bone development and targets Dnpep to modulate bone morphogenetic protein signaling. Mol. Cell. Biol. 2011, 31, 3019–3028. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; He, X.; Kato, H.; Wakitani, S.; Kobayashi, T.; Watanabe, S.; Iida, A.; Tahara, H.; Warman, M.L.; Watanapokasin, R. Sox9 is upstream of microRNA-140 in cartilage. Appl. Biochem. Biotechnol. 2012, 166, 64–71. [Google Scholar] [CrossRef]
- Yamashita, S.; Miyaki, S.; Kato, Y.; Yokoyama, S.; Sato, T.; Barrionuevo, F.; Akiyama, H.; Scherer, G.; Takada, S.; Asahara, H. L-Sox5 and Sox6 proteins enhance chondrogenic miR-140 microRNA expression by strengthening dimeric Sox9 activity. J. Biol. Chem. 2012, 287, 22206–22215. [Google Scholar] [CrossRef]
- Karlsen, T.A.; Jakobsen, R.B.; Mikkelsen, T.S.; Brinchmann, J.E. microRNA-140 targets RALA and regulates chondrogenic differentiation of human mesenchymal stem cells by translational enhancement of SOX9 and ACAN. Stem Cells Dev. 2013, 23, 290–304. [Google Scholar] [CrossRef]
- Tuddenham, L.; Wheeler, G.; Ntounia-Fousara, S.; Waters, J.; Hajihosseini, M.K.; Clark, I.; Dalmay, T. The cartilage specific microRNA-140 targets histone deacetylase 4 in mouse cells. FEBS Lett. 2006, 580, 4214–4217. [Google Scholar] [CrossRef]
- Li, C.; Hu, Q.; Chen, Z.; Shen, B.; Yang, J.; Kang, P.; Zhou, Z.; Pei, F. MicroRNA-140 Suppresses Human Chondrocytes Hypertrophy by Targeting SMAD1 and Controlling the Bone Morphogenetic Protein Pathway in Osteoarthritis. Am. J. Med. Sci. 2018, 355, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Vega, R.B.; Matsuda, K.; Oh, J.; Barbosa, A.C.; Yang, X.; Meadows, E.; McAnally, J.; Pomajzl, C.; Shelton, J.M.; Richardson, J.A. Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell 2004, 119, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Colnot, C. Cellular and molecular interactions regulating skeletogenesis. J. Cell. Biochem. 2005, 95, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, J.D.; Sergeeva, O.; Somoza, R.A.; Li, M.; Caplan, A.I.; Khalil, A.M.; Lee, Z. Analysis of-5p and-3p strands of miR-145 and miR-140 during mesenchymal stem cell chondrogenic differentiation. Tissue Eng. Part A 2019, 25, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, J.; Pan, Y.; Shan, Y.; Jiang, L.; Qi, X.; Jia, L. miR-140–5p/miR-149 affects chondrocyte proliferation, apoptosis, and autophagy by targeting FUT1 in osteoarthritis. Inflammation 2018, 41, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, I.; Mourmoura, E.; Malizos, K.; Tsezou, A. Functional role of MIR-140 and MIR-146A in inflammation and catabolic processes in osteoarthritis. Osteoarthr. Cartil. 2019, 27, S284. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Weng, Y.; Jiang, Y.; Zhao, S.; Zhou, D.; Xu, N. Overexpression of miR-140–5p inhibits lipopolysaccharide-induced human intervertebral disc inflammation and degeneration by downregulating toll-like receptor 4. Oncol. Rep. 2018, 40, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Nakasa, T.; Miyaki, S.; Ishikawa, M.; Deie, M.; Adachi, N.; Yasunaga, Y.; Asahara, H.; Ochi, M. Expression of microRNA-146a in osteoarthritis cartilage. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2009, 60, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, S.; Yang, H.; Zhang, C.; Kang, Q.; Deng, J.; Xu, Y.; Ding, Y.; Li, S. Potential Novel Prediction of TMJ-OA: MiR-140–5p Regulates Inflammation Through Smad/TGF-β Signaling. Front. Pharmacol. 2019, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zhang, D.; Yu, K.; Sun, L.; Yang, J.; Zhao, C.; Li, X.; Chen, Y. Detection of miR-22, miR-140 and Bone Morphogenetic Proteins (BMP)-2 Expression Levels in Synovial Fluid of Osteoarthritis Patients Before and After Arthroscopic Debridement. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 863. [Google Scholar] [CrossRef] [PubMed]
- Miyaki, S.; Nakasa, T.; Otsuki, S.; Grogan, S.P.; Higashiyama, R.; Inoue, A.; Kato, Y.; Sato, T.; Lotz, M.K.; Asahara, H. MicroRNA-140 is expressed in differentiated human articular chondrocytes and modulates interleukin-1 responses. Arthritis Rheum. 2009, 60, 2723–2730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyaki, S.; Sato, T.; Inoue, A.; Otsuki, S.; Ito, Y.; Yokoyama, S.; Kato, Y.; Takemoto, F.; Nakasa, T.; Yamashita, S. MicroRNA-140 plays dual roles in both cartilage development and homeostasis. Genes Dev. 2010, 24, 1173–1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tardif, G.; Hum, D.; Pelletier, J.-P.; Duval, N.; Martel-Pelletier, J. Regulation of the IGFBP-5 and MMP-13 genes by the microRNAs miR-140 and miR-27a in human osteoarthritic chondrocytes. BMC Musculoskelet. Disord. 2009, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Guo, H.; Zhang, Y.; Chen, L.; Ying, D.; Dong, S. MicroRNA-145 regulates chondrogenic differentiation of mesenchymal stem cells by targeting Sox9. PLoS ONE 2011, 6, e21679. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Sanchez, A.; Dudek, K.A.; Murphy, C.L. Regulation of human chondrocyte function through direct inhibition of cartilage master regulator SOX9 by microRNA-145 (miRNA-145). J. Biol. Chem. 2012, 287, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Hata, K. Epigenetic regulation of chondrocyte differentiation. Jpn. Dent. Sci. Rev. 2015, 51, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Karlsen, T.A.; de Souza, G.A.; Ødegaard, B.; Engebretsen, L.; Brinchmann, J.E. microRNA-140 inhibits inflammation and stimulates chondrogenesis in a model of interleukin 1β-induced osteoarthritis. Mol. Ther. -Nucleic Acids 2016, 5, e373. [Google Scholar] [CrossRef] [PubMed]
- Le, L.; Swingler, T.; Crowe, N.; Niu, L.; Dalmay, T.; Barter, M.; Young, D.; Donell, S.; Clark, I. The role of microRNA 3085 in chondrocytes. Osteoarthr. Cartil. 2018, 26, S161–S162. [Google Scholar] [CrossRef]
- Rasheed, Z.; Rasheed, N.; Al Abdulmonem, W.; Khan, M.I. MicroRNA-125b-5p regulates IL-1β induced inflammatory genes via targeting TRAF6-mediated MAPKs and NF-κB signaling in human osteoarthritic chondrocytes. Sci. Rep. 2019, 9, 6882. [Google Scholar] [CrossRef]
- Zheng, H.; Ramnaraign, D.; Anderson, B.A.; Tycksen, E.; Nunley, R.; McAlinden, A. MicroRNA-138 Inhibits Osteogenic Differentiation and Mineralization of Human Dedifferentiated Chondrocytes by Regulating RhoC and the Actin Cytoskeleton. JBMR Plus 2019, 3, e10071. [Google Scholar] [CrossRef]
- Chen, S.; Xu, Z.; Shao, J.; Fu, P.; Wu, H. MicroRNA-218 promotes early chondrogenesis of mesenchymal stem cells and inhibits later chondrocyte maturation. BMC Biotechnol. 2019, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cheng, P.; Hu, W.; Yin, W.; Guo, F.; Chen, A.; Huang, H. Downregulated microRNA-340–5p promotes proliferation and inhibits apoptosis of chondrocytes in osteoarthritis mice through inhibiting the extracellular signal-regulated kinase signaling pathway by negatively targeting the FMOD gene. J. Cell. Physiol. 2019, 234, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Tian, X.Y.; Huang, X.X.; He, L.L.; Xu, F. microRNA-186 inhibition of PI3K–AKT pathway via SPP1 inhibits chondrocyte apoptosis in mice with osteoarthritis. J. Cell. Physiol. 2019, 234, 6042–6053. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, S.; Chen, P.; Yang, B.; Yang, J.; Liu, R.; Li, J.; Xia, D. MicroRNA-27b-3p inhibits apoptosis of chondrocyte in rheumatoid arthritis by targeting HIPK2. Artif. CellsNanomed. Biotechnol. 2019, 47, 1766–1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Z.k.; Meng, F.g.; Zhang, Z.q.; Mao, G.p.; Huang, Z.y.; Liao, W.m.; He, A.S. MicroRNA-193b-3p regulates matrix metalloproteinase 19 expression in interleukin-1β-induced human chondrocytes. J. Cell. Biochem. 2018, 119, 4775–4782. [Google Scholar] [CrossRef]
- Wu, Y.H.; Liu, W.; Zhang, L.; Liu, X.Y.; Wang, Y.; Xue, B.; Liu, B.; Duan, R.; Zhang, B.; Ji, Y. Effects of microRNA-24 targeting C-myc on apoptosis, proliferation, and cytokine expressions in chondrocytes of rats with osteoarthritis via MAPK signaling pathway. J. Cell. Biochem. 2018, 119, 7944–7958. [Google Scholar] [CrossRef]
- Chen, H.; Yang, J.; Tan, Z. Upregulation of microRNA-9–5p inhibits apoptosis of chondrocytes through downregulating Tnc in mice with osteoarthritis following tibial plateau fracture. J. Cell. Physiol. 2019. [Google Scholar] [CrossRef]
- Liu, W.; Zha, Z.; Wang, H. Upregulation of microRNA-27a inhibits synovial angiogenesis and chondrocyte apoptosis in knee osteoarthritis rats through the inhibition of PLK2. J. Cell. Physiol. 2019. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, T.; Cai, B.; Wang, X.; Feng, W.; Han, Y.; Li, D.; Li, S.; Liu, J. MicroRNA-495 enhances chondrocyte apoptosis, senescence and promotes the progression of osteoarthritis by targeting AKT1. Am. J. Transl. Res. 2019, 11, 2232. [Google Scholar]
- Ding, Y.; Wang, L.; Zhao, Q.; Wu, Z.; Kong, L. MicroRNA-93 inhibits chondrocyte apoptosis and inflammation in osteoarthritis by targeting the TLR4/NF-κB signaling pathway. Int. J. Mol. Med. 2019, 43, 779–790. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, S.; Xie, X.; Ding, M.; Zhou, Q.; Zhou, X. MicroRNA-31 promotes chondrocyte proliferation by targeting C-X-C motif chemokine ligand 12. Mol. Med. Rep. 2019, 19, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Song, W.; Ma, T.; Wang, K. microRNA-590–5p targets transforming growth factor β1 to promote chondrocyte apoptosis and autophagy in response to mechanical pressure injury. J. Cell. Biochem. 2018, 119, 9931–9940. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhao, B.; He, Q.; Zhang, Y.; Peng, X.-B. microRNA-206 is required for osteoarthritis development through its effect on apoptosis and autophagy of articular chondrocytes via modulating the phosphoinositide 3-kinase/protein kinase B-mTOR pathway by targeting insulin-like growth factor-1. J. Cell. Biochem. 2019, 120, 5287–5303. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Wang, B.; Qu, Y.; Zheng, C.; Xu, J.; Xie, Z.; Ma, Y. MicroRNA-23c inhibits articular cartilage damage recovery by regulating MSCs differentiation to chondrocytes via reducing FGF2. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 941–948. [Google Scholar] [PubMed]
- Yu, C.; Wang, Y. MicroRNA-19a promotes cell viability and migration of chondrocytes via up-regulating SOX9 through NF-κB pathway. Biomed. Pharmacother. 2018, 98, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, H.; Wang, L. MicroRNA-107 regulates autophagy and apoptosis of osteoarthritis chondrocytes by targeting TRAF3. Int. Immunopharmacol. 2019, 71, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, L.; Jin, S.; Lin, J.; Zheng, H.; Zhang, H.; Fan, H.; He, F.; Ma, S.; Li, Q. Altered expression of microRNA-98 in IL-1β-induced cartilage degradation and its role in chondrocyte apoptosis Corrigendum in/10.3892/mmr. 2018.8794. Mol. Med. Rep. 2017, 16, 3208–3216. [Google Scholar] [CrossRef]
- Zhou, J.; Tian, Z.; Zhu, L.; Wu, W.; Zhou, S.; Zhao, Y.; Huang, S. MicroRNA-615–3p promotes the osteoarthritis progression by inhibiting chondrogenic differentiation of bone marrow mesenchymal stem cells. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6212–6220. [Google Scholar]
- Zhao, C.; Miao, Y.; Cao, Z.; Shi, J.; Li, J.; Kang, F.; Dou, C.; Xie, Z.; Xiang, Q.; Dong, S. MicroRNA-29b regulates hypertrophy of murine mesenchymal stem cells induced toward chondrogenesis. J. Cell. Biochem. 2019, 120, 8742–8753. [Google Scholar] [CrossRef]
- Hu, S.; Mao, G.; Zhang, Z.; Wu, P.; Wen, X.; Liao, W.; Zhang, Z. MicroRNA-320c inhibits development of osteoarthritis through downregulation of canonical Wnt signaling pathway. Life Sci. 2019, 228, 242–250. [Google Scholar] [CrossRef]
- Li, H.; Miao, D.; Zhu, Q.; Huang, J.; Lu, G.; Xu, W. MicroRNA-17–5p contributes to osteoarthritis progression by binding p62/SQSTM1. Exp. Ther. Med. 2018, 15, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Liu, J.; Tycksen, E.; Nunley, R.; McAlinden, A. MicroRNA-181a/b-1 over-expression enhances osteogenesis by modulating PTEN/PI3K/AKT signaling and mitochondrial metabolism. Bone 2019. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Li, Y.; Wei, S. miR-4262 regulates chondrocyte viability, apoptosis, autophagy by targeting SIRT1 and activating PI3K/AKT/mTOR signaling pathway in rats with osteoarthritis. Exp. Ther. Med. 2018, 15, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Penolazzi, L.; Lambertini, E.; Bergamin, L.S.; Roncada, T.; De Bonis, P.; Cavallo, M.; Piva, R. MicroRNA-221 silencing attenuates the degenerated phenotype of intervertebral disc cells. Aging (Albany NY) 2018, 10, 2001. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Wang, J.; Zhang, Z.; Yang, J. miR-107 modulates chondrocyte proliferation, apoptosis, and extracellular matrix synthesis by targeting PTEN. Int. J. Clin. Exp. Pathol. 2019, 12, 488–497. [Google Scholar]
- Zhang, W.; Hsu, P.; Zhong, B.; Guo, S.; Zhang, C.; Wang, Y.; Luo, C.; Zhan, Y.; Zhang, C. MiR-34a enhances chondrocyte apoptosis, senescence and facilitates development of osteoarthritis by targeting DLL1 and regulating PI3K/AKT pathway. Cell. Physiol. Biochem. 2018, 48, 1304–1316. [Google Scholar] [CrossRef]
- Zhang, W.; Zhong, B.; Zhang, C.; Luo, C.; Zhan, Y. miR-373 regulates inflammatory cytokine-mediated chondrocyte proliferation in osteoarthritis by targeting the P2X7 receptor. FEBS Open Bio 2018, 8, 325–331. [Google Scholar] [CrossRef]
- Zhou, J.; Liang, A.; Hong, J.; Sun, J.; Lin, X.; Peng, Y.; Wang, X.; Sun, S.; Xiao, D.; Xu, K. MicroRNA-155 suppresses the catabolic effect induced by TNF-α and IL-1β by targeting C/EBPβ in rat nucleus pulposus cells. Connect. Tissue Res. 2019, 60, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.; Gao, J.; Si, Y.; Zhao, D. Combination of circulating miR-19b-3p, miR-122-5p and miR-486-5p expressions correlates with risk and disease severity of knee osteoarthritis. Am. J. Trans. Res. 2017, 9, 2852. [Google Scholar]
- Shi, J.; Guo, K.; Su, S.; Li, J.; Li, C. miR4865p is upregulated in osteoarthritis and inhibits chondrocyte proliferation and migration by suppressing SMAD2. Mol. Med. Rep. 2018, 18, 502–508. [Google Scholar]
- Liang, J.; Xu, L.; Zhou, F.; Liu, A.m.; Ge, H.x.; Chen, Y.y.; Tu, M. MALAT1/miR-127–5p regulates osteopontin (OPN)-mediated proliferation of human chondrocytes through PI3K/Akt pathway. J. Cell. Biochem. 2018, 119, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Zhang, X.; Hu, X.; Zhou, C.; Ao, Y. Silencing of microRNA-101 prevents IL-1β-induced extracellular matrix degradation in chondrocytes. Arthritis Res. Ther. 2012, 14, R268. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yoon, D.S.; Paik, S.; Lee, K.-M.; Jang, Y.; Lee, J.W. microRNA-495 inhibits chondrogenic differentiation in human mesenchymal stem cells by targeting Sox9. Stem Cells Dev. 2014, 23, 1798–1808. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Sanchez, A.; Murphy, C.L. miR-1247 functions by targeting cartilage transcription factor SOX9. J. Biol. Chem. 2013, 288, 30802–30814. [Google Scholar] [CrossRef] [PubMed]
- Guerit, D.; Philipot, D.; Chuchana, P.; Toupet, K.; Brondello, J.-M.; Mathieu, M.; Jorgensen, C.; Noel, D. Sox9-regulated miRNA-574–3p inhibits chondrogenic differentiation of mesenchymal stem cells. PLoS ONE 2013, 8, e62582. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, G.; Mirzamohammadi, F.; Kobayashi, T. MicroRNAs involved in bone formation. Cell. Mol. Life Sci. 2014, 71, 4747–4761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudek, K.A.; Lafont, J.E.; Martinez-Sanchez, A.; Murphy, C.L. Type II collagen expression is regulated by tissue-specific miR-675 in human articular chondrocytes. J. Biol. Chem. 2010, 285, 24381–24387. [Google Scholar] [CrossRef]
- Lin, E.A.; Kong, L.; Bai, X.-H.; Luan, Y.; Liu, C.-j. miR-199a*, a bone morphogenic protein 2-responsive microRNA, regulates chondrogenesis via direct targeting to Smad1. J. Biol. Chem. 2009, 284, 11326–11335. [Google Scholar] [CrossRef]
- Xu, J.; Lv, S.; Hou, Y.; Xu, K.; Sun, D.; Zheng, Y.; Zhang, Z.; Li, X.; Li, Y.; Chi, G. miR-27b promotes type II collagen expression by targetting peroxisome proliferator-activated receptor-γ2 during rat articular chondrocyte differentiation. Biosci. Rep. 2018, 38, BSR20171109. [Google Scholar] [CrossRef]
- Jee, Y.H.; Wang, J.; Yue, S.; Jennings, M.; Clokie, S.J.; Nilsson, O.; Lui, J.C.; Baron, J. Mir-374–5p, mir-379–5p, and mir-503–5p regulate proliferation and hypertrophic differentiation of growth plate chondrocytes in male rats. Endocrinology 2018, 159, 1469–1478. [Google Scholar] [CrossRef]
- Song, J.; Lee, M.; Kim, D.; Han, J.; Chun, C.-H.; Jin, E.-J. MicroRNA-181b regulates articular chondrocytes differentiation and cartilage integrity. Biochem. Biophys. Res. Commun. 2013, 431, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Ukai, T.; Sato, M.; Akutsu, H.; Umezawa, A.; Mochida, J. MicroRNA-199a-3p, microRNA-193b, and microRNA-320c are correlated to aging and regulate human cartilage metabolism. J. Orthop. Res. 2012, 30, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Buxton, P.; Edwards, C.; Archer, C.W.; Francis-West, P. Growth/differentiation factor-5 (GDF-5) and skeletal development. J. Bone Jt. Surg. Am. 2001, 83, S23–S30. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, J.; Yang, S.; Liu, X.; Ye, S.; Tian, H. MicroRNA-21 controls the development of osteoarthritis by targeting GDF-5 in chondrocytes. Exp. Mol. Med. 2014, 46, e79. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Huang, Z.; Wu, P.; Chang, Z.; Liao, W.; Zhang, Z. CDK6 and miR-320c Co-Regulate Chondrocyte Catabolism Through NF-κB Signaling Pathways. Cell. Physiol. Biochem. 2018, 51, 909–923. [Google Scholar] [CrossRef] [PubMed]
- SHAO, J.S.; Al Aly, Z.; LAI, C.F.; CHENG, S.L.; Cai, J.; Huang, E.; Behrmann, A.; Towler, D.A. Vascular Bmp–Msx2–Wnt signaling and oxidative stress in arterial calcification. Ann. N. Y. Acad. Sci. 2007, 1117, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Ko, J.-Y.; Kim, H.Y.; Park, J.-W.; Guilak, F.; Im, G.-I. MiR-892b Inhibits Hypertrophy by Targeting KLF10 in the Chondrogenesis of Mesenchymal Stem Cells. Mol. Ther. -Nucleic Acids 2019. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, D.; Sun, H.; Qi, Y.; Xu, W.; Jin, X.; Li, C.; Lin, Z.; Li, G. MiR-132–3p regulates ADAMTS-5 expression and promotes chondrogenic differentiation of rat mesenchymal stem cells. J. Cell. Biochem. 2018, 119, 2579–2587. [Google Scholar] [CrossRef]
- Brocker, C.N.; Vasiliou, V.; Nebert, D.W. Evolutionary divergence and functions of the ADAM and ADAMTS gene families. Hum. Genom. 2009, 4, 43. [Google Scholar] [CrossRef]
- Xu, X.; Li, X.; Liang, Y.; Ou, Y.; Huang, J.; Xiong, J.; Duan, L.; Wang, D. Estrogen Modulates Cartilage and Subchondral Bone Remodeling in an Ovariectomized Rat Model of Postmenopausal Osteoarthritis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 3146. [Google Scholar] [CrossRef]
- Wu, Z.; Qiu, X.; Gao, B.; Lian, C.; Peng, Y.; Liang, A.; Xu, C.; Gao, W.; Zhang, L.; Su, P. Melatonin-mediated miR-526b-3p and miR-590–5p upregulation promotes chondrogenic differentiation of human mesenchymal stem cells. J. Pineal Res. 2018, 65, e12483. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Estrada, K.D.; Lyons, K.M. Smad signaling in skeletal development and regeneration. Cytokine Growth Factor Rev. 2009, 20, 379–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Wu, X. miR-134 inhibits chondrogenic differentiation of bone marrow mesenchymal stem cells by targetting SMAD6. Biosci. Rep. 2019, 39, BSR20180921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Z.; Chang, Z.; Mao, G.; Hu, S.; Zeng, A.; Fu, M. miR-193b-5p regulates chondrocytes metabolism by directly targeting histone deacetylase 7 in interleukin-1β-induced osteoarthritis. J. Cell. Biochem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dong, S. The signaling pathways involved in chondrocyte differentiation and hypertrophic differentiation. Stem Cells Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Hu, S.; Zhang, Z.; Wu, P.; Zhao, X.; Lin, R.; Liao, W.; Kang, Y. Exosomal miR-95–5p regulates chondrogenesis and cartilage degradation via histone deacetylase 2/8. J. Cell. Mol. Med. 2018, 22, 5354–5366. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, X.; Zhang, C.; Zhang, Z.; Lun, J.; Liao, W.; Zhang, Z. MiR-455–3p inhibits the degenerate process of chondrogenic differentiation through modification of DNA methylation. Cell Death Dis. 2018, 9, 537. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Li, Y.; Li, Y.; Jiang, Z.; Shen, D.; Zhao, Z.; Wang, F. MiR-181a regulates the chondrogenic differentiation in pig peripheral blood mesenchymal stem cells. Int. J. Clin. Exp. Pathol. 2018, 11, 947–955. [Google Scholar]
- Huang, J.; Zhao, L.; Fan, Y.; Liao, L.; Ma, P.X.; Xiao, G.; Chen, D. The microRNAs miR-204 and miR-211 maintain joint homeostasis and protect against osteoarthritis progression. Nat. Commun. 2019, 10, 2876. [Google Scholar] [CrossRef]
- Qureshi, I.A.; Mehler, M.F. Long non-coding RNAs: Novel targets for nervous system disease diagnosis and therapy. Neurotherapeutics 2013, 10, 632–646. [Google Scholar] [CrossRef]
- Liu, Y.; Li, G.; Zhang, J.-f. The role of long non-coding RNA H19 in musculoskeletal system: A new player in an old game. Exp. Cell Res. 2017, 360, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Fang, F.; Lu, S.; Li, X.; Yang, Y.; Wang, Z. lncRNA-HIT promotes cell proliferation of non-small cell lung cancer by association with E2F1. Cancer Gene Ther. 2017, 24, 221. [Google Scholar] [CrossRef] [PubMed]
- Richards, E.J.; Zhang, G.; Li, Z.-P.; Permuth-Wey, J.; Challa, S.; Li, Y.; Kong, W.; Dan, S.; Bui, M.M.; Coppola, D. Long Non-coding RNAs (LncRNA) Regulated by Transforming Growth Factor (TGF) β LncRNA-HIT-MEDIATED TGFβ-INDUCED EPITHELIAL TO MESENCHYMAL TRANSITION IN MAMMARY EPITHELIA. J. Biol. Chem. 2015, 290, 6857–6867. [Google Scholar] [CrossRef] [PubMed]
- Carlson, H.L.; Quinn, J.J.; Yang, Y.W.; Thornburg, C.K.; Chang, H.Y.; Stadler, H.S. LncRNA-HIT functions as an epigenetic regulator of chondrogenesis through its recruitment of p100/CBP complexes. PLoS Genet. 2015, 11, e1005680. [Google Scholar] [CrossRef]
- Sun, H.; Peng, G.; Ning, X.; Wang, J.; Yang, H.; Deng, J. Emerging roles of long noncoding RNA in chondrogenesis, osteogenesis, and osteoarthritis. Am. J. Transl. Res. 2019, 11, 16. [Google Scholar] [PubMed]
- Huynh, N.P.; Gloss, C.C.; Lorentz, J.; Tang, R.; McAlinden, A.; Zhang, B.; Brunger, J.; Guilak, F. Long non-coding RNA GRASLND enhances chondrogenesis via suppression of interferon type II signaling pathway. bioRxiv 2019, 650010. [Google Scholar]
- Liang, Z.; Ren, C. Emodin attenuates apoptosis and inflammation induced by LPS through up-regulating lncRNA TUG1 in murine chondrogenic ATDC5 cells. Biomed. Pharmacother. 2018, 103, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Meulenbelt, I.M.; Bhutani, N.; den Hollander, W.; Gay, S.; Oppermann, U.; Reynard, L.N.; Skelton, A.J.; Young, D.A.; Beier, F.; Loughlin, J. The first international workshop on the epigenetics of osteoarthritis. Connect. Tissue Res. 2017, 58, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Steck, E.; Boeuf, S.; Gabler, J.; Werth, N.; Schnatzer, P.; Diederichs, S.; Richter, W. Regulation of H19 and its encoded microRNA-675 in osteoarthritis and under anabolic and catabolic in vitro conditions. J. Mol. Med. 2012, 90, 1185–1195. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, S.; Bao, N.; Yang, C.; Ti, Y.; Zhou, L.; Zhao, J. Sox4 enhances chondrogenic differentiation and proliferation of human synovium-derived stem cell via activation of long noncoding RNA DANCR. J. Mol. Histol. 2015, 46, 467–473. [Google Scholar] [CrossRef]
- Ou, F.; Su, K.; Sun, J.; Liao, W.; Yao, Y.; Zheng, Y.; Zhang, Z. The LncRNA ZBED3-AS1 induces chondrogenesis of human synovial fluid mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2017, 487, 457–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barter, M.J.; Gomez, R.; Hyatt, S.; Cheung, K.; Skelton, A.J.; Xu, Y.; Clark, I.M.; Young, D.A. The long non-coding RNA ROCR contributes to SOX9 expression and chondrogenic differentiation of human mesenchymal stem cells. Development 2017, 144, 4510–4521. [Google Scholar] [CrossRef] [PubMed]
- Shu, T.; He, L.; Wang, X.; Pang, M.; Yang, B.; Feng, F.; Wu, Z.; Liu, C.; Zhang, S.; Liu, B. Long noncoding RNA UCA1 promotes chondrogenic differentiation of human bone marrow mesenchymal stem cells via miRNA-145–5p/SMAD5 and miRNA-124–3p/SMAD4 axis. Biochem. Biophys. Res. Commun. 2019, 514, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, M.; Chen, L.; Sun, T.; Wang, H.; Zhao, L.; Zhao, Q. LncRNA PMS2L2 protects ATDC5 chondrocytes against lipopolysaccharide-induced inflammatory injury by sponging miR-203. Life Sci. 2019, 217, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.-l.; Zhao, Q.-q.; Ma, Y.; Song, Y.-l.; Min, J.; Lu, J.-r.; Li, H.; Zhao, D.-Q. Long Noncoding RNA H19 Participates in the Regulation of Adipose-Derived Stem Cells Cartilage Differentiation. Stem Cells Int. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Z.; Li, Z.; Yu, B.; Wang, Y. Long noncoding RNAs expression signatures in chondrogenic differentiation of human bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2015, 456, 459–464. [Google Scholar] [CrossRef]
- Hu, K.; Jiang, W.; Sun, H.; Li, Z.; Rong, G.; Yin, Z. Long noncoding RNA ZBED3-AS1 induces the differentiation of mesenchymal stem cells and enhances bone regeneration by repressing IL-1beta via Wnt/beta-catenin signaling pathway. J. Cell. Physiol. 2019. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, C.; Chen, S.; Wang, G.; Shi, B.; Tao, X.; Zhou, L.; Zhao, J. Long noncoding RNA DANCR is a positive regulator of proliferation and chondrogenic differentiation in human synovium-derived stem cells. DNA Cell Biol. 2017, 36, 136–142. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X.; Chen, S.; Yang, C.; Shi, B.; Zhou, L.; Zhao, J. Long noncoding RNA DANCR regulates miR-1305-Smad 4 axis to promote chondrogenic differentiation of human synovium-derived mesenchymal stem cells. Biosci. Rep. 2017, 37, BSR20170347. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, P.; Jiang, P.; Lv, Y.; Dong, C.; Dai, X.; Tan, L.; Wang, Z. Upregulation of lncRNA HOTAIR contributes to IL-1β-induced MMP overexpression and chondrocytes apoptosis in temporomandibular joint osteoarthritis. Gene 2016, 586, 248–253. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Luo, Y.; Liu, Y.; Yu, N. LncRNA PVT1 regulates chondrocyte apoptosis in osteoarthritis by acting as a sponge for miR-488–3p. DNA Cell Biol. 2017, 36, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Luo, M.; Huang, Y. lncRNA-CIR regulates cell apoptosis of chondrocytes in osteoarthritis. J. Cell. Biochem. 2019, 120, 7229–7237. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Liu, J.; Luo, T.; Chen, Q.; Lu, M.; Meng, D. LncRNA PACER is down-regulated in osteoarthritis and regulates chondrocyte apoptosis and lncRNA HOTAIR expression. Biosci. Rep. 2019, 39, BSR20190404. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Wang, J.; Zhou, Y.; Zhao, Y.; Hang, D.; Cao, Y. LncRNA CASC2 is up-regulated in osteoarthritis and participates in the regulation of IL-17 expression and chondrocyte proliferation and apoptosis. Biosci. Rep. 2019, 39, BSR20182454. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, J.A. Article Menu Download PDF [PDF] Full Article Content List Abstract Introduction Wine Sensory Attributes Declaration of Conflicting Interests Funding References Figures & Tables Article Metrics Toggle Citation Dialog Cite Toggle Share Dialog Share Toggle Permissions Dialog Request Permissions Related Articles Assessing Olive Oil Peroxide Value by NIRS, and on Reference Methods; Sage Publications: Thousand Oaks, CA, USA, 2017. [Google Scholar]
- Shen, H.; Wang, Y.; Shi, W.; Sun, G.; Hong, L.; Zhang, Y. LncRNA SNHG5/miR-26a/SOX2 signal axis enhances proliferation of chondrocyte in osteoarthritis. Acta Biochim. Et Biophys. Sin. 2018, 50, 191–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Liu, L.; Yang, J.; Ding, J.; Xu, X. lncRNA DILC is downregulated in osteoarthritis and regulates IL-6 expression in chondrocytes. J. Cell. Biochem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, Z.; Guo, S.; Tang, G.; Lu, W.; Qi, X. LncRNA ANCR is positively correlated with transforming growth factor-β1 in patients with osteoarthritis. J. Cell. Biochem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lv, G.; Wang, B.; Kuang, L. The role of lncRNA XIST/miR-211 axis in modulating the proliferation and apoptosis of osteoarthritis chondrocytes through CXCR4 and MAPK signaling. Biochem. Biophys. Res. Commun. 2018, 503, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, L.; Wang, Q.; Huang, J.; Xu, S. LncRNA FOXD2-AS1 induces chondrocyte proliferation through sponging miR-27a-3p in osteoarthritis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1241–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Ai, H.; Liu, J.; Xu, M.; Zhou, Z.; Qian, C.; Xie, Y.; Yan, J. Characterization of novel lnc RNAs in the spinal cord of rats with lumbar disc herniation. J. Pain Res. 2019, 12, 501. [Google Scholar] [CrossRef] [PubMed]
- Rogler, L.E.; Kosmyna, B.; Moskowitz, D.; Bebawee, R.; Rahimzadeh, J.; Kutchko, K.; Laederach, A.; Notarangelo, L.D.; Giliani, S.; Bouhassira, E. Small RNAs derived from lncRNA RNase MRP have gene-silencing activity relevant to human cartilage–hair hypoplasia. Hum. Mol. Genet. 2013, 23, 368–382. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Sun, F.; Ran, J.; Wu, L. Identify CRNDE and LINC00152 as the key lncRNAs in age-related degeneration of articular cartilage through comprehensive and integrative analysis. PeerJ 2019, 7, e7024. [Google Scholar] [CrossRef] [PubMed]
- Maass, P.G.; Rump, A.; Schulz, H.; Stricker, S.; Schulze, L.; Platzer, K.; Aydin, A.; Tinschert, S.; Goldring, M.B.; Luft, F.C.; et al. A misplaced lncRNA causes brachydactyly in humans. J. Clin. Investig. 2012, 122, 3990–4002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, M.J.; Philp, A.M.; Heward, J.A.; Roux, B.T.; Walsh, D.A.; Davis, E.T.; Lindsay, M.A.; Jones, S.W. Long intergenic noncoding RNAs mediate the human chondrocyte inflammatory response and are differentially expressed in osteoarthritis cartilage. Arthritis Rheumatol. 2016, 68, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.F.; Tycksen, E.D.; Cai, L.; Yu, J.; Wright, R.; Brophy, R. Distinct degenerative phenotype of articular cartilage from knees with meniscus tear compared to knees with osteoarthritis. Osteoarthr. Cartil. 2019, 27, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Mleczko, A.M.; Bąkowska-Żywicka, K. When small RNAs become smaller: Non-canonical functions of snoRNAs and their derivatives. Acta Biochim. Pol. 2016, 63, 601–607. [Google Scholar] [CrossRef]
- Narla, A.; Ebert, B.L. Ribosomopathies: Human disorders of ribosome dysfunction. Blood 2010, 115, 3196–3205. [Google Scholar] [CrossRef]
- Caron, M.; Steinbusch, M.; Reicherter, K.; Mattijssen, S.; Surtel, D.; van Rhijn, L.; Pruijn, G.; Lausch, E.; Zabel, B.; Welting, T. RNase MRP is a novel regulator of endochondral ossification. Osteoarthr. Cartil. 2013, 21, S12–S13. [Google Scholar] [CrossRef] [Green Version]
- Steinbusch, M.M.; Fang, Y.; Milner, P.I.; Clegg, P.D.; Young, D.A.; Welting, T.J.; Peffers, M.J. Serum snoRNAs as biomarkers for joint ageing and post traumatic osteoarthritis. Sci. Rep. 2017, 7, 43558. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, M.; Marks, P.; White, L.M.; Hurtig, M.; Mi, Q.S.; Divine, G.; Gibson, G. Serum non-coding RNAs as biomarkers for osteoarthritis progression after ACL injury. Osteoarthr. Cartil. 2012, 20, 1631–1637. [Google Scholar] [CrossRef] [Green Version]
- Baraniskin, A.; Nöpel-Dünnebacke, S.; Ahrens, M.; Jensen, S.G.; Zöllner, H.; Maghnouj, A.; Wos, A.; Mayerle, J.; Munding, J.; Kost, D. Circulating U2 small nuclear RNA fragments as a novel diagnostic biomarker for pancreatic and colorectal adenocarcinoma. Int. J. Cancer 2013, 132, E48–E57. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-P.; Zhang, J.-L.; Wang, J.-Y.; Cui, M.-X.; Jia, J.-L.; Liu, X.-H.; Liang, Q.-D. MiR-1246 promotes LPS-induced inflammatory injury in chondrogenic cells ATDC5 by targeting HNF4γ. Cell. Physiol. Biochem. 2017, 43, 2010–2021. [Google Scholar] [CrossRef] [PubMed]
- Horton, W.A.; Hall, J.G.; Hecht, J.T. Achondroplasia. Lancet 2007, 370, 162–172. [Google Scholar] [CrossRef]
- Zelzer, E.; Olsen, B.R. The genetic basis for skeletal diseases. Nature 2003, 423, 343. [Google Scholar] [CrossRef] [PubMed]
- Horton, W.A.; Laederich, M.B. Treatment for Achondroplasia. US8426396B2, 1 August 2008. [Google Scholar]
- Hermanns, P.; Bertuch, A.A.; Bertin, T.K.; Dawson, B.; Schmitt, M.E.; Shaw, C.; Zabel, B.; Lee, B. Consequences of mutations in the non-coding RMRP RNA in cartilage-hair hypoplasia. Hum. Mol. Genet. 2005, 14, 3723–3740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grigelioniene, G.; Suzuki, H.I.; Taylan, F.; Mirzamohammadi, F.; Borochowitz, Z.U.; Ayturk, U.M.; Tzur, S.; Horemuzova, E.; Lindstrand, A.; Weis, M.A. Gain-of-function mutation of microRNA-140 in human skeletal dysplasia. Nat. Med. 2019, 1. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Watkins, G.; Le Good, N.; Roberts, S.; Murphy, C.; Brockbank, S.; Needham, M.; Read, S.; Newham, P. The identification of differentially expressed microRNA in osteoarthritic tissue that modulate the production of TNF-α and MMP13. Osteoarthr. Cartil. 2009, 17, 464–472. [Google Scholar] [CrossRef]
- Iliopoulos, D.; Malizos, K.N.; Oikonomou, P.; Tsezou, A. Integrative microRNA and proteomic approaches identify novel osteoarthritis genes and their collaborative metabolic and inflammatory networks. PLoS ONE 2008, 3, e3740. [Google Scholar] [CrossRef]
- Díaz-Prado, S.; Cicione, C.; Muiños-López, E.; Hermida-Gómez, T.; Oreiro, N.; Fernández-López, C.; Blanco, F.J. Characterization of microRNA expression profiles in normal and osteoarthritic human chondrocytes. BMC Musculoskelet. Disord. 2012, 13, 144. [Google Scholar] [CrossRef]
- Su, W.; Xie, W.; Shang, Q.; Su, B. The long noncoding RNA MEG3 is downregulated and inversely associated with VEGF levels in osteoarthritis. BioMed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Xu, J.; Xu, Y. The lncRNA MEG3 downregulation leads to osteoarthritis progression via miR-16/SMAD7 axis. Cell Biosci. 2017, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Ahn, C.; Chun, C.H.; Jin, E.J. A long non-coding RNA, GAS5, plays a critical role in the regulation of miR-21 during osteoarthritis. J. Orthop. Res. 2014, 32, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Liang, J.q.; Li, Y.; Lu, J.; Jia, H.b.; Xu, L.y.; Ma, X.l. Identification of long noncoding RNA associated with osteoarthritis in humans. Orthop. Surg. 2014, 6, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Yu, J.; Han, L.; Tian, S.; Xu, B.; Gong, X.; Zhao, Q.; Wang, Y. Knockdown of long non-coding RNA RP11–445H22. 4 alleviates LPS-induced injuries by regulation of MiR-301a in osteoarthritis. Cell. Physiol. Biochem. 2018, 45, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, Y.; Wang, Y.; Huang, X.; Zhao, W.; Zhao, Z. Long non-coding RNA XIST promotes extracellular matrix degradation by functioning as a competing endogenous RNA of miR-1277–5p in osteoarthritis. Int. J. Mol. Med. 2019, 44, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, Z.; Shan, Y.; Pan, Y.; Ma, J.; Jia, L. Long non-coding RNA HOTAIR promotes osteoarthritis progression via miR-17–5p/FUT2/β-catenin axis. Cell Death Dis. 2018, 9. [Google Scholar] [CrossRef]
- Xiao, K.; Yang, Y.; Bian, Y.; Feng, B.; Li, Z.; Wu, Z.; Qiu, G.; Weng, X. Identification of differentially expressed long noncoding RNAs in human knee osteoarthritis. J. Cell. Biochem. 2019, 120, 4620–4633. [Google Scholar] [CrossRef]
- Ali, S.; Shestopaloff, K.; Gandhi, R.; Kapoor, M. Circulating microRNA signatures identified in early versus late knee osteoarthritis. Osteoarthr. Cartil. 2019, 27, S285–S286. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Song, Q.; Huang, X.; Chen, Z.; Zhang, F.; Wang, K.; Huang, G.; Shen, H. Long noncoding RNA GAS5 promotes apoptosis in primary nucleus pulposus cells derived from the human intervertebral disc via Bcl-2 downregulation and caspase-3 upregulation. Mol. Med. Rep. 2019, 19, 2164–2172. [Google Scholar] [CrossRef]
- Tao, S.-C.; Yuan, T.; Zhang, Y.-L.; Yin, W.-J.; Guo, S.-C.; Zhang, C.-Q. Exosomes derived from miR-140–5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 2017, 7, 180–195. [Google Scholar] [CrossRef]
- Bao, X.; Ren, T.; Huang, Y.; Sun, K.; Wang, S.; Liu, K.; Zheng, B.; Guo, W. Knockdown of long non-coding RNA HOTAIR increases miR-454–3p by targeting Stat3 and Atg12 to inhibit chondrosarcoma growth. Cell Death Dis. 2017, 8, e2605. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.W.; Zhang, X.; Qian, G.B.; Jiang, M.J.; Wang, P.; Wang, K.Z. Downregulation of long noncoding RNA LOC101928134 inhibits the synovial hyperplasia and cartilage destruction of osteoarthritis rats through the activation of the Janus kinase/signal transducers and activators of transcription signaling pathway by upregulating IFNA1. J. Cell. Physiol. 2019, 234, 10523–10534. [Google Scholar] [PubMed]
- Zhao, R.-l.; Zhang, X.-m.; Jia, L.-n.; Song, W.; Sun, Y.-l.; Meng, X.-y.; Peng, X.-X. pNNS-Conjugated Chitosan Mediated IGF-1 and miR-140 Overexpression in Articular Chondrocytes Improves Cartilage Repair. BioMed Res. Int. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Wang, S.; Jia, L.; Li, Q.; Qiao, J.; Peng, X. Interleukin-1 receptor antagonist protein (IL-1Ra) and miR-140 overexpression via pNNS-conjugated chitosan-mediated gene transfer enhances the repair of full-thickness cartilage defects in a rabbit model. Bone Jt. Res. 2019, 8, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, X.; Hu, X.; Dai, L.; Fu, X.; Zhang, J.; Ao, Y. Circular RNA related to the chondrocyte ECM regulates MMP13 expression by functioning as a MiR-136 ‘Sponge’in human cartilage degradation. Sci. Rep. 2016, 6, 22572. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-f.; Zhang, Y.; Xiao, J.; Wang, F.; Li, M.; Guo, X.-z.; Xie, H.-b.; Xia, H.; Chen, B. Hsa_circ_0045714 regulates chondrocyte proliferation, apoptosis and extracellular matrix synthesis by promoting the expression of miR-193b target gene IGF1R. Hum. Cell 2017, 30, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, J.; Sen, S. MicroRNA as biomarkers and diagnostics. J. Cell. Physiol. 2016, 231, 25–30. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, H.; Tan, Z.; Zhang, C.; Fu, X. Bottleneck limitations for microRNA-based therapeutics from bench to the bedside. Die Pharm. Int. J. Pharm. Sci. 2015, 70, 147–154. [Google Scholar]
- Ekimler, S.; Sahin, K. Computational methods for microRNA target prediction. Genes 2014, 5, 671–683. [Google Scholar] [CrossRef]
- Bardin, P.; Sonneville, F.; Corvol, H.; Tabary, O. Emerging microRNA therapeutic approaches for cystic fibrosis. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]


| miRNA | Abnormal Expression (↑ Or ↓) | Target | Cell Type | Year of Publication | References |
|---|---|---|---|---|---|
| miR-140 | - | Targets RALA, histone deacetylase 4, aggrecanases and syndecan 4 | Mouse chondrocytes | - | [40,41,58] |
| miR-3085 | ↑ | Regulates negatively transforming growth factor β1/Smad, IL-1β/NFκB, and Wnt3a/β-catenin signaling pathways | Mouse and rat chondrocytes | 2018 | [59] |
| miR-125b-5p | - | Targets TRAF6/MAPKs/NF-κB pathway | Human chondrocytes | 2019 | [60] |
| miR-138 | ↑ | Downregulates RhoC and the Actin Cytoskeleton | Human chondrocytes | 2018 | [61] |
| miR-218 | ↑ | Suppresses ALP, BSP, collagen type II alpha 1, OCN, and osteopontin. Promotes SOX9, COL2A1, ACAN, GAG, and COMP | Human synovium-derived MSCs | 2019 | [62] |
| miR-340–5p | - | Inhibits the ERK signaling pathway via the FMOD gene | Mouse chondrocytes | 2018 | [63] |
| miR-186 | ↓ | Interacts with SPP1 and regulates PI3K–AKT pathway | Mouse chondrocytes | 2018 | [64] |
| miR--27b-3p | ↓ | Inhibits HIPK2 expression | Human chondrocytes | 2018 | [65] |
| miR-193b-3p | ↑ | Inhibits matrix metalloproteinase-19 | Human chondrocytes | 2018 | [66] |
| miR-24 | ↑ | Targets C-myc on apoptosis and cytokine expressions in chondrocytes of osteoarthritis rats via MAPK signaling pathway | Rat chondrocytes | 2017 | [67] |
| miR-9–5 | ↑ | Inhibits apoptosis of chondrocytes through downregulating Tnc in mice with osteoarthritis | Mouse chondrocytes | 2019 | [68] |
| miR-27a | ↑ | Inhibits PLK2 | Knee arthritis rat model | 2019 | [69] |
| miR-495 | ↑ | Targets AKT1 | Rat | 2019 | [70] |
| miR-93 | ↑ | Targets TLR4/NF-κB signaling pathway | Mouse cartilage cells | 2018 | [71] |
| miR-31 | ↑ | Targets C-X-C motif chemokine ligand 12 | Human chondrocytes | 2019 | [72] |
| miR-590–5p | ↑ | Targets TGF-β1 to promote chondrocyte apoptosis and autophagy in response to mechanical pressure injury | Human chondrocytes | 2018 | [73] |
| miR-206 | ↓ | Influences apoptosis and autophagy of articular chondrocytes via modulating the PI3K/protein kinase B-mTOR pathway by targeting insulin-like growth factor-1 | Rat chondrocytes | 2018 | [74] |
| miR-23 | - | Inhibits articular cartilage damage recovery by regulating MSCs differentiation to chondrocytes via reducing fibroblast growth factor 2 | Rat MSCs | 2019 | [75] |
| miR-19a | - | Promotes cell viability and migration of chondrocytes via up-regulating SOX9 through NF-κB pathway | Human synovium-derived chondrocytes | 2018 | [76] |
| miR-107 | - | Regulates autophagy and apoptosis of OA chondrocytes by targeting TRAF3 | Rat cartilage cells | 2019 | [77] |
| miR-98 | - | Targets the 3́ untranslated region of Bcl-2 | Human chondrocytes | 2018 | [78] |
| miR-615–3p | ↑ | Increases the expressions of inflammatory cytokines and inhibiting chondrogenic differentiation of hBMSCs. | Human bone marrow stem cells (hBMSCs) | 2019 | [79] |
| miR- 29b | ↑ | Regulates chondrogenesis homeostasis and enhance hypertrophic phenotype. | Murine MSCs | 2019 | [80] |
| miR-4784 | ↑ | Promotes the expression of Col2a1 and inhibits the MMP-3 expression in chondrocytes. | Human cartilage cells | 2018 | |
| miR-320c | ↑ | Regulates the expression of β-catenin by directly targeting 3′UTR of β-catenin mRNA and decreasing the relative transcriptional activity of the β-catenin/TCF complex | human adipose-derived stromal/stem cells (hADSCs) | 2019 | [81] |
| miR-17-5p | ↓ | Induces autophagy mainly through suppressing the expression of p62 | Mouse model of OA | 2018 | [82] |
| miR-181a/b-1 | ↑ | Modulates PTEN/PI3K/AKT signaling and mitochondrial metabolism | Human chondrocytes | 2019 | [83] |
| miR-4262 | - | Regulates chondrocyte viability, apoptosis, and autophagy by targeting SIRT1 and activating PI3K/AKT/mTOR signaling pathway | Rat model of OA | 2018 | [84] |
| miR-221 | ↓ | Increases the expression of typical chondrogenic markers including COL2A1, ACAN, and SOX9 | Human cartilage cells derived from intervertebral disk | 2018 | [85] |
| miR-107 | - | Modulates chondrocyte proliferation, apoptosis, and ECM synthesis by targeting PTEN | Human cartilage cells | 2019 | [86] |
| miR-34a | ↑ | Enhances chondrocyte apoptosis, senescence and facilitates the development of OA by targeting DLL1 and regulating PI3K/AKT pathway | Rat chondrocytes | 2019 | [87] |
| miR-373 | ↓ | Regulates inflammatory cytokine-mediated chondrocyte proliferation in OA by targeting the P2X7 receptor | Human chondrocytes | 2018 | [88] |
| miR-155 | - | It is a sustainable factor for intervertebral disk and suppresses the expression of catabolic genes induced by TNF-α and IL-1β by targeting C/EBPβ in rat NP cells | Mouse cartilage cells derived from intervertebral disk | 2018 | [89] |
| miR-486 | ↑ | Inhibits chondrocyte proliferation and migration by suppressing SMAD2 | Human cartilage cells | 2018 | [90,91] |
| miR-127–5p | - | MALAT1/miR-127–5p regulates osteopontin-mediated proliferation of human chondrocytes through PI3K/Akt Pathway | Human cartilage cells | 2018 | [92] |
| lncRNAs | Downstream Targets | Cell Type | Effects | Reference |
|---|---|---|---|---|
| GRASLND | SOX9 | MSC | It has a protective effect in engineered cartilage against interferon type II across different sources of chondroprogenitor cells. | [127] |
| lncRNA TUG1 | Notch and NF-κB pathways | murine chondrogenic ATDC5 cells | It suppresses Notch and NF-κB pathways. | [128] |
| SOX9nc2 | SOX9 and TGFβ | OA cartilage | Depletion of the SOX9nc2 transcript, by RNAi, prevents chondrogenesis and concomitant induction of SOX9 expression. | [129] |
| H19 | COL2A1 | OA-affected cartilage | This lncRNA stimulates chondrocyte anabolism. | [130] |
| DANCR | Smad 4, miR-1305 STAT3, Smad3, myc | SMSCs | SOX4 could directly bind to the promoter of DANCR lncRNA and increases its expression and knockdown of DANCR could reverse these effects. | [131] |
| HIT | p100, CBP | mouse embryos | LncRNA-HIT is essential for chondrogenic differentiation in the limb mesenchyme. | [125] |
| ZBED3-AS1 | zbed3 | SFMSCs | It promotes chondrogenesis and could directly increase zbed3 expression. | [132] |
| ROCR | SOX9 | human BMSCs | SOX9 induction is significantly ablated in the absence of ROCR. Thus, ROCR contributes to SOX9 expression and chondrogenic differentiation. | [133] |
| UCA1 | miRNA-145–5p/miRNA-124–3p | human BMSCs | It promotes chondrogenic differentiation of human BMSCs via miRNA-145–5p/SMAD5 and miRNA-124–3p/SMAD4 axis. | [134] |
| PMS2L2 | miR-203. | ATDC5 chondrocytes | PMS2L2 has a protective role in LPS-induced inflammatory injury in chondrocytes. | [135] |
| Disease | Non-coding RNA | Abnormal Expression | Targets | Effects | Reference |
|---|---|---|---|---|---|
| Osteoarthritis | lncRNA H19 | - | Regulates the balance between ECM anabolism and regeneration | [126] | |
| lncRNA HOTAIR | ↑ | matrix metalloproteinase (MMP)-1, MMP3, MMP9 | Contributes to IL-1β-induced MMP overexpression and chondrocytes apoptosis in temporomandibular joint osteoarthritis. | [141] | |
| lncRNA PVT1 | ↓ | miR-488–3p | Promotes apoptosis of OA and normal chondrocytes through miR-488–3p | [142] | |
| lncRNA-CIR | - | Bim and miR-130a | lncRNA-CIR/miR-130a/Bim axis is involved in oxidative stress-related apoptosis of chondrocytes in OA. | [143] | |
| lncRNA PACER | ↓ | down-regulates lncRNA HOTAIR. | Regulates chondrocyte apoptosis | [144] | |
| LncRNA CASC2 | ↑ | IL-17 | Participates in the regulation of IL-17 expression and chondrocyte proliferation and apoptosis. | [145] | |
| lncRNA Nespas | ↑ | ACSL6 | suppresses miRs targeting ACSL6 and subsequent ACSL6 upregulation. | [146] | |
| LncRNA SNHG5 | - | SOX2 | LncRNA SNHG5/miR-26a/SOX2 signal axis enhances proliferation of chondrocyte in osteoarthritis | [147] | |
| lncRNA DILC | ↓ | IL-6 | Regulates IL-6 expression in chondrocytes. | [148] | |
| LncRNA ANCR | ↑ | TGF-β1 | ANCR might participate in OA by downregulating TGF-β1 and promote the proliferation of chondrocytes. | [149] | |
| lncRNA XIST | - | CXCR4 and MAPK signaling | lncRNA XIST can promote the proliferation of OA chondrocytes and promote apoptosis through the miR-211/CXCR4 axis | [150] | |
| LncRNA-p21 | ↑ | miR-451 | Negatively regulates the expression of miR-451 and promotes the apoptosis of chondrocytes in OA by acting as a sponge for miR-451 | [90] | |
| LncRNA FOXD2-AS1 | ↑ | miR-27a-3p | miR-27a-3p mimics could abolish the effects of FOXD2-AS1 overexpression on cell proliferation, inflammation, and ECM degradation in chondrocytes | [151] | |
| Spinal disc herniation | lncRNAs TCONS | - | - | These lncRNAs in the spinal cord of rats in a radicular pain model of LDH have different expression patterns and their roles in unknown. | [152] |
| Human cartilage-hair hypoplasia | lncRNA RNase MRP | - | PTCH2 and SOX4 | have gene-silencing activity relevant to human cartilage–hair hypoplasia | [153] |
| Age-related degeneration of articular cartilage | CRNDE and LINC00152 as the key lncRNAs | - | - | Are involved in the process of age-related degeneration of articular cartilage | [154] |
| Brachydactyly type E | DA125942 | ↑ | PTHLH and SOX9 | Upregulation of DA125942 downregulates SOX9. | [155] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razmara, E.; Bitaraf, A.; Yousefi, H.; Nguyen, T.H.; Garshasbi, M.; Cho, W.C.-s.; Babashah, S. Non-Coding RNAs in Cartilage Development: An Updated Review. Int. J. Mol. Sci. 2019, 20, 4475. https://doi.org/10.3390/ijms20184475
Razmara E, Bitaraf A, Yousefi H, Nguyen TH, Garshasbi M, Cho WC-s, Babashah S. Non-Coding RNAs in Cartilage Development: An Updated Review. International Journal of Molecular Sciences. 2019; 20(18):4475. https://doi.org/10.3390/ijms20184475
Chicago/Turabian StyleRazmara, Ehsan, Amirreza Bitaraf, Hassan Yousefi, Tina H. Nguyen, Masoud Garshasbi, William Chi-shing Cho, and Sadegh Babashah. 2019. "Non-Coding RNAs in Cartilage Development: An Updated Review" International Journal of Molecular Sciences 20, no. 18: 4475. https://doi.org/10.3390/ijms20184475
APA StyleRazmara, E., Bitaraf, A., Yousefi, H., Nguyen, T. H., Garshasbi, M., Cho, W. C. -s., & Babashah, S. (2019). Non-Coding RNAs in Cartilage Development: An Updated Review. International Journal of Molecular Sciences, 20(18), 4475. https://doi.org/10.3390/ijms20184475