Interpretation of Fiber Supplementation on Offspring Testicular Development in a Pregnant Sow Model from a Proteomics Perspective
Abstract
:1. Introduction
2. Results
2.1. Effect of Maternal Fiber Intake on Body Weight and Testicular Development of Offspring
2.2. Effect of Maternal Fiber Intake on Offspring Biochemical Parameters
2.3. DEPs Analysis in Proteomics
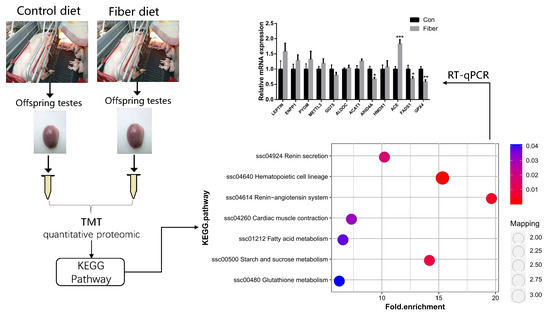
2.4. Functional Enrichment Analysis of DEPs
2.5. Relative Expression of mRNAs
3. Discussion
4. Materials and Methods
4.1. Animals and Diets
4.2. Sample Collection
4.3. Physiology and Biochemistry of Testes
4.4. TMT Quantitative Proteomic
4.5. Bioinformatics Analysis
4.5.1. Annotation Methods
4.5.2. Functional Enrichment
4.5.3. Quantitative PCR Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fang, L.Y.; Du, J.W.; Tao, X.R.; Liu, G.L.; Wang, F.; Chen, X.R. The expression of c-fos in spermatogonia and mesenchymal cells during the development of fetal testis. J. Anat. 2007, 30, 805–808. [Google Scholar]
- Lording, D.W.; De kretser, D.M. Comparative ultrastructural and histochemical studies of the interstitial cells of the rat testis during fetal and postnatal development. Reproduction 1972, 29, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.B.; Knell, C.M. The fate of fetal Leydig cells during the development of the fetal and postnatal rat testis. Development 1988, 103, 535–544. [Google Scholar] [PubMed]
- Orth, J.M. Proliferation of sertoli cells in fetal and postnatal rats: A quantitative autoradiographic study. Anat. Rec. 1982, 203, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Van Straaten, H.W.; Wensing, C.J. Leydig cell development in the testis of the pig. Biol. Reprod. 1978, 18, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ge, R.-S.; Zirkin, B.R. Leydig cells: From stem cells to aging. Mol. Cell Endocrinol. 2009, 306, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, T.; Hedinger, C.; Groscurth, P. Ultrastructure of developing germ cells in the fetal human testis. Cell Tissue Res. 1975, 161, 55–70. [Google Scholar] [CrossRef]
- Griswold, M.D. The central role of Sertoli cells in spermatogenesis. Semin. Cell Dev. Biol. 1998, 9, 411–416. [Google Scholar] [CrossRef] [Green Version]
- Xun, W.; Yueying, W. Animal Physiology; China Forestry Press: Beijing, China, 2016; Volume 328. (In Chinese) [Google Scholar]
- Bielli, A.; Pérez, R.; Pedrana, G. Low maternal nutrition during pregnancy reduces the number of Sertoli cells in the newborn lamb. Reprod. Fertil. Dev. 2002, 14, 333–337. [Google Scholar] [CrossRef]
- Ramos, C.d.F.; Lima, S.S.; Rocha, M.L.M. Maternal malnutrition during lactation alters gonadotropin-releasing hormone expression in the hypothalamus of weaned male rat pups. Nutr. Neurosci. 2010, 13, 170–174. [Google Scholar] [CrossRef]
- Vilanova Teixeira, C.; Silandre, D.; Marcelly de Souza Santos, A. Effects of maternal undernutrition during lactation on aromatase, estrogen, and androgen receptors expression in rat testis at weaning. J. Endocrinol. 2007, 192, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Rehfeldt, C.; Nissen, P.M.; Kuhn, G. Effects of maternal nutrition and porcine growth hormone (pGH) treatment during gestation on endocrine and metabolic factors in sows, fetuses and pigs, skeletal muscle development, and postnatal growth. Domest. Anim. Endocrinol. 2004, 27, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, E.; Rodríguez-González, G.L.; Guzmán, C. A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development. J. Physiol 2005, 563, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Rae, M.T.; Rhind, S.M.; Fowler, P.A.; Miller, D.W.; Kyle, C.E.; Brooks, A.N. Effect of maternal undernutrition on fetal testicular steroidogenesis during the CNS androgen-responsive period in male sheep fetuses. Reproduction 2002, 124, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Zhang, Z. Food restriction affects reproduction and survival of F1 and F2 offspring of Rat-like hamster (Cricetulus triton). Physiol. Behav. 2006, 87, 607–613. [Google Scholar] [CrossRef]
- Edwards, L.J.; Bryce, A.E.; Coulter, C.L.; McMillen, I.C. Maternal undernutrition throughout pregnancy increases adrenocorticotrophin receptor and steroidogenic acute regulatory protein gene expression in the adrenal gland of twin fetal sheep during late gestation. Mol. Cell. Endocrinol. 2002, 196, 1–10. [Google Scholar] [CrossRef]
- Iwasa, T.; Matsuzaki, T.; Yano, K.; Mayila, Y.; Irahara, M. Prenatal undernutrition attenuates fasting-induced reproductive dysfunction in pre-pubertal male rats. Int. J. Dev. Neurosci. 2018, 71, 30–33. [Google Scholar] [CrossRef]
- Jazwiec, P.A.; Sloboda, D.M. Nutritional adversity, sex and reproduction: 30 years of DOHaD and what have we learned? J. Endocrinol. 2019, 242, T51–T68. [Google Scholar] [CrossRef]
- Longcope, C.; Feldman, H.A.; McKinlay, J.B. Diet and Sex Hormone-Binding Globulin. J. Clin. Endorc. Metab. 2000, 85, 293–296. [Google Scholar] [CrossRef]
- Yu, B.; Khan, G.; Foxworth, A.; Huang, K.; Hilakivi-Clarke, L. Maternal dietary exposure to fiber during pregnancy and mammary tumorigenesis among rat offspring. Int. J. Cancer 2006, 119, 2279–2286. [Google Scholar] [CrossRef]
- Dai, F.J.; Chau, C.F. Classification and regulatory perspectives of dietary fiber. J. Food Drug Anal. 2017, 25, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Ötles, S.; Ozgoz, S. Health effects of dietary fiber. Acta Sci. Pol. Technol. Aliment. 2014, 13, 191–202. [Google Scholar]
- Le Bourgot, C.; Ferret-Bernard, S.; Blat, S.; Apper, E.; Huërou-Luron, I.L. Short-chain fructooligosaccharide supplementation during gestation and lactation or after weaning differentially impacts pig growth and IgA response to influenza vaccination. J. Funct. Foods 2016, 24, 307–315. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, X.L.; Ding, L.L.; Wang, L.X.; Xu, Q.S.; Wang, X.W. Effects of complex oligosaccharides on testicular development and reproductive hormones in young mice. J. Nutr. 2014, 2, 135–140. [Google Scholar]
- Pastuszewska, B.; Taciak, M.; Tuśnio, A. Physiological effects of long-term feeding diets supplemented with potato fibre or cellulose to adult rats. Arch. Anim. Nutr. 2010, 64, 155–169. [Google Scholar] [CrossRef]
- Cheng, L.K.; Wang, L.X.; Xu, Q.S.; Huang, L.J.; Zhou, D.S.; Li, Z.; Li, S.G.; Du, Y.G.; Yin, H. Chitooligosaccharide supplementation improves the reproductive performance and milk composition of sows. Livest. Sci. 2015, 174, 74–81. [Google Scholar] [CrossRef]
- Ferguson, E.; Slevin, J.; Hunter, M.; Edwards, S.; Ashworth, C. Beneficial effects of a high fibre diet on oocyte maturity and embryo survival in gilts. Reproduction 2007, 133, 433–439. [Google Scholar] [CrossRef]
- Ji, X.M. The Effects of maternal diet supplement with high fiber and sodium butyrate during gestation and lactation on longissimus dorsi muscle characteristic of piglet. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2016. [Google Scholar]
- Yan, L. Effects of maternal fiber nutrition on fetal and postnatal growth and antioxidant capacity. Ph.D. Thesis, Sichuan Agricultural University, Sichuan, China, 2011. [Google Scholar]
- Alves, M.G.; Martins, A.D.; Cavaco, J.E.; Socorro, S.; Oliveira, P.F. Diabetes, insulin-mediated glucose metabolism and Sertoli/blood-testis barrier function. Tissue Barriers 2013, 1, e23992. [Google Scholar] [CrossRef] [Green Version]
- Malama, E.; Zeron, Y.; Janett, F.; Siuda, M.; Roth, Z.; Bollwein, H. Use of computer-assisted sperm analysis and flow cytometry to detect seasonal variations of bovine semen quality. Theriogenology 2017, 87, 79–90. [Google Scholar] [CrossRef]
- Shin, B.-C.; Fujikura, K.; Takata, K.; Tanaka, S.; Suzuki, T. Glucose Transporter GLUT3 in the Rat Placental Barrier: A Possible Machinery for the Transplacental Transfer of Glucose. Endocrinology 1997, 138, 3997–4004. [Google Scholar] [CrossRef]
- Alves, M.G.; Rato, L.; Carvalho, R.A.; Moreira, P.I.; Socorro, S.; Oliveira, P.F. Hormonal control of Sertoli cell metabolism regulates spermatogenesis. Cell. Mol. Life Sci. 2013, 70, 777–793. [Google Scholar] [CrossRef] [PubMed]
- Courtens, J.L.; Plöen, L. Improvement of Spermatogenesis in Adult Cryptorchid Rat Testis by Intratesticular Infusion of Lactate1. Biol. Reprod. 1999, 61, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Riera, M.F.; Galardo, M.N.; Pellizzari, E.H. Molecular mechanisms involved in Sertoli cell adaptation to glucose deprivation. Am. J. Physiol.-Endoc. Metab. 2009, 297, E907–E914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera, E. Lipid metabolism in pregnancy and its consequences in the fetus and newborn. Endocrine 2002, 19, 43–55. [Google Scholar] [CrossRef]
- Chen, L.; Lafond, J.; Pelletier, R.M. A novel technical approach for the measurement of individual ACAT-1 and ACAT-2 enzymatic activity in the testis. Hum. Embryog. 2009, 550, 169–177. [Google Scholar]
- Mei, X. The expression of FADS1 and FADS2 genes and their effects on fatty acid composition of high-quality chickens under different feeding methods Thesis. Master’s Thesis, Sichuan Agricultural University, Sichuan, China, 2012. [Google Scholar]
- Pelletier, R.M. The blood-testis barrier: The junctional permeability, the proteins and the lipids. Prog. Histochem. Cyto. 2011, 46, 49–127. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ge, X.; Liang, W.; Shao, Y.; Jing, J.; Wang, C.; Zeng, R.; Yao, B. Effects of saturated palmitic acid and omega-3 polyunsaturated fatty acids on Sertoli cell apoptosis. Syst. Biol. Reprod. Med. 2018, 64, 368–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datar, J.; Regassa, A.; Kim, W.-K.; Taylor, C.G.; Zahradka, P.; Suh, M.J.L. Lipid Metabolism is Closely Associated with Normal Testicular Growth Based on Global Transcriptome Profiles in Normal and Underdeveloped Testis of Obese Zucker (fa/fa) Rats. Lipids 2017, 52, 951–960. [Google Scholar] [CrossRef]
- Hermo, L.; Chung, S.; Mitchell, G.; Pan, L.; Wang, S.P.; Trasler, J. Infertility and Testicular Defects in Hormone-Sensitive Lipase-Deficient Mice. Endocrinology 2001, 142, 4272–4281. [Google Scholar]
- Li, W.; Wu, Z.Q.; Zhang, S.; Cao, R.; Zhao, J.; Sun, Z.J.; Zou, W. Augmented expression of gamma-glutamyl transferase 5 (GGT5) impairs testicular steroidogenesis by deregulating local oxidative stress. Cell Tissue Res. 2016, 366, 467–481. [Google Scholar] [CrossRef]
- Puglisi, R.; Bevilacqua, A.; Carlomagno, G.; Fau-Lenzi, A.; Gandini, L.; Stefanini, M.; Mangia, F.; Boitani, C. Mice overexpressing the mitochondrial phospholipid hydroperoxide glutathione peroxidase in male germ cells show abnormal spermatogenesis and reduced fertility. Endocrinology 2007, 148, 4302–4309. [Google Scholar] [CrossRef]
- Bauché, F.; Fouchard, M.-H.; Jégou, B. Antioxidant system in rat testicular cells. Febs. Lett. 1994, 349, 392–396. [Google Scholar] [CrossRef] [Green Version]
- Baumber, J.; Ball, B.A.; Gravance, C.G.; Medina, V.; Davies-Morel, M.C. The effect of reactive oxygen species on equine sperm motility, viability, acrosomal integrity, mitochondrial membrane potential, and membrane lipid peroxidation. J. Androl. 2000, 21, 895–902. [Google Scholar] [PubMed]
- Stradaioli, G.; Noro, T.; Sylla, L.; Monaci, M. Decrease in glutathione (GSH) content in bovine sperm after cryopreservation: Comparison between two extenders. Theriogenology 2007, 67, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Gadea, J.n.; Sellés, E.; Marco, M.A.; Coy, P.; Matás, C.; Romar, R.; Ruiz, S. Decrease in glutathione content in boar sperm after cryopreservation: Effect of the addition of reduced glutathione to the freezing and thawing extenders. Theriogenology 2004, 62, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Mata-Campuzano, M.; Álvarez-Rodríguez, M.; Tamayo-Canul, J.; López-Urueña, E.; de Paz, P.; Anel, L.; Martínez-Pastor, F.; Álvarez, M. Refrigerated storage of ram sperm in presence of Trolox and GSH antioxidants: Effect of temperature, extender and storage time. Anim. Reprod. Sci. 2014, 151, 137–147. [Google Scholar] [CrossRef]
- Langford, K.G.; Shai, S.Y.; Howard, T.E.; Kovac, M.J.; Overbeek, P.A.; Bernstein, K.E. Transgenic mice demonstrate a testis-specific promoter for angiotensin-converting enzyme. J. Biol. Chem. 1991, 266, 15559–15562. [Google Scholar]
- Ganong, W.F. Reproduction and the renin-angiotensin system. Neurosci. Biobehav. Rev. 1995, 19, 241–250. [Google Scholar] [CrossRef]
- Vinson, G.P.; Saridogan, E.; Puddefoot, J.R.; Djahanbakhch, O. Tissue renin-angiotensin systems and reproduction. Hum. Reprod. 1997, 12, 651–662. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; Yang, Y.; Feng, G.-H.; Sun, B.-F.; Chen, J.-Q.; Li, Y.-F.; Chen, Y.-S.; Zhang, X.-X.; Wang, C.-X.; Jiang, L.-Y.; et al. Mettl3-mediated m6A regulates spermatogonial differentiation and meiosis initiation. Cell Res. 2017, 27, 1100. [Google Scholar] [CrossRef]
- Wu, R.-C.; Jiang, M.; Beaudet, A.L.; Wu, M.-Y. ARID4A and ARID4B regulate male fertility, a functional link to the AR and RB pathways. Proc. Natl. Acad. Sci. USA 2013, 110, 4616–4621. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, T.; Fujioka, H.; Ishimura, T.; Takenaka, A.; Fujisawa, M. Expression of leptin and leptin receptor in the testis of fertile and infertile patients. Andrologia 2007, 39, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Swierstra, E. Cytology and duration of the cycle of the seminiferous epithelium of the boar; duration of spermatozoan transit through the epididymis. Anat. Rec. 1968, 161, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]







| Protein Accession | Protein Description | Fiber/Con Ratio | Fiber/Con p Value | Gene Name |
|---|---|---|---|---|
| A0A287A1D6 | Methyltransferase like 3 | 0.625 | 0.0488 | METTL3 |
| F1RJ25 | Fructose-bisphosphate aldolase | 0.757 | 0.025 | ALDOC |
| P36968 | Phospholipid hydroperoxide glutathione peroxidase | 0.76 | 0.0039 | GPX4 |
| F1RLR8 | Gamma-glutamyltransferase 5 | 0.802 | 0.0158 | GGT5 |
| K7N7E5 | Uncharacterized protein | 0.825 | 0.015 | FADS1 |
| F1SSK5 | Uncharacterized protein | 0.828 | 0.0164 | ARID4A |
| I3LP02 | Acetyl-CoA acetyltransferase 1 | 1.226 | 0.01 | ACAT1 |
| F1RQQ8 | Alpha-1,4 glucan phosphorylase | 1.247 | 0.0234 | PYGM |
| B9TRX0 | Leptin receptor gene-related protein | 1.249 | 0.0378 | LEPROT |
| F1RRW5 | Angiotensin-converting enzyme | 1.273 | 0.0286 | ACE |
| A0A286ZUF1 | Ectonucleotide pyrophosphatase/phosphodiesterase 1 | 1.321 | 0.00702 | ENPP1 |
| A0A287AWS9 | Heme oxygenase | 1.432 | 0.0239 | HMOX1 |
| A0A287BLE1 | Sequestosome 1 | 1.216 | 0.0184 | SQSTM1 |
| F1SAZ0 | Sperm associated antigen 17 | 0.351 | 0.0000791 | SPAG17 |
| A0A287A7G0 | COX assembly mitochondrial protein | 1.91 | 0.0139 | CMC2 |
| F1CNZ4 | STEAP family member 4 | 1.318 | 0.0464 | STEAP4 |
| Material and Composition, % | Nutritive Composition | ||
|---|---|---|---|
| Corn | 62.39 | DE, Mcal/kg | 3.36 |
| Peeled soybean meal | 13.10 | CP, % | 13.39 |
| Fish meal | 2.00 | CF, % | 2.90 |
| Flour | 10.00 | CF, % | 1.41 |
| Corn starch | 10.00 | Soluble fiber, % | 1.13 |
| Lys | 0.10 | Insoluble fiber, % | 9.08 |
| Thr | 0.02 | Insoluble/soluble | 8.03 |
| CaCO3 | 0.84 | Dietary fiber, % | 10.21 |
| CaHCO3 | 0.46 | Ca, % | 0.60 |
| NaCl | 0.40 | Available P, % | 0.27 |
| Choline | 0.14 | Lys, % | 0.60 |
| Sow multivitamin 1 | 0.05 | Met, % | 0.21 |
| Mineral addition 2 | 0.50 | Thr, % | 0.46 |
| Total | 100.00 | Trp, % | 0.14 |
| Gene Name | Forward Sequence (5′→3′) | Reverse Sequence (5′→3′) | Accession No | Product Length (Base Pair) |
|---|---|---|---|---|
| ACAT1 | GGCTTACCTATTTCTACTCCGTGC | CCATTCCACCTGCCACCAT | XM_005667301.3 | 127 |
| FADS1 | AGCCTTGCTGCCTGCCTACT | CAGTGGCACATAAGTGAGGAAGAT | NM_001113041.1 | 124 |
| GGT5 | AACACGGTTCACCTGTGGATG | CCTGTGGTCGCGTTGTAGATAGT | XM_021074378.1 | 118 |
| GPX4 | AACCAGTTTGGGAGGCAGGAG | GGACTTTCATCCACTTCCACAGAG | NM_214407.1 | 142 |
| PYGM | ACGTGGACGACGAAGCCTTTA | TTGATGTGGACTTTGTATTCCCTCT | XM_003122588.5 | 103 |
| ALDOC | GATAAAGGCATTGTCGTGGGC | GCAAAGTCGGCACCATCCT | XM_005656989.3 | 140 |
| METTL3 | CTTGCCCTTACACAGAGCGTTG | CAAACTTGCCCAAGATACTGACGT | XM_003128580.5 | 112 |
| ARID4A | ATCTGCTCTTTCACCAAACATGC | TTCCATTCCATTTGACAGAGGTG | XM_021088206.1 | 124 |
| HMOX1 | CAGGCTGAGAATGCCGAGTT | CTTGTTGTGCTCAATCTCCTCCT | NM_001004027.1 | 129 |
| ACE | ACGCCAACAGCACTTGTCTTC | ATGGCTCTGCCCACCTTGTC | NM_001033015.3 | 121 |
| LEPTIN | TCATCAAGACGATTGTCACCAGG | TGGATCACATTTCTGGAAGGCA | XM_021078503.1 | 184 |
| ENPP1 | CACATCCCAGATTCCCTCACA | GCCTCAACAACTCTTCAACCCAT | XM_021087944.1 | 131 |
| β-actin | TCTGGCACCACACCTTCT | TGATCTGGGTCATCTTCTCAC | XM_021086047.1 | 114 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Li, L.; Li, Y.; Wang, K.; Wei, D.; Xu, S.; Feng, B.; Che, L.; Fang, Z.; Li, J.; et al. Interpretation of Fiber Supplementation on Offspring Testicular Development in a Pregnant Sow Model from a Proteomics Perspective. Int. J. Mol. Sci. 2019, 20, 4549. https://doi.org/10.3390/ijms20184549
Lin Y, Li L, Li Y, Wang K, Wei D, Xu S, Feng B, Che L, Fang Z, Li J, et al. Interpretation of Fiber Supplementation on Offspring Testicular Development in a Pregnant Sow Model from a Proteomics Perspective. International Journal of Molecular Sciences. 2019; 20(18):4549. https://doi.org/10.3390/ijms20184549
Chicago/Turabian StyleLin, Yan, Lujie Li, Yang Li, Ke Wang, Dongqin Wei, Shengyu Xu, Bin Feng, Lianqiang Che, Zhengfeng Fang, Jian Li, and et al. 2019. "Interpretation of Fiber Supplementation on Offspring Testicular Development in a Pregnant Sow Model from a Proteomics Perspective" International Journal of Molecular Sciences 20, no. 18: 4549. https://doi.org/10.3390/ijms20184549
APA StyleLin, Y., Li, L., Li, Y., Wang, K., Wei, D., Xu, S., Feng, B., Che, L., Fang, Z., Li, J., Zhuo, Y., & Wu, D. (2019). Interpretation of Fiber Supplementation on Offspring Testicular Development in a Pregnant Sow Model from a Proteomics Perspective. International Journal of Molecular Sciences, 20(18), 4549. https://doi.org/10.3390/ijms20184549