Angiotensin-II Drives Human Satellite Cells Toward Hypertrophy and Myofibroblast Trans-Differentiation by Two Independent Pathways
Abstract
:1. Introduction
2. Results
2.1. Characterization of hSC Phenotype
2.2. Activated hSCs Express a Functional RAS Protein Panel
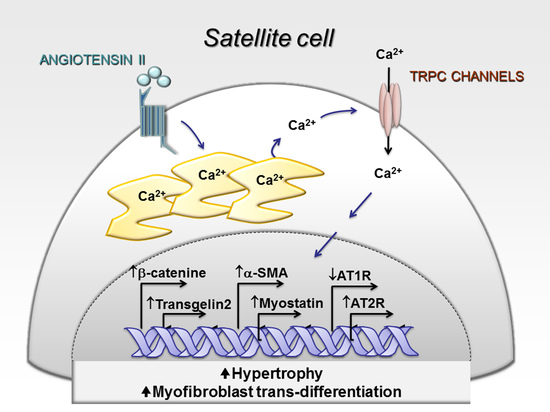
2.3. Sub-Chronic Conditioning of Activated hSCs with Ang Leads to Hypertrophy and Myofibroblast Trans-Differentiation Without Modification of Cell Viability
2.4. Sub-Chronic Conditioning of Activated hSC with Ang Enhances TRPC Channel Function and Modifies Expression Patterns of AT Receptors
3. Discussion
4. Materials and Methods
4.1. hSC Preparation
4.2. Immunofluorescence
4.3. Western Blot Analysis
4.4. Quantitative PCR Analysis
4.5. Assessment of Cell Viability/Growth
4.6. TRPC-Mediated Ca2+ Imaging
4.7. Solutions
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SC | satellite cell |
| hSC | hSC |
| Ang | angiotensin-II |
| RAS | renin angiotensin system |
| TRPC | transient receptor potential canonical |
| AT1R | Ang type-1 receptor |
| AT2R | Ang type-2 receptor |
| ACE | angiotensin-converting enzyme |
| ACEi | angiotensin-converting enzyme inhibitors, |
| ARB | AT1 receptor antagonist |
| Pax7 | paired box protein 7 |
| Myf5 | myogenic factor 5 |
| MyoD | myogenic factor 3 |
| Myogenin | Myogenin |
| MHC | myosin heavy chain |
| ACE1 | type-1 angiotensin-converting enzyme |
| ACE2 | type-2 angiotensin-converting enzyme |
| Irb | Irbesartan |
| α-SMA | α-smooth muscle actin |
References
- Almada, A.E.; Wagers, A.J. Molecular circuitry of stem cell fate in skeletal muscle regeneration, ageing and disease. Nat. Rev. Mol. Cell Biol. 2016, 17, 267–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delafontaine, P.; Yoshida, T. The renin-angiotensin system and the biology of skeletal muscle: Mechanisms of muscle wasting in chronic disease states. Trans. Am. Clin. Climatol. Assoc. 2016, 127, 245–258. [Google Scholar] [PubMed]
- Yoshida, T.; Galvez, S.; Tiwari, S.; Rezk, B.M.; Semprun-Prieto, L.; Higashi, Y.; Sukhanov, S.; Yablonka-Reuveni, Z.; Delafontaine, P. Angiotensin II inhibits satellite cell proliferation and prevents skeletal muscle regeneration. J. Biol. Chem. 2013, 288, 23823–23832. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.P.; Baker, J.; Bellamy, L.M.; McKay, B.R.; de Lisio, M.; Parise, G. Regulation of muscle satellite cell activation and chemotaxis by angiotensin II. PLoS ONE 2010, 5, e15212. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.P.; Bellamy, L.M.; Lisio, M.D.; Parise, G. Captopril treatment induces hyperplasia but inhibits myonuclear accretion following severe myotrauma in murine skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Huq, T.S.; Delafontaine, P. Angiotensin type 2 receptor signaling in satellite cells potentiates skeletal muscle regeneration. J. Biol. Chem. 2014, 289, 26239–26248. [Google Scholar] [CrossRef]
- Messerli, F.H.; Bangalore, S.; Bavishi, C.; Rimoldi, S.F. Angiotensin-Converting Enzyme Inhibitors in Hypertension: To Use or Not to Use? J. Am. Coll Cardiol. 2018, 71, 1474–1482. [Google Scholar] [CrossRef]
- Arumugam, S.; Sreedhar, R.; Thandavarayan, R.A.; Karuppagounder, V.; Krishnamurthy, P.; Suzuki, K.; Nakamura, M.; Watanabe, K. Angiotensin receptor blockers: Focus on cardiac and renal injury. Trends Cardiovasc Med. 2016, 26, 221–228. [Google Scholar] [CrossRef]
- Burks, T.N.; Andres-Mateos, E.; Marx, R.; Mejias, R.; van Erp, C.; Simmers, J.L.; Walston, J.D.; Ward, C.W.; Cohn, R.D. Losartan restores skeletal muscle remodeling and protects against disuse atrophy in sarcopenia. Sci. Transl. Med. 2011, 3, 82ra37. [Google Scholar] [CrossRef]
- Lin, Y.L.; Chen, S.Y.; Lai, Y.H.; Wang, C.H.; Kuo, C.H.; Liou, H.H.; Hsu, B.G. Angiotensin II receptor blockade is associated with preserved muscle strength in chronic hemodialysis patients. BMC Nephrol. 2019, 20, 54. [Google Scholar] [CrossRef]
- Collamati, A.; Marzetti, E.; Calvani, R.; Tosato, M.; D’Angelo, E.; Sisto, A.N.; Landi, F. Sarcopenia in heart failure: Mechanisms and therapeutic strategies. J. Geriatr. Cardiol. 2016, 13, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Mitch, W.E. Mechanisms of muscle wasting in chronic kidney disease. Nat. Rev. Nephrol. 2014, 10, 504–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostka, J.; Sikora, J.; Kostka, T. Relationship of quadriceps muscle power and optimal shortening velocity with angiotensin-converting enzyme activity in older women. Clin. Interv. Aging. 2017, 12, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Sumukadas, D.; Witham, M.D.; Struthers, A.D.; McMurdo, M.E. Effect of perindopril on physical function in elderly people with functional impairment: A randomized controlled trial. CMAJ 2007, 177, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Coelho, V.A.; Probst, V.S.; Nogari, B.M.; Teixeira, D.C.; Felcar, J.M.; Santos, D.C.; Gomes, M.V.; Andraus, R.A.; Fernandes, K.B. Angiotensin-II blockage, muscle strength, and exercise capacity in physically independent older adults. J. Phys. Ther. Sci. 2016, 28, 547–552. [Google Scholar] [CrossRef] [Green Version]
- Gray, S.L.; Aragaki, A.K.; LaMonte, M.J.; Cochrane, B.B.; Kooperberg, C.; Robinson, J.G.; Woods, N.F.; LaCroix, A.Z. Statins, angiotensin-converting enzyme inhibitors, and physical performance in older women. J. Am. Geriatr. Soc. 2012, 60, 2206–2214. [Google Scholar] [CrossRef]
- Buford, T.W.; Manini, T.M.; Hsu, F.C.; Cesari, M.; Anton, S.D.; Nayfield, S.; Stafford, R.S.; Church, T.S.; Pahor, M.; Carter, C.S. Angiotensin-converting enzyme inhibitor use by older adults is associated with greater functional responses to exercise. J. Am. Geriatr. Soc. 2012, 60, 1244–1252. [Google Scholar] [CrossRef]
- Vaghy, P.L.; Russell, J.S.; Lantry, L.E.; Stephens, R.E.; Ward, P.E. Angiotensin and bradykinin metabolism by peptidases identified in cultured human skeletal muscle myocytes and fibroblasts. Peptides 1995, 16, 1367–1373. [Google Scholar] [CrossRef]
- Qi, J.S.; Minor, L.K.; Smith, C.; Hu, B.; Yang, J.; Andrade-Gordon, P.; Damiano, B. Characterization of functional urotensin II receptors in human skeletal muscle myoblasts: Comparison with angiotensin II receptors. Peptides 2005, 26, 683–690. [Google Scholar] [CrossRef]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef]
- Saliba, Y.; Karam, R.; Smayra, V.; Aftimos, G.; Abramowitz, J.; Birnbaumer, L.; Farès, N. Evidence of a Role for Fibroblast Transient Receptor Potential Canonical 3 Ca2+ Channel in Renal Fibrosis. J. Am. Soc. Nephrol. 2015, 26, 1855–1876. [Google Scholar] [CrossRef] [PubMed]
- Stiber, J.A.; Tang, Y.; Li, T.; Rosenberg, P.B. Cytoskeletal regulation of TRPC channels in the cardiorenal system. Curr. Hypertens. Rep. 2012, 14, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Camacho Londono, J.E.; Tian, Q.; Hammer, K.; Schröder, L.; Camacho Lodoño, J.; Reil, J.C.; He, T.; Oberhofer, M.; Mannebach, S.; Mathar, I.; et al. A background Ca2+ entry pathway mediated by TRPC1/TRPC4 is critical for development of pathological cardiac remodelling. Eur. Heart J. 2015, 36, 2257–2266. [Google Scholar] [CrossRef]
- Seo, K.; Rainer, P.P.; Shalkey Hahn, V.; Lee, D.I.; Jo, S.H.; Andersen, A.; Liu, T.; Xu, X.; Willette, R.N.; Lepore, J.J.; et al. Combined TRPC3 and TRPC6 blockade by selective small-molecule or genetic deletion inhibits pathological cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 2014, 111, 1551–1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makarewich, C.A.; Zhang, H.; Davis, J.; Correll, R.N.; Trappanese, D.M.; Hoffman, N.E.; Troupes, C.D.; Berretta, R.M.; Kubo, H.; Madesh, M.; et al. Transient receptor potential channels contribute to pathological structural and functional remodeling after myocardial infarction. Circ. Res. 2014, 115, 567–580. [Google Scholar] [CrossRef]
- Ilatovskaya, D.V.; Blass, G.; Palygin, O.; Levchenko, V.; Pavlov, T.S.; Grzybowski, M.N.; Winsor, K.; Shuyskiy, L.S.; Geurts, A.M.; Cowley, A.W., Jr.; et al. A NOX4/TRPC6 Pathway in Podocyte Calcium Regulation and Renal Damage in Diabetic Kidney Disease. J. Am. Soc. Nephrol. 2018, 29, 1917–1927. [Google Scholar] [CrossRef] [Green Version]
- Staruschenko, A.; Spires, D.; Palygin, O. Role of TRPC6 in Progression of Diabetic Kidney Disease. Curr. Hypertens. Rep. 2019, 21, 48. [Google Scholar] [CrossRef] [PubMed]
- Rubaiy, H.N. Treasure troves of pharmacological tools to study transient receptor potential canonical 1/4/5 channels. Br. J. Pharmacol. 2019, 176, 832–846. [Google Scholar] [CrossRef]
- Tu, M.K.; Levin, J.B.; Hamilton, A.M.; Borodinsky, L.N. Calcium signaling in skeletal muscle development, maintenance and regeneration. Cell Calcium 2016, 59, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Michelucci, A.; García-Castañeda, M.; Boncompagni, S.; Dirksen, R.T. Role of STIM1/ORAI1-mediated store-operated Ca2+ entry in skeletal muscle physiology and disease. Cell Calcium 2018, 76, 101–115. [Google Scholar] [CrossRef]
- Kuang, S.; Rudnicki, M.A. The emerging biology of satellite cells and their therapeutic potential. Trends Mol. Med. 2008, 14, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Delfini, M.; Hirsinger, E.; Pourquié, O.; Duprez, D. Delta 1-activated notch inhibits muscle differentiation without affecting Myf5 and Pax3 expression in chick limb myogenesis. Development 2000, 127, 5213–5224. [Google Scholar] [PubMed]
- Smith, L.R.; Barton, E.R. Regulation of fibrosis in muscular dystrophy. Matrix Biol. 2018, 68, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Mann, C.J.; Perdiguero, E.; Kharraz, Y.; Aguilar, S.; Pessina, P.; Serrano, A.L.; Muñoz-Cánoves, P. Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle 2011, 1, 21. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Price, R.L.; Nakayama, M.; Ito, K.; Schuldt, A.J.; Manning, W.J.; Sanbe, A.; Borg, T.K.; Robbins, J.; Lorell, B.H. Ventricular-specific expression of angiotensin II type 2 receptors causes dilated cardiomyopathy and heart failure in transgenic mice. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2179–H2187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Sun, Y.; Carretero, O.A.; Zhu, L.; Harding, P.; Shesely, E.G.; Dai, X.; Rhaleb, N.E.; Peterson, E.; Yang, X.P. Effects of cardiac overexpression of the angiotensin II type 2 receptor on remodeling and dysfunction in mice post-myocardial infarction. Hypertension 2014, 63, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Li, H.; Shenoy, V.; Li, Q.; Wong, F.; Zhang, L.; Raizada, M.K.; Sumners, C.; Katovich, M.J. Moderate cardiac-selective overexpression of angiotensin II type 2 receptor protects cardiac functions from ischaemic injury. Exp. Physiol. 2012, 97, 89–101. [Google Scholar] [CrossRef]
- Teixeira, L.B.; Parreiras-E-Silva, L.T.; Bruder-Nascimento, T.; Duarte, D.A.; Simões, S.C.; Costa, R.M.; Rodriguez, D.Y.; Ferreira, P.A.B.; Silva, C.A.A.; Abrao, E.P.; et al. Ang-(1-7) is an endogenous β-arrestin-biased agonist of the AT1 receptor with protective action in cardiac hypertrophy. Sci. Rep. 2017, 7, 11903. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, J.; Yue, Z.; Liu, S.; Ma, Y.; Yu, N.; Gao, Y.; Sun, S.; Chen, S.; Liu, P. Store-Operated Ca2+ Entry (SOCE) contributes to angiotensin II-induced cardiac fibrosis in cardiac fibroblasts. J. Pharmacol. Sci. 2016, 132, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.D.; Morgan, J.P.; Yan, X. Genetically modified mouse models used for studying the role of the AT2 receptor in cardiac hypertrophy and heart failure. J. Biomed. Biotechnol. 2011, 2011, 141039. [Google Scholar] [CrossRef]
- Feng, S. TRPC Channel Structure and Properties. Adv. Exp. Med. Biol. 2017, 976, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Ilatovskaya, D.V.; Palygin, O.; Levchenko, V.; Endres, B.T.; Staruschenko, A. The Role of Angiotensin II in Glomerular Volume Dynamics and Podocyte Calcium Handling. Sci. Rep. 2017, 7, 299. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Cherednichenko, G.; Pessah, I.N.; Allen, P.D. Functional coupling between TRPC3 and RyR1 regulates the expressions of key triadic proteins. J. Biol. Chem. 2006, 281, 10042–10048. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.G.; Cabrera, D.; Céspedes, C.; Vio, C.P.; Vazquez, Y.; Brandan, E.; Cabello-Verrugio, C. Inhibition of the angiotensin-converting enzyme decreases skeletal muscle fibrosis in dystrophic mice by a diminution in the expression and activity of connective tissue growth factor (CTGF/CCN-2). Cell Tissue Res. 2013, 353, 173–187. [Google Scholar] [CrossRef] [PubMed]









| Antibodies | Dilution | Supplier |
|---|---|---|
| Anti-ACE1 | 1:50 (IF) | Santa Cruz Biotechnology, Heidelberg, Germany |
| Anti-ACE2 | 1:50 (IF) | Santa Cruz Biotechnology, Heidelberg, Germany |
| Anti-ATR1 | 1:100 (IF) 1:500 (WB) | Alomon Labs, Jerusalem, Israel |
| Anti-ATR2 | 1:200 (WB) | Alomon Labs, Jerusalem, Israel |
| Anti-GAPDH | 1:7000 (WB) | Merk-Millipore, Darmstadt, Germany |
| Anti-Myf5 | 1:100 (IF) | Santa Cruz Biotechnology, Heidelberg, Germany |
| Anti-MyoD | 1: 100 (IF) | Santa Cruz Biotechnology, Heidelberg, Germany |
| Anti-myogenin | 1:100 (IF) | Developmental Studies Hybridoma Bank, Iowa City, Iowa, United States of America |
| Anti-myostatin | 1:300 (WB) | Biorbyt Explore, Cambridge, United Kingdom |
| Anti-myosin heavy chain | 1:100 (IF) | BD Pharmingen, San Jose, California, United States of America |
| Anti-Pax7 | 1:100 (IF) | Developmental Studies Hybridoma Bank, Iowa City, Iowa, United States of America |
| Anti-p-AKT | 1:1000 (WB) | Cell Signaling Technology, Leiden, The Netherlands |
| Anti-p-ERK1/2 (Thr202/Tyr204) | 1:1000 (WB) | Cell Signaling Technology, Leiden, The Netherlands |
| Anti-p-mTOR | 1:1000 (WB) | Cell Signaling Technology, Leiden, The Netherlands |
| Anti-p-P38 | 1: 400 (WB) | Cell Signaling Technology, Leiden, The Netherlands |
| Anti-TRPC1 | 1:100 (IF) 1:200 (WB) | Alomon Labs, Jerusalem, Israel |
| Anti-TRPC3 | 1:100 (IF) 1:200 (WB) | Alomon Labs, Jerusalem, Israel |
| Anti-TRPC4 | 1:100 (IF) 1:200 (WB) | Alomon Labs, Jerusalem, Israel |
| Anti-TRPC5 | 1:100 (IF) 1:200 (WB) | Alomon Labs, Jerusalem, Israel |
| Anti-TRPC7 | 1:100 (IF) 1:200(WB) | Alomon Labs, Jerusalem, Israel |
| Anti-Mas receptor | 1:100 (IF) | Santa Cruz Biotech, Heidelberg, Germany |
| Anti-smooth muscle actin | 1:100 (IF) | Dako, Santa Clara, California, United States of America |
| Anti-catenin | 1:1000 (IF) | BD Pharmingen, San Jose, California, United States of America |
| Anti-transgelin 2 | 1:100 (IF) | Everest Biotech, Upper Heyford, United Kingdom |
| Anti-vimentin | 1:100 (IF) | Santa Cruz Biotech, Heidelberg, Germany |
| Anti-rabbit IgG-Alexa Fluor 594 | 1:500 (IF) | Thermo Fisher scientific, Bengaluru, India |
| Anti-chicken IgG-Alexa Fluor 647 | 1:500 (IF) | Thermo Fisher scientific, Bengaluru, India |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurino, A.; Spinelli, V.; Gencarelli, M.; Balducci, V.; Dini, L.; Diolaiuti, L.; Ghionzoli, M.; Messineo, A.; Mugelli, A.; Cerbai, E.; et al. Angiotensin-II Drives Human Satellite Cells Toward Hypertrophy and Myofibroblast Trans-Differentiation by Two Independent Pathways. Int. J. Mol. Sci. 2019, 20, 4912. https://doi.org/10.3390/ijms20194912
Laurino A, Spinelli V, Gencarelli M, Balducci V, Dini L, Diolaiuti L, Ghionzoli M, Messineo A, Mugelli A, Cerbai E, et al. Angiotensin-II Drives Human Satellite Cells Toward Hypertrophy and Myofibroblast Trans-Differentiation by Two Independent Pathways. International Journal of Molecular Sciences. 2019; 20(19):4912. https://doi.org/10.3390/ijms20194912
Chicago/Turabian StyleLaurino, Annunziatina, Valentina Spinelli, Manuela Gencarelli, Valentina Balducci, Leonardo Dini, Lorenzo Diolaiuti, Marco Ghionzoli, Antonio Messineo, Alessandro Mugelli, Elisabetta Cerbai, and et al. 2019. "Angiotensin-II Drives Human Satellite Cells Toward Hypertrophy and Myofibroblast Trans-Differentiation by Two Independent Pathways" International Journal of Molecular Sciences 20, no. 19: 4912. https://doi.org/10.3390/ijms20194912
APA StyleLaurino, A., Spinelli, V., Gencarelli, M., Balducci, V., Dini, L., Diolaiuti, L., Ghionzoli, M., Messineo, A., Mugelli, A., Cerbai, E., Raimondi, L., & Sartiani, L. (2019). Angiotensin-II Drives Human Satellite Cells Toward Hypertrophy and Myofibroblast Trans-Differentiation by Two Independent Pathways. International Journal of Molecular Sciences, 20(19), 4912. https://doi.org/10.3390/ijms20194912