Encapsulation of Micro- and Milli-Sized Particles with a Hollow-Type Spherical Bacterial Cellulose Gel via Particle-Preloaded Droplet Cultivation
Abstract
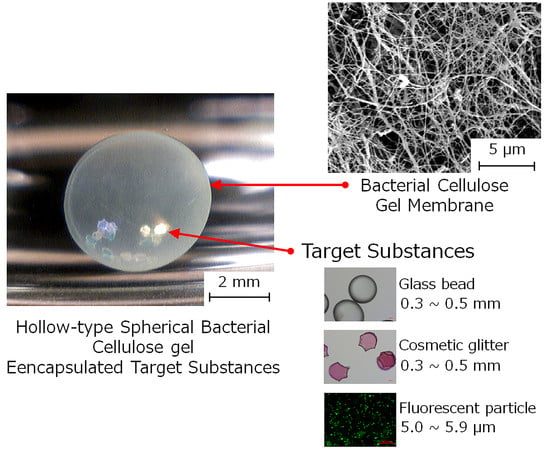
:1. Introduction
2. Results
2.1. Preparation of the HSBC Gel Encapsulating Large Particles Using Method A
2.2. Optimization of the Dissolving Process of the Ca-Alg Gel and Silicone Oil
2.3. Preparation of the HSBC Gel Encapsulating Millimeter-Size Particles with a Controlled Shape Using Method A
2.4. Preparation of the HSBC Gel Encapsulating Fluorescent Particles Using Method B
3. Discussions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Spherical Alginate Gel Including Millimeter-Sized Particles
4.3. Preparation of the Alginate Gel Including Fluorescent Particles
4.4. Preparation of the Hollow-Type Spherical BC Gels Encapsulated Target Substances
4.5. Preparation of HSBC Aerogel Using Supercritical CO2
4.6. Characterization of HSBC Gels
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Brown, A.J. XLIII.—On an Acetic Ferment which Forms Cellulose. J. Chem. Soc. 1886, 49, 432–439. [Google Scholar] [CrossRef]
- Nishi, Y.; Uryu, M.; Yamanaka, S.; Watanabe, K.; Kitamura, N.; Iguchi, M.; Mitsuhashi, S. The Structure and Mechanical Properties of Sheets Prepared from Bacterial Cellulose. J. Mater. Sci. 1990, 25, 2997–3001. [Google Scholar] [CrossRef]
- Okiyama, A.; Motoki, M.; Yamanaka, S. Bacterial Cellulose II. Processing of the Gelatinous Cellulose for Food Materials. Food Hydrocoll. 1992, 6, 479–487. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and Surface Engineering of Iron Oxide Nanoparticles for Biomedical Applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Eto, Y.; Takano, S.; Nakamori, S.; Shibai, H.; Yamanaka, S. A New Bacterial Cellulose Substrate for Mammalian Cell Culture. Cytotechnology 1993, 13, 107–114. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Stoica-Guzun, A.; Stroescu, M.; Tache, F.; Zaharescu, T.; Grosu, E. Effect of electron beam irradiation on bacterial cellulose membranes used as transdermal drug delivery systems. Nucl. Instr. Methods Phys. Res. B 2007, 265, 434–438. [Google Scholar] [CrossRef]
- Weyella, P.; Beekmanna, U.; Küpperb, C.; Dederichsb, M.; Thamma, J.; Fischera, D.; Kralischa, D. Tailor-made material characteristics of bacterial cellulose for drug delivery applications in dentistry. Carbohydr. Polym. 2019, 207, 1–10. [Google Scholar] [CrossRef]
- Wanga, B.; Lv, X.; Chen, S.; Lia, Z.; Yao, J.; Peng, X.; Feng, C.; Xu, C.; Wang, H. Use of heparinized bacterial cellulose based scaffold for improving angiogenesis in tissue regeneration. Carbohydr. Polym. 2018, 181, 948–956. [Google Scholar] [CrossRef]
- Esguerra, M.; Fink, H.; Laschke, M.W.; Jeppsson, A.; Delbro, D.; Gatenholm, P.; Menger, M.D.; Risberg, B. Intravital fluorescent microscopic evaluation of bacterial cellulose as scaffold for vascular grafts. J. Biomed. Mater. Res. Part A 2010, 93, 140–149. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, K.; Feng, J.; Liu, J.; Huang, R.; Chen, Z.; Yang, J.; Dai, Z.; Chen, Y.; Wang, N.; et al. Construction of Small-Diameter Vascular Graft by Shape-Memory and Self-Rolling Bacterial Cellulose Membrane. Adv. Healthc. Mater. 2017, 6, 1601343. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Catchmark, J.M.; Vogler, E.A. Factors Impacting the Formation of Sphere-Like Bacterial Cellulose Particles and Their Biocompatibility for Human Osteoblast Growth. Biomacromolecules 2013, 14, 3444–3452. [Google Scholar] [CrossRef] [PubMed]
- Czaja, W.; Romanovicz, D.; Brown, R.M., Jr. Structural Investigations of Microbial Cellulose Produced in Stationary and Agitated Culture. Cellulose 2004, 11, 403–411. [Google Scholar] [CrossRef]
- Putra, A.; Kakugo, A.; Furukawa, H.; Gong, J.P.; Osada, Y. Tubular Bacterial Cellulose Gel with Oriented Fibrils on the Curved Surface. Polymer 2008, 49, 1885–1891. [Google Scholar] [CrossRef]
- Nimeskern, L.; Ávila, H.M.; Sundberg, J.; Gatenholm, P.; Müller, R.; Stok, K.S. Mechanical Evaluation of Bacterial Nanocellulose as an Implant Material for Ear Cartilage Replacement. J. Mech. Behav. Biomed. Mater. 2013, 22, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, T.; Yamazaki, K.; Sato, Y.; Shida, T.; Aoyagi, T. Production of hollow-type spherical bacterial cellulose as a controlled release device by newly designed floating cultivation. Heliyon 2018, 4, e00873. [Google Scholar] [CrossRef] [Green Version]
- Lim, F.S.; Sun, A.M. Microencapsulated islets as bioartificial endocrine pancreas. Science 1980, 210, 908–910. [Google Scholar] [CrossRef]
- O’Shea, G.M.; Goosen, M.F.; Sun, A.M. Prolonged survival of transplanted islets of Langerhans encapsulated in a biocompatible membrane. Biochim. Biophys. Acta 1984, 804, 133–136. [Google Scholar] [CrossRef]
- Klöck, G.; Frank, H.; Houben, R.; Zekorn, T.; Horcher, A.; Siebers, U.; Whörle, M.; Federlin, K.; Zimmermann, U. Production of purified alginates suitable for use in immunoisolated transplantation. Appl. Microbiol. Biotechnol. 1994, 40, 638–643. [Google Scholar] [CrossRef]
- Otterlei, M.; Ostgaard, K.; Skjåk-Braek, G.; Smidsrød, O.; Soon-Shiong, P.; Espevik, T. Induction of cytokine production from human monocytes stimulated with alginate. J. Immunother. 1991, 10, 286–291. [Google Scholar] [CrossRef]
- Vaithilingam, V.; Bal, S.; Touch, B.E. Encapsulated Islet Transplantation: Where Do We Stand? Rev. Diabet. Stud. 2017, 14, 51–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Lacik, I.; Brissová, M.; Anilkumar, A.V.; Prokop, A.; Hunkeler, D.; Green, R.; Shahrokhi, K.; Powers, A.C. An encapsulation system for the immunoisolation of pancreatic islets. Nat. Biotechnol. 1997, 15, 358–362. [Google Scholar] [CrossRef] [PubMed]
- De Vos, P.; Lazarjani, H.A.; Poncelet, D.; Faas, M.M. Polymers in cell encapsulation from an enveloped cell perspective. Adv. Drug Deliv. Rev. 2014, 67–68, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Nyitray, C.E.; Chang, R.; Faleo, G.; Lance, K.D.; Bernards, D.A.; Tang, Q.; Desai, T.A. Polycaprolactone thin-film micro and nanoporous cell-encapsulation devices. ACS Nano 2015, 9, 5675–5682. [Google Scholar] [CrossRef] [PubMed]
- Erickson, H.P. Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol. Proced. Online 2009, 11, 32–51. [Google Scholar] [CrossRef]
- Jachimska, B.; Wasilewska, M.; Adamczyk, Z. Characterization of Globular Protein Solutions by Dynamic Light Scattering, Electrophoretic Mobility, and Viscosity Measurements. Langmuir 2008, 24, 6866–6872. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, J.G.; Huertas, M.L.; Carrasco, B. Calculation of Hydrodynamic Properties of Globular Proteins from Their Atomic-Level Structure. Biophys. J. 2000, 78, 719–730. [Google Scholar] [CrossRef] [Green Version]
- Supplier’s Data. Available online: https://www.sigmaaldrich.com/technical-documents/protocols/biology/fluorescein-isothiocyanate-dextran.html (accessed on 20 August 2019).
- Desai, T.A.; West, T.; Cohen, M.; Boiarski, T.; Rampersaud, A. Nanoporous microsystems for islet cell replacement. Adv. Drug Deliv. Rev. 2004, 56, 1661–1673. [Google Scholar] [CrossRef]
- Kikuchi, A.; Kawabuchi, M.; Sugihara, M.; Sakurai, Y.; Okano, T. Pulsed dextrane release from calcium-alginate gel beads. J. Control. Release 1997, 447, 21–29. [Google Scholar] [CrossRef]
- Backdahl, H.; Helenius, G.; Bodin, A.; Nannmark, U.; Johansson, B.R.; Risberg, B.; Gatenholm, P. Mechanical properties of bacterial cellulose and interactions with smooth muscle cells. Biomaterials 2006, 27, 2141–2149. [Google Scholar] [CrossRef]
- Andersson, J.; Stenhamre, H.; Bäckdahl, H.; Gatenholm, P. Behavior of human articular chondrocytes on microporous bacterial cellulose scaffolds. J. Biomed. Mater. Res. A 2010, 94, 1124–1132. [Google Scholar] [PubMed]
- Bäckdahl, H.; Esguerra, M.; Delbro, D.; Risberg, B.; Gatenholm, P. Engineering microporosity in bacterial cellulose scaffolds. J. Tissue Eng. Regen. Med. 2006, 2, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Bodin, A.; Bharadwaj, S.; Wu, S.; Gatenholm, P.; Atala, A.; Zhang, Y. Tissue-engineered conduit using urine-derived stem cells seeded bacterial cellulose polymer in urinary reconstruction and diversion. Biomaterials 2010, 31, 8889–8901. [Google Scholar] [CrossRef] [PubMed]
- Svensson, A.; Nicklasson, E.; Harrah, T.; Panilaitis, B.; Kaplan, D.L.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 2005, 26, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Helenius, G.; Bäckdahl, H.; Bodin, A.; Nannmark, U.; Gatenholm, P.; Risberg, B. In vivo biocompatibility of bacterial cellulose. J. Biomed. Mater. Res. A 2006, 76, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Desai, T.; Shea, L.D. Advances in islet encapsulation technologies. Nat. Rev. Drug Discov. 2017, 16, 338–350. [Google Scholar] [CrossRef]
- Hestrin, S.; Schramm, M. Synthesis of cellulose by Acetobacter xylinum. 2. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem. J. 1954, 58, 345–352. [Google Scholar] [CrossRef]
- Buchtová, N.; Budtova, T. Cellulose Aero-, Cryo- and Xerogels: Towards Understanding of Morphology Control. Cellulose 2016, 23, 2585–2595. [Google Scholar] [CrossRef]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoshi, T.; Suzuki, M.; Ishikawa, M.; Endo, M.; Aoyagi, T. Encapsulation of Micro- and Milli-Sized Particles with a Hollow-Type Spherical Bacterial Cellulose Gel via Particle-Preloaded Droplet Cultivation. Int. J. Mol. Sci. 2019, 20, 4919. https://doi.org/10.3390/ijms20194919
Hoshi T, Suzuki M, Ishikawa M, Endo M, Aoyagi T. Encapsulation of Micro- and Milli-Sized Particles with a Hollow-Type Spherical Bacterial Cellulose Gel via Particle-Preloaded Droplet Cultivation. International Journal of Molecular Sciences. 2019; 20(19):4919. https://doi.org/10.3390/ijms20194919
Chicago/Turabian StyleHoshi, Toru, Masashige Suzuki, Mayu Ishikawa, Masahito Endo, and Takao Aoyagi. 2019. "Encapsulation of Micro- and Milli-Sized Particles with a Hollow-Type Spherical Bacterial Cellulose Gel via Particle-Preloaded Droplet Cultivation" International Journal of Molecular Sciences 20, no. 19: 4919. https://doi.org/10.3390/ijms20194919
APA StyleHoshi, T., Suzuki, M., Ishikawa, M., Endo, M., & Aoyagi, T. (2019). Encapsulation of Micro- and Milli-Sized Particles with a Hollow-Type Spherical Bacterial Cellulose Gel via Particle-Preloaded Droplet Cultivation. International Journal of Molecular Sciences, 20(19), 4919. https://doi.org/10.3390/ijms20194919