Protective Effects of Melatonin on the Skin: Future Perspectives
Abstract
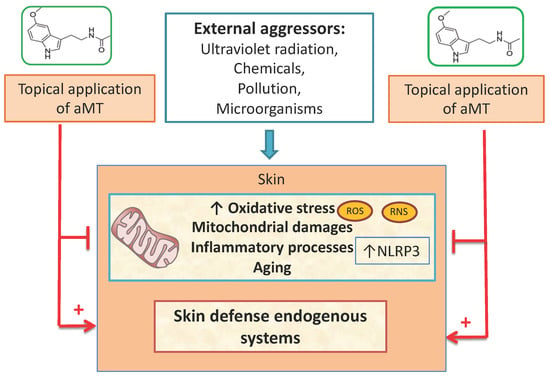
:1. Introduction
2. Melatonin as Skin Cellular Protector
3. Synthesis and Metabolism of Melatonin in the Skin
4. Mechanism of Action of Melatonin in the Skin
4.1. Melatonin Receptors in the Skin
4.2. Receptor-Independent Functions of Melatonin
4.3. Melatonin as a Potent Antioxidant
4.4. Melatonin as a Potent Mitochondrial Protector
4.5. Melatonin as a Potent Anti-Inflammatory Agent
5. Functions of Melatonin in the Skin
5.1. Melatonin as a Photoprotector
5.2. Melatonin as a Radioprotector
5.3. Melatonin as a Protector against Skin Damage
6. Topical Application of Melatonin
7. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 5MT | 5-methoxytryptamine |
| AANAT | Aralkylamine N-acetyltransferase |
| AD | Atopic dermatitis |
| AFMK | N1-acetyl-N2-formyl-5-methoxykynuramine |
| AMK | N1-acetyl-5-methoxykynuramine |
| aMT | N-acetyl-5-methoxytryptamine, melatonin |
| AP-1 | Activator protein-1 |
| ASMT | Acetylserotonin O-methyltransferase |
| CAT | Catalase |
| CRP | C-reactive porotein |
| COX-2 | Cyclooxygenase-2 |
| ETC | Electron transport chain |
| FGF-β | Fibroblast growth factor-β |
| GLI1 | Glioma-associated oncogene transcription factor |
| GPx | Glutathione peroxidase |
| GRd | Glutathione reductase |
| GST | Glutathionine S-transferase |
| HH | Hedgehog pathway |
| HO-1 | Heme oxygenase-1 |
| IFN-γ | Interferon type II (IFN-γ in humans) |
| ILs | Interleukins |
| iNOS | Inducible nitric oxide synthase |
| MAPKs | Mitogen-activated protein kinases |
| MDA | Malondialdehyde |
| MMPs | Metalloproteinases |
| MPTP | Mitochondrial permeability transition pores |
| MT1 | Membrane receptors type I |
| MT2 | Membrane receptors type II |
| MT3 | Membrane receptors type III |
| mtNOS | Mitochondrial nitric oxide synthase |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLCs | Nanostructured lipid carriers |
| NQO1 | NAD(P)H dehydrogenase [quinone] 1 |
| NQO2 | NAD(P)H dehydrogenase [quinone] 2, flavoprotein quinone oxidoreductase II |
| Nrf2 | Nuclear factor erythroid-2-related factor 2 |
| RNS | Reactive nitrogen species |
| RORα | Retinoic acid related orphan receptor alfa |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| γ-GCS | γ-glutamyl-cystein synthase |
References
- Slominski, A.T.; Hardeland, R.; Zmijewski, M.A.; Slominski, R.M.; Reiter, R.J.; Paus, R. Melatonin: A cutaneous perspective on its production, metabolism, and functions. J. Investig. Dermatol. 2018, 138, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Tobin, D.J.; Zmijewski, M.A.; Wortsman, J.; Paus, R. Melatonin in the skin: Synthesis, metabolism and functions. Trends Endocrinol. Metab. 2008, 19, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Sayed, R.K.A.; Fernández-Ortiz, M.; Diaz-Casado, M.E.; Aranda-Martínez, P.; Fernández-Martínez, J.; Guerra-Librero, A.; Escames, G.; López, L.C.; Alsaadawy, R.M.; Acuña-Castroviejo, D. Lack of NLRP3 inflammasome activation reduces age-dependentsSarcopenia and mitochondrial dysfunction, favoring the prophylactic effect of melatonin. J. Gerontol. Ser. A 2019. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Kleszczynśki, K.; Janjetovic, Z.; Sweatman, T.; Lin, Z.; Li, W.; Reiter, R.J.; Fischer, T.W.; Slominski, A.T. Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells. FASEB J. 2013, 27, 2742–2755. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Pisarchik, A.; Semak, I.; Sweatman, T.; Wortsman, J.; Szczesniewski, A.; Slugocki, G.; McNulty, J.; Kauser, S.; Tobin, D.J.; et al. Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J. 2002, 16, 896–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milani, M.; Sparavigna, A. Antiaging efficacy of melatonin-based day and night creams: A randomized, split-face, assessor-blinded proof-of-concept trial. Clin. Cosmet. Investig. Dermatol. 2018, 11, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Pugazhenthi, K.; Kapoor, M.; Clarkson, A.N.; Hall, I.; Appleton, I. Melatonin accelerates the process of wound repair in full-thickness incisional wounds. J. Pineal Res. 2008, 44, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Ren, L.; Ma, H.; Hu, R.; Gao, H.; Wang, L.; Chen, X.; Zhao, Z.; Liu, J. Melatonin promotes diabetic wound healing in vitro by regulating keratinocyte activity. Am. J. Transl. Res. 2016, 8, 4682–4693. [Google Scholar]
- Soybir, G.; Topuzlu, C.; Odabaş, Ö.; Dolay, K.; Bilir, A.; Köksoy, F. The effects of melatonin on angiogenesis and wound healing. Surg. Today 2003, 33, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.D.; García-Moreno, H.; González-Yanes, C.; Calvo, J.R. Possible involvement of the inhibition of NF-κB factor in anti-inflammatory actions that melatonin exerts on mast cells. J. Cell. Biochem. 2016, 117, 1926–1933. [Google Scholar] [CrossRef]
- Marseglia, L.; D’Angelo, G.; Barberi, I.; Manti, S.; Salpietro, C.; Arrigo, T.; Reiter, R.J.; Gitto, E. Melatonin and atopy: Role in atopic dermatitis and asthma. Int. J. Mol. Sci. 2014, 15, 13482–13493. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, L.; Cuppari, C.; Manti, S.; D’Angelo, G.; Salpietro, C.; Reiter, R.J.; Gitto, E. Atopic dermatitis: Melatonin as potential treatment. J. Biol. Regul. Homeost. Agents 2015, 29, 142–149. [Google Scholar] [PubMed]
- Park, G.; Lee, S.H.; Oh, D.S.; Kim, Y.U. Melatonin inhibits neuronal dysfunction-associated with neuroinflammation by atopic psychological stress in NC/Nga atopic-like mouse models. J. Pineal Res. 2017, 63. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.; Trüeb, R.; Hänggi, G.; Innocenti, M.; Elsner, P. Topical melatonin for treatment of androgenetic alopecia. Int. J. Trichology 2012, 4, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Tsiskarishvili, N.I.; Katsitadze, A.; Tsiskarishvili, N.V.; Tsiskarishvil, T.; Chitanava, L. Melatonin concentration in the blood of vitiligo patients with stress in anamnesis. Georgian Med. News 2016, 254, 47–53. [Google Scholar]
- Romić, M.D.; Klarić, M.Š.; Lovrić, J.; Pepić, I.; Cetina-Čižmek, B.; Filipović-Grčić, J.; Hafner, A. Melatonin-loaded chitosan/Pluronic® F127 microspheres as in situ forming hydrogel: An innovative antimicrobial wound dressing. Eur. J. Pharm. Biopharm. 2016, 107, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, S.J.; Slominski, A.; Etminan, M.; Pruski, D.; Paus, R.; Namboodiri, M.A.A. Identification and characterization of two isozymic forms of arylamine n-acetyltransferase in syrian hamster skin. J. Investig. Dermatol. 1993, 101, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Bubenik, G.A.; Smith, P.S.; Schams, D. The Effect of Orally Administered Melatonin on the Seasonality of Deer Pelage Exchange, Antler Development, LH, FSH, Prolactin, Testosterone, T3, T4, Cortisol, and Alkaline Phosphatase. J. Pineal Res. 1986, 3, 331–349. [Google Scholar] [CrossRef]
- Lerchl, A.; Schlatt, S. Influence of photoperiod on pineal melatonin synthesis, fur color, body weight, and reproductive function in the female Djungarian hamster, Phodopus sungorus. Neuroendocrinology 1993, 57, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Lin, Z.; Tidwell, W.J.; Li, W.; Slominski, A.T. Melatonin and its metabolites accumulate in the human epidermis in vivo and inhibit proliferation and tyrosinase activity in epidermal melanocytes in vitro. Mol. Cell. Endocrinol. 2015, 404, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Pisarchik, A.; Johansson, O.; Jing, C.; Semak, I.; Slugocki, G.; Wortsman, J. Tryptophan hydroxylase expression in human skin cells. Biochim. Biophys. Acta-Mol. Basis Dis. 2003, 1639, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Holm-Schou, A.S.S.; Philipsen, P.A.; Wulf, H.C. Skin cancer phototype: A new classification directly related to skin cancer and based on responses from 2869 individuals. Photodermatol. Photoimmunol. Photomed. 2019, 35, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Pisarchik, A.; Semak, I.; Sweatman, T.; Wortsman, J. Characterization of the serotoninergic system in the C57BL/6 mouse skin. Eur. J. Biochem. 2003, 270, 3335–3344. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Semak, I.; Kim, T.K.; Janjetovic, Z.; Slominski, R.M.; Zmijewski, J.W. Melatonin, mitochondria, and the skin. Cell. Mol. Life Sci. 2017, 74, 3913–3925. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Schulz-Douglas, V.; Bünz, A.; Beazley, W.; Körner, C. Pteridines in the control of pigmentation. J. Investig. Dermatol. 1997, 109, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.W.; Slominski, A.; Zmijewski, M.A.; Reiter, R.J.; Paus, R. Melatonin as a major skin protectant: From free radical scavenging to DNA damage repair. Exp. Dermatol. 2008, 17, 713–730. [Google Scholar] [CrossRef]
- Venegas, C.; García, J.A.; Doerrier, C.; Volt, H.; Escames, G.; Lõpez, L.C.; Reiter, R.J.; Acuña-Castroviejo, D. Analysis of the daily changes of melatonin receptors in the rat liver. J. Pineal Res. 2013, 54, 313–321. [Google Scholar] [CrossRef]
- Dubocovich, M.L.; Markowska, M. Functional MT 1 and MT 2 melatonin receptors in mammals. Endocrine 2005, 27, 101–110. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Galano, A. Melatonin: Exceeding expectations. Physiology (Bethesda) 2014, 29, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Pisarchik, A.; Zbytek, B.; Tobin, D.J.; Kauser, S.; Wortsman, J. Functional activity of serotoninergic and melatoninergic systems expressed in the skin. J. Cell. Physiol. 2003, 186, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Fischer, T.W.; Zmijewski, M.A.; Wortsman, J.; Semak, I.; Zbytek, B.; Slominski, R.M.; Tobin, D.J. On the role of melatonin in skin physiology and pathology. Endocrine 2005, 27, 137–147. [Google Scholar] [CrossRef]
- Fischer, T.W.; Slominski, A.; Tobin, D.J.; Paus, R. Melatonin and the hair follicle. J. Pineal Res. 2008, 44, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Semak, I.; Fischer, T.W.; Kim, T.K.; Kleszczyński, K.; Hardeland, R.; Reiter, R.J. Metabolism of melatonin in the skin: Why is it important? Exp. Dermatol. 2017, 26, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Janjetovic, Z.; Jarrett, S.G.; Lee, E.F.; Duprey, C.; Reiter, R.J.; Slominski, A.T. Melatonin and its metabolites protect human melanocytes against UVB-induced damage: Involvement of NRF2-mediated pathways. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Nosjean, O.; Ferro, M.; Cogé, F.; Beauverger, P.; Henlin, J.M.; Lefoulon, F.; Fauche, J.L.; Delagrange, P.; Canet, E.; Boutin, J.A. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J. Biol. Chem. 2000, 275, 31311–31317. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kromminga, A.; Dunlop, T.W.; Tychsen, B.; Conrad, F.; Suzuki, N.; Memezawa, A.; Bettermann, A.; Aiba, S.; Carlberg, C.; et al. A role of melatonin in neuroectodermal-mesodermal interactions: The hair follicle synthesizes melatonin and expresses functional melatonin receptors. FASEB J. 2005, 19, 1710–1712. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kleszczyński, K.; Semak, I.; Janjetovic, Z.; Żmijewski, M.A.; Kim, T.K.; Slominski, R.M.; Reiter, R.J.; Fischer, T.W. Local melatoninergic system as the protector of skin integrity. Int. J. Mol. Sci. 2014, 15, 17705–17732. [Google Scholar] [CrossRef]
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.S.; Slominski, A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell. Endocrinol. 2012, 351, 152–166. [Google Scholar] [CrossRef] [Green Version]
- Skobowiat, C.; Brożyna, A.A.; Janjetovic, Z.; Jeayeng, S.; Oak, A.S.W.; Kim, T.K.; Panich, U.; Reiter, R.J.; Slominski, A.T. Melatonin and its derivatives counteract the ultraviolet B radiation-induced damage in human and porcine skin ex vivo. J. Pineal Res. 2018, 65, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Choo, M.K.; Park, J.M.; Fisher, D.E. Topical ROR inverse agonists suppress inflammation in mouse models of atopic dermatitis and acute irritant dermatitis. J. Investig. Dermatol. 2017, 137, 2523–2531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Reiter, R.J.; Manchester, L.C.; Yan, M.; El-Sawi, M.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Allegra, M.; Hardeland, R. Chemical and physical properties and potential mechanisms: Melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2002, 2, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Tan, D.X.; Reiter, R.J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.W.; Zmijewski, M.A.; Zbytek, B.; Sweatman, T.W.; Slominski, R.M.; Wortsman, J.; Slominski, A. Oncostatic effects of the indole melatonin and expression of its cytosolic and nuclear receptors in cultured human melanoma cell lines. Int. J. Oncol. 2006, 29, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Magesh, S.; Chen, Y.; Hu, L. Small Molecule Modulators of Keap1-Nrf2-ARE Pathway as Potential Preventive and Therapeutic Agents. Med. Res. Rev. 2012, 32, 687–726. [Google Scholar] [CrossRef] [Green Version]
- García, J.A.; Ortiz, F.; Miana, J.; Doerrier, C.; Fernández-Ortiz, M.; Rusanova, I.; Escames, G.; García, J.J.; Acuña-Castroviejo, D. Contribution of inducible and neuronal nitric oxide synthases to mitochondrial damage and melatonin rescue in LPS-treated mice. J. Physiol. Biochem. 2017, 73, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Sayed, R.K.A.; Fernández-Ortiz, M.; Diaz-Casado, M.E.; Rusanova, I.; Rahim, I.; Escames, G.; López, L.C.; Mokhtar, D.M.; Acuña-Castroviejo, D. The protective effect of melatonin against age-associated, sarcopenia-dependent tubular aggregate formation, lactate depletion, and mitochondrial changes. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 1330–1338. [Google Scholar] [CrossRef]
- Venegas, C.; García, J.A.; Escames, G.; Ortiz, F.; López, A.; Doerrier, C.; García-Corzo, L.; López, L.C.; Reiter, R.J.; Acuña-Castroviejo, D. Extrapineal melatonin: Analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 2012, 52, 217–227. [Google Scholar] [CrossRef]
- Chen, H.H.; Chen, Y.T.; Yang, C.C.; Chen, K.H.; Sung, P.H.; Chiang, H.J.; Chen, C.H.; Chua, S.; Chung, S.Y.; Chen, Y.L.; et al. Melatonin pretreatment enhances the therapeutic effects of exogenous mitochondria against hepatic ischemia–reperfusion injury in rats through suppression of mitochondrial permeability transition. J. Pineal Res. 2016, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Doerrier, C.; García, J.A.; Volt, H.; Díaz-Casado, M.E.; Lima-Cabello, E.; Ortiz, F.; Luna-Sánchez, M.; Escames, G.; López, L.C.; Acuña-Castroviejo, D. Identification of mitochondrial deficits and melatonin targets in liver of septic mice by high-resolution respirometry. Life Sci. 2015, 121, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Volt, H.; García, J.A.; Doerrier, C.; Díaz-Casado, M.E.; Guerra-Librero, A.; Lõpez, L.C.; Escames, G.; Tresguerres, J.A.; Acuña-Castroviejo, D. Same molecule but different expression: Aging and sepsis trigger NLRP3 inflammasome activation, a target of melatonin. J. Pineal Res. 2016, 60, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Casado, M.E.; Rusanova, I.; Aranda, P.; Fernández-Ortiz, M.; Sayed, R.K.A.; Fernández-Gil, B.I.; Hidalgo-Gutiérrez, A.; Escames, G.; López, L.C.; Acuña-Castroviejo, D. In Vivo Determination of Mitochondrial Respiration in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Treated Zebrafish Reveals the Efficacy of Melatonin in Restoring Mitochondrial Normalcy. Zebrafish 2018, 15. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Lee, H.-J.; Lee, H.; Kim, J.-H.; Hwang, J.; Koo, J.; Kim, S.-H. The anti-wrinkle mechanism of melatonin in UVB treated HaCaT keratinocytes and hairless mice via inhibition of ROS and sonic hedgehog mediated inflammatory proteins. Int. J. Mol. Sci. 2018, 19, 1995. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, F.; Acuña-Castroviejo, D.; Doerrier, C.; Dayoub, J.C.; Lõpez, L.C.; Venegas, C.; García, J.A.; Lõpez, A.; Volt, H.; Luna-Sánchez, M.; et al. Melatonin blunts the mitochondrial/NLRP3 connection and protects against radiation-induced oral mucositis. J. Pineal Res. 2015, 58, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, F.; García, J.A.; Acuña-Castroviejo, D.; Doerrier, C.; Lõpez, A.; Venegas, C.; Volt, H.; Luna-Sánchez, M.; Lõpez, L.C.; Escames, G. The beneficial effects of melatonin against heart mitochondrial impairment during sepsis: Inhibition of i NOS and preservation of n NOS. J. Pineal Res. 2014, 56, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Castroviejo, D.; Rahim, I.; Acuña-Fernández, C.; Fernández-Ortiz, M.; Solera-Marín, J.; Sayed, R.K.A.; Díaz-Casado, M.E.; Rusanova, I.; López, L.C.; Escames, G. Melatonin, clock genes and mitochondria in sepsis. Cell. Mol. Life Sci. 2017, 74, 3965–3987. [Google Scholar] [CrossRef] [PubMed]
- Svobodova, A.; Walterova, D.; Vostalova, J. Ultraviolet light induced alteration to the skin. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech. Repub. 2006, 150, 25–38. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, G.; You, Y.; Besaratinia, A. Mutations induced by ultraviolet light. Mutat. Res. 2005, 571, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Marto, J.; Ascenso, A.; Gonçalves, L.M.; Gouveia, L.F.; Manteigas, P.; Pinto, P.; Oliveira, E.; Almeida, A.J.; Ribeiro, H.M. Melatonin-based pickering emulsion for skin’s photoprotection. Drug Deliv. 2016, 23, 1594–1607. [Google Scholar] [CrossRef] [PubMed]
- Cleaver, J.E.; Crowley, E. UV damage, DNA repair and skin carcinogenesis. Front Biosci 2002, 7, d1024–d1043. [Google Scholar] [PubMed]
- Ryoo, Y.W.; Suh, S.I.; Mun, K.C.; Kim, B.C.; Lee, K.S. The effects of the melatonin on ultraviolet-B irradiated cultured dermal fibroblasts. J. Dermatol. Sci. 2001, 27, 162–169. [Google Scholar] [CrossRef]
- Janjetovic, Z.; Nahmias, Z.P.; Hanna, S.; Jarrett, S.G.; Kim, T.K.; Reiter, R.J.; Slominski, A.T. Melatonin and its metabolites ameliorate ultraviolet B-induced damage in human epidermal keratinocytes. J. Pineal Res. 2014, 57, 90–102. [Google Scholar] [CrossRef]
- Lee, J.H.; Moon, J.H.; Nazim, U.M.; Lee, Y.J.; Seol, J.W.; Eo, S.K.; Lee, J.H.; Park, S.Y. Melatonin protects skin keratinocyte from hydrogen peroxide-mediated cell death; the SIRT1 pathway. Oncotarget 2016, 7, 12075–12088. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, D.; Avitabile, D.; Shiota, M.; Yokomizo, A.; Naito, S.; Bizzarri, M.; Torrisi, M.R. Nuclear redox imbalance affects circadian oscillation in HaCaT keratinocytes. Int. J. Biochem. Cell Biol. 2015, 65, 113–124. [Google Scholar] [CrossRef]
- Lim, H.D.; Kim, Y.S.; Ko, S.H.; Yoon, I.J.; Cho, S.G.; Chun, Y.H.; Choi, B.J.; Kim, E.C. Cytoprotective and anti-inflammatory effects of melatonin in hydrogen peroxide-stimulated CHON-001 human chondrocyte cell line and rabbit model of osteoarthritis via the SIRT1 pathway. J. Pineal Res. 2012, 53, 225–237. [Google Scholar] [CrossRef]
- Scheuer, C.; Pommergaard, H.C.; Rosenberg, J.; Gögenur, I. Dose dependent sun protective effect of topical melatonin: A randomized, placebo-controlled, double-blind study. J. Dermatol. Sci. 2016, 84, 178–185. [Google Scholar] [CrossRef]
- Fischer, T.W.; Zbytek, B.; Sayre, R.M.; Apostolov, E.O.; Basnakian, A.G.; Sweatman, T.W.; Wortsman, J.; Elsner, P.; Slominski, A. Melatonin increases survival of HaCaT keratinocytes by suppressing UV-induced apoptosis. J. Pineal Res. 2006, 40, 18–26. [Google Scholar] [CrossRef]
- Fischer, T.W.; Scholz, G.; Knöll, B.; Hipler, U.C.; Eisner, P. Melatonin suppresses reactive oxygen species induced by UV irradiation in leukocytes. J. Pineal Res. 2004, 37, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Chun Kim, B.; Sung Shon, B.; Wook Ryoo, Y.; Pyo Kim, S.; Suk Lee, K. Melatonin reduces X-ray irradiation-induced oxidative damages in cultured human skin fibroblasts. J. Dermatol. Sci. 2001, 26, 194–200. [Google Scholar] [CrossRef]
- Hussein, M.R.; Abu-Dief, E.E.; Abd El-Reheem, M.H.; Abd-Elrahman, A. Ultrastructural evaluation of the radioprotective effects of melatonin against X-ray-induced skin damage in Albino rats. Int. J. Exp. Pathol. 2005, 86, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Shabeeb, D.; Najafi, M.; Musa, A.E.; Keshavarz, M.; Shirazi, A.; Hassanzadeh, G.; Hadian, M.R.; Samandari, H. Biochemical and histopathological evaluation of the radioprotective effects of melatonin against gamma ray-induced skin damage. Curr. Radiopharm. 2019, 12, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, M.A.; Elkayam, R.; Gelernter, I.; Pfeffer, R.M. Melatonin for prevention of breast radiation dermatitis: A phase II, prospective, double-blind randomized trial. Isr. Med. Assoc. J. 2016, 18, 188–192. [Google Scholar] [PubMed]
- Fernandez-Gil, B.; Abdel Moneim, A.E.; Ortiz, F.; Shen, Y.Q.; Soto-Mercado, V.; Mendivil-Perez, M.; Guerra-Librero, A.; Acuña-Castroviejo, D.; Molina-Navarro, M.M.; Garcia-Verdugo, J.M.; et al. Melatonin protects rats from radiotherapyinduced small intestine toxicity. PLoS ONE 2017, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Adhikari, J.S.; Rizvi, M.A.; Chaudhury, N.K. Melatonin attenuates 60Co γ-ray-induced hematopoietic, immunological and gastrointestinal injuries in C57BL/6 male mice. Environ. Toxicol. 2017, 32, 501–518. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Adjaye, J.; Akamatsu, H.; Moe-Behrens, G.; Niemann, C. Human skin stem cells and the ageing process. Exp. Gerontol. 2008, 43, 986–997. [Google Scholar] [CrossRef] [Green Version]
- Imokawa, G.; Ishida, K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging I: Reduced skin elasticity, highly associated with enhanced dermal elastase activity, triggers wrinkling and sagging. Int. J. Mol. Sci. 2015, 16, 7753–7775. [Google Scholar] [CrossRef]
- Khan, N.; Syed, D.N.; Pal, H.C.; Mukhtar, H.; Afaq, F. Pomegranate fruit extract inhibits UVB-induced inflammation and proliferation by modulating NF-κB and MAPK signaling pathways in mouse skin. Photochem. Photobiol. 2012, 88, 1126–1134. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Kanimozhi, G.; Prasad, N.R.; Agilan, B.; Ganesan, M.; Mohana, S.; Srithar, G. 7-Hydroxycoumarin prevents UVB-induced activation of NF-κB and subsequent overexpression of matrix metalloproteinases and inflammatory markers in human dermal fibroblast cells. J. Photochem. Photobiol. B Biol. 2016, 161, 170–176. [Google Scholar] [CrossRef]
- Zhang, M.; Hwang, E.; Lin, P.; Gao, W.; Ngo, H.T.T.; Yi, T.-H. Prunella vulgaris L. Exerts a Protective Effect Against Extrinsic Aging Through NF-κB, MAPKs, AP-1, and TGF-β/Smad Signaling Pathways in UVB-Aged Normal Human Dermal Fibroblasts. Rejuvenation Res. 2018, 21, 313–322. [Google Scholar] [CrossRef]
- Abe, Y.; Tanaka, N. Roles of the Hedgehog Signaling Pathway in Epidermal and Hair Follicle Development, Homeostasis, and Cancer. J. Dev. Biol. 2017, 5, 12. [Google Scholar] [CrossRef]
- Brechbiel, J.; Miller-Moslin, K.; Adjei, A.A. Crosstalk between hedgehog and other signaling pathways as a basis for combination therapies in cancer. Cancer Treat. Rev. 2014, 40, 750–759. [Google Scholar] [CrossRef]
- Hall, G.; Phillips, T.J. Estrogen and skin: The effects of estrogen, menopause, and hormone replacement therapy on the skin. J. Am. Acad. Dermatol. 2005, 53, 555–568. [Google Scholar] [CrossRef]
- Ehrhardt, C.; Wessels, J.T.; Wuttke, W.; Seidlová-Wuttke, D. The effects of 20-hydroxyecdysone and 17β-estradiol on the skin of ovariectomized rats. Menopause 2011, 18, 323–327. [Google Scholar] [CrossRef]
- Uslu, S.; Oktem, G.; Uysal, A.; Soner, B.C.; Arbak, S.; Ince, U. Stem cell and extracellular matrix-related molecules increase following melatonin treatment in the skin of postmenopausal rats. Cell Biol. Int. 2014, 38, 924–932. [Google Scholar] [CrossRef]
- Masaki, H.; Ishikawa, T.; Takahashi, S.; Okumura, M.; Sakai, N.; Haga, M.; Kominami, K.; Migita, H.; McDonald, F.; Shimada, F.; et al. Heterogeneity of pluripotent marker gene expression in colonies generated in human iPS cell induction culture. Stem Cell Res. 2008, 1, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Fan, Y.; Zhou, B.; Xu, Z.; Chen, Y.; Sun, X. Generation of induced pluripotent stem cells from skin fibroblasts of a patient with olivopontocerebellar atrophy. Tohoku J. Exp. Med. 2012, 226, 151–159. [Google Scholar] [CrossRef]
- Marinowic, D.R.; Majolo, F.; Sebben, A.D.; Da Silva, V.D.; Lopes, T.G.; Paglioli, E.; Palmini, A.; MacHado, D.C.; Da Costa, J.C. Induced pluripotent stem cells from patients with focal cortical dysplasia and refractory epilepsy. Mol. Med. Rep. 2017, 15, 2049–2056. [Google Scholar] [CrossRef] [Green Version]
- Poon, R.; Nik, S.A.; Ahn, J.; Slade, L.; Alman, B.A. β-catenin and transforming growth factor β have distinct roles regulating fibroblast cell motility and the induction of collagen lattice contraction. BMC Cell Biol. 2009, 11, 1–10. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.E.; Leung, D.Y.M. Pathophysiology of atopic dermatitis: Clinical implications. Allergy Asthma Proc. 2019, 40, 84–92. [Google Scholar] [CrossRef]
- Chang, Y.S.; Chou, Y.T.; Lee, J.H.; Lee, P.L.; Dai, Y.S.; Sun, C.; Lin, Y.T.; Wang, L.C.; Yu, H.H.; Yang, Y.H.; et al. Atopic Dermatitis, Melatonin, and Sleep Disturbance. Pediatrics 2014, 17, 1–11. [Google Scholar] [CrossRef]
- Cano Barquilla, P.; Pagano, E.S.; Jiménez-Ortega, V.; Fernández-Mateos, P.; Esquifino, A.I.; Cardinali, D.P. Melatonin normalizes clinical and biochemical parameters of mild inflammation in diet-induced metabolic syndrome in rats. J. Pineal Res. 2014, 57, 280–290. [Google Scholar] [CrossRef]
- Agil, A.; Reiter, R.J.; Jiménez-Aranda, A.; Ibán-Arias, R.; Navarro-Alarcón, M.; Marchal, J.A.; Adem, A.; Fernández-Vázquez, G. Melatonin ameliorates low-grade inflammation and oxidative stress in young Zucker diabetic fatty rats. J. Pineal Res. 2013, 54, 381–388. [Google Scholar] [CrossRef]
- Kim, T.H.; Jung, J.A.; Kim, G.D.; Jang, A.H.; Ahn, H.J.; Park, Y.S.; Park, C.S. Melatonin inhibits the development of 2,4-dinitrofluorobenzene-induced atopic dermatitis-like skin lesions in NC/Nga mice. J. Pineal Res. 2009, 47, 324–329. [Google Scholar] [CrossRef]
- Taghavi Ardakani, A.; Farrehi, M.; Sharif, M.R.; Ostadmohammadi, V.; Mirhosseini, N.; Kheirkhah, D.; Moosavi, S.G.A.; Behnejad, M.; Reiter, R.J.; Asemi, Z. The effects of melatonin administration on disease severity and sleep quality in children with atopic dermatitis: A randomized, double-blinded, placebo-controlled trial. Pediatr. Allergy Immunol. 2018, 29, 834–840. [Google Scholar] [CrossRef]
- Bangha, E.; Lauth, D.; Kistler, G.S.; Elsner, P. Daytime serum levels of melatonin after topical application onto the human skin. Skin Pharmacol. Physiol. 1997, 10, 298–302. [Google Scholar] [CrossRef]
- Fischer, T.W.; Greif, C.; Fluhr, J.W.; Wigger-Alberti, W.; Elsner, P. Percutaneous penetration of topically applied melatonin in a cream and an alcoholic solution. Skin Pharmacol. Physiol. 2004, 17, 190–194. [Google Scholar] [CrossRef]
- Flo Sierra, A.; Garduño Ramírez, M.L.; Calpena Campmany, A.C.; Martínez, A.R.; Naveros, B.C. In vivo and in vitro evaluation of the use of a newly developed melatonin loaded emulsion combined with UV filters as a protective agent against skin irradiation. J. Dermatol. Sci. 2013, 69, 202–214. [Google Scholar] [CrossRef]
- Hatem, S.; Nasr, M.; Moftah, N.H.; Ragai, M.H.; Geneidi, A.S.; Elkheshen, S.A. Clinical cosmeceutical repurposing of melatonin in androgenic alopecia using nanostructured lipid carriers prepared with antioxidant oils. Expert Opin. Drug Deliv. 2018, 15, 927–935. [Google Scholar] [CrossRef]



| Type of Cell | Melatonin Dosage | Effects | Authors |
|---|---|---|---|
| Human keratinocytes (HaCaT) | Cells were preincubated with melatonin at graded concentrations from 10−9 to 10−3 M for 30 min prior to UV irradiation at doses of 25 and 50 mJ/cm2. | Pretreatment with melatonin inhibited apoptosis, increasing DNA synthesis, and number of colonies. | [71] |
| Human keratinocytes (HaCaT) and normal human epidermal keratinocytes (NHEK) | Before UVR, cells were pre-incubated for 1 h with melatonin (10-3 M) and irradiated with increasing UVR doses (0, 10, 25, 50 mJ/cm2). | At 48 h post-UVR, melatonin effectively protected cells, decreased disturbances in plasma membrane potential and changed intracellular pH, caused by irradiation (25 or 50 mJ/cm2). The presence of melatonin significantly protected the cells -12% (HaCaT) and 14% (NHEK) | [72] |
| Ex vivo full human skin thickness | Skin was preincubated with melatonin (10−3 M) and exposed to UVR in a dose- (0, 100, 300 mJ/cm2) and time-dependent manner (0, 24, 48 h post UVR). | Pre-incubation of skin samples with melatonin led to significant reductions in 8-OHdG-positive cells and prevention and depletion of antioxidative enzymes (CAT, GPx, Cu/ZnSOD, MnSOD). | [73] |
| Human full-thickness skin in organ culture and cultured normal human epidermal keratinocytes (NHEK) | Human skin and cells were preincubated with melatonin (10−3 M) and exposed to UVR in a dose (0, 100, 300 mJ/cm2)- and time-dependent manner (0, 24, 48 h post-UVR). | Melatonin inverted the increase in Hsp70 gene expression and Hsp70 protein levels in skin, as well as the decrease in enhanced gene expression of pro-inflammatory cytokines (IL-1b, IL-6, Casp-1) and pro-apoptotic protein (Casp-3) in NHEK. | [74] |
| Human keratinocytes (HaCaT) | Cells were exposed to formulations with 1% w/v melatonin solutions and controls for 2 h and then irradiated with a single dose of UVB (26 mJ/cm2). | Reduced generation of ROS and lower caspase 3 and 7 enzymes activities in cells previously treated with melatonin. | [62] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusanova, I.; Martínez-Ruiz, L.; Florido, J.; Rodríguez-Santana, C.; Guerra-Librero, A.; Acuña-Castroviejo, D.; Escames, G. Protective Effects of Melatonin on the Skin: Future Perspectives. Int. J. Mol. Sci. 2019, 20, 4948. https://doi.org/10.3390/ijms20194948
Rusanova I, Martínez-Ruiz L, Florido J, Rodríguez-Santana C, Guerra-Librero A, Acuña-Castroviejo D, Escames G. Protective Effects of Melatonin on the Skin: Future Perspectives. International Journal of Molecular Sciences. 2019; 20(19):4948. https://doi.org/10.3390/ijms20194948
Chicago/Turabian StyleRusanova, Iryna, Laura Martínez-Ruiz, Javier Florido, César Rodríguez-Santana, Ana Guerra-Librero, Darío Acuña-Castroviejo, and Germaine Escames. 2019. "Protective Effects of Melatonin on the Skin: Future Perspectives" International Journal of Molecular Sciences 20, no. 19: 4948. https://doi.org/10.3390/ijms20194948
APA StyleRusanova, I., Martínez-Ruiz, L., Florido, J., Rodríguez-Santana, C., Guerra-Librero, A., Acuña-Castroviejo, D., & Escames, G. (2019). Protective Effects of Melatonin on the Skin: Future Perspectives. International Journal of Molecular Sciences, 20(19), 4948. https://doi.org/10.3390/ijms20194948