Cordycepin Enhances Radiosensitivity in Oral Squamous Carcinoma Cells by Inducing Autophagy and Apoptosis Through Cell Cycle Arrest
Abstract
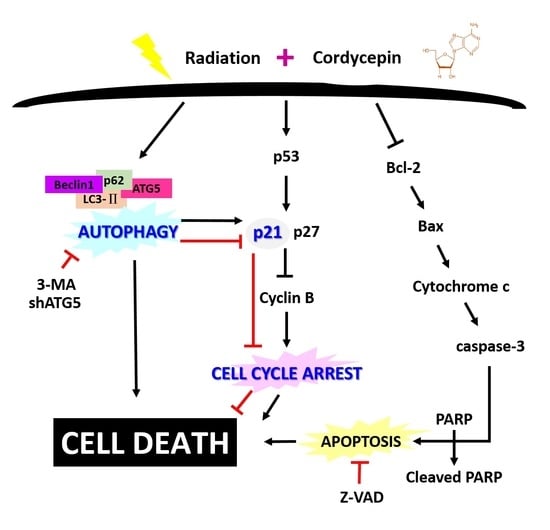
:1. Introduction
2. Results
2.1. Cell Death Dosage Effects of Cordycepin and/or IR on SCC-9, SCC-25, and SAS Cells
2.2. Apoptotic Effect of Combined IR and Cordycepin on SAS Cells
2.3. Autophagic Effect of Combined IR and Cordycepin on SAS Cells
2.4. Cell Cycle Effect of Combined IR and Cordycepin on SAS Cells
2.5. Cordycepin and/or IR Induce S Phase Arrest and Regulate the Expression of Cell Cycle Regulatory Proteins in SAS Cells
2.6. Cordycepin Combined with IR Induced G2/M Cell Cycle Arrest through Autophagy Induction in SAS Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Irradiation Treatment, Cell Viability, and Synergistic Interaction Analysis
4.3. Clonogenic Assay
4.4. Early Apoptosis and Autophagy Detections
4.5. Stable Knockdown Clone Selection
4.6. Annexin V/Propidium Iodide (PI) Double Staining Assay
4.7. Immunofluorescence and Confocal Microscopy
4.8. Western Blotting
4.9. Cell Cycle Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ART | artesunate |
| AVO | acidic vesicular organelle |
| BAF | bafilomycin A1 |
| CDK | cyclin-dependent kinase |
| CI | combination index |
| EMT | epithelial-mesenchymal transition |
| IR | irradiation |
| IR | ionizing radiation |
| OSCC | oral squamous cell carcinoma |
| PARP | poly (ADP-ribose) polymerase |
| PE | plating efficiency |
| pRB | retinoblastoma protein |
| SF | survival fraction |
| shRNA | short hairpin RNA |
| UPS | ubiquitin-proteasome system |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; Abdulle, A.S.M.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019. [Google Scholar] [CrossRef]
- Petruzzi, M.N.; Cherubini, K.; Salum, F.G.; de Figueiredo, M.A. Role of tumour-associated macrophages in oral squamous cells carcinoma progression: An update on current knowledge. Diagn. Pathol. 2017, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, J.; Wu, Y.; Ge, H.; Song, Y.; Wang, D.; Yuan, H.; Jiang, H.; Wang, Y.; Cheng, J. TEAD4 overexpression promotes epithelial-mesenchymal transition and associates with aggressiveness and adverse prognosis in head neck squamous cell carcinoma. Cancer Cell Int. 2018, 18, 178. [Google Scholar] [CrossRef]
- Montagnani, F.; Fornaro, L.; Frumento, P.; Vivaldi, C.; Falcone, A.; Fioretto, L. Multimodality treatment of locally advanced squamous cell carcinoma of the oesophagus: A comprehensive review and network meta-analysis. Crit. Rev. Oncol. Hematol. 2017, 114, 24–32. [Google Scholar] [CrossRef]
- Lo Nigro, C.; Denaro, N.; Merlotti, A.; Merlano, M. Head and neck cancer: Improving outcomes with a multidisciplinary approach. Cancer Manag. Res. 2017, 9, 363–371. [Google Scholar] [CrossRef]
- Burdak-Rothkamm, S.; Prise, K.M. New molecular targets in radiotherapy: DNA damage signalling and repair in targeted and non-targeted cells. Eur. J. Pharmacol. 2009, 625, 151–155. [Google Scholar] [CrossRef] [Green Version]
- Lomax, M.E.; Folkes, L.K.; O’Neill, P. Biological consequences of radiation-induced DNA damage: Relevance to radiotherapy. Clin. Oncol. (R. Coll. Radiol.) 2013, 25, 578–585. [Google Scholar] [CrossRef]
- McDonnell, T.J.; Meyn, R.E.; Robertson, L.E. Implications of apoptotic cell death regulation in cancer therapy. Semin. Cancer Biol. 1995, 6, 53–60. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Keyomarsi, K. Role of cell cycle in mediating sensitivity to radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 928–942. [Google Scholar] [CrossRef]
- Withers, H.R.; Mason, K.; Reid, B.O.; Dubravsky, N.; Barkley, H.T., Jr.; Brown, B.W.; Smathers, J.B. Response of mouse intestine to neutrons and gamma rays in relation to dose fractionation and division cycle. Cancer 1974, 34, 39–47. [Google Scholar] [CrossRef]
- Chiu, H.W.; Ho, S.Y.; Guo, H.R.; Wang, Y.J. Combination treatment with arsenic trioxide and irradiation enhances autophagic effects in U118-MG cells through increased mitotic arrest and regulation of PI3K/Akt and ERK1/2 signaling pathways. Autophagy 2009, 5, 472–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, S.Y.; Chen, W.C.; Chiu, H.W.; Lai, C.S.; Guo, H.R.; Wang, Y.J. Combination treatment with arsenic trioxide and irradiation enhances apoptotic effects in U937 cells through increased mitotic arrest and ROS generation. Chem.-Biol. Interact. 2009, 179, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.J.; Park, I.C.; Park, M.J.; Woo, S.H.; Hong, S.I.; Chung, H.Y.; Kim, T.H.; Lee, Y.S.; Rhee, C.H.; Lee, S.J. Enhancement of radiation response in human cervical cancer cells in vitro and in vivo by arsenic trioxide (As2O3). FEBS. Lett. 2002, 519, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Seong, D.B.; Hong, S.; Muthusami, S.; Kim, W.D.; Yu, J.R.; Park, W.Y. Cordycepin increases radiosensitivity in cervical cancer cells by overriding or prolonging radiation-induced G2/M arrest. Eur. J. Pharmacol. 2016, 771, 77–83. [Google Scholar] [CrossRef]
- Cunningham, K.G.; Manson, W.; Spring, F.S.; Hutchinson, S.A. Cordycepin, a metabolic product isolated from cultures of Cordyceps militaris (Linn.) Link. Nature 1950, 166, 949. [Google Scholar] [CrossRef]
- Jeong, J.W.; Jin, C.Y.; Kim, G.Y.; Lee, J.D.; Park, C.; Kim, G.D.; Kim, W.J.; Jung, W.K.; Seo, S.K.; Choi, I.W.; et al. Anti-inflammatory effects of cordycepin via suppression of inflammatory mediators in BV2 microglial cells. Int. Immunopharmacol. 2010, 10, 1580–1586. [Google Scholar] [CrossRef]
- Leu, S.F.; Poon, S.L.; Pao, H.Y.; Huang, B.M. The in vivo and in vitro stimulatory effects of cordycepin on mouse leydig cell steroidogenesis. Biosci. Biotechnol. Biochem. 2011, 75, 723–731. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, Y.H.; Pan, B.S.; Chang, M.M.; Huang, B.M. Functional study of Cordyceps sinensis and cordycepin in male reproduction: A review. J. Food Drug Anal. 2017, 25, 197–205. [Google Scholar] [CrossRef]
- Wu, W.C.; Hsiao, J.R.; Lian, Y.Y.; Lin, C.Y.; Huang, B.M. The apoptotic effect of cordycepin on human OEC-M1 oral cancer cell line. Cancer Chemoth. Pharm. 2007, 60, 103–111. [Google Scholar] [CrossRef]
- Yoshikawa, N.; Kunitomo, M.; Kagota, S.; Shinozuka, K.; Nakamura, K. Inhibitory Effect of Cordycepin on Hematogenic Metastasis of B16-F1 Mouse Melanoma Cells Accelerated by Adenosine-5 ‘-diphosphate. Anticancer Res. 2009, 29, 3857–3860. [Google Scholar] [PubMed]
- He, W.; Zhang, M.F.; Ye, J.; Jiang, T.T.; Fang, X.; Song, Y. Cordycepin induces apoptosis by enhancing JNK and p38 kinase activity and increasing the protein expression of Bcl-2 pro-apoptotic molecules. J. Zhejiang Univ. -Sci. B 2010, 11, 654–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baik, J.S.; Kwon, H.Y.; Kim, K.S.; Jeong, Y.K.; Cho, Y.S.; Lee, Y.C. Cordycepin induces apoptosis in human neuroblastoma SK-N-BE(2)-C and melanoma SK-MEL-2 cells. Indian J. Biochem. Biophys. 2012, 49, 86–91. [Google Scholar] [PubMed]
- Lin, L.T.; Lai, Y.J.; Wu, S.C.; Hsu, W.H.; Tai, C.J. Optimal conditions for cordycepin production in surface liquid-cultured Cordyceps militaris treated with porcine liver extracts for suppression of oral cancer. J. Food Drug Anal. 2018, 26, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Su, N.W.; Wu, S.H.; Chi, C.W.; Liu, C.J.; Tsai, T.H.; Chen, Y.J. Metronomic Cordycepin Therapy Prolongs Survival of Oral Cancer-Bearing Mice and Inhibits Epithelial-Mesenchymal Transition. Molecules 2017, 22, 629. [Google Scholar] [CrossRef]
- Aldridge, D.R.; Radford, I.R. Explaining differences in sensitivity to killing by ionizing radiation between human lymphoid cell lines. Cancer Res. 1998, 58, 2817–2824. [Google Scholar]
- Shackelford, R.E.; Kaufmann, W.K.; Paules, R.S. Cell cycle control, checkpoint mechanisms, and genotoxic stress. Environ. Health Persp. 1999, 107, 5–24. [Google Scholar]
- Tuli, H.S.; Sharma, A.K.; Sandhu, S.S.; Kashyap, D. Cordycepin: A bioactive metabolite with therapeutic potential. Life Sci. 2013, 93, 863–869. [Google Scholar] [CrossRef]
- Chen, K.; Shou, L.M.; Lin, F.; Duan, W.M.; Wu, M.Y.; Xie, X.; Xie, Y.F.; Li, W.; Tao, M. Artesunate induces G2/M cell cycle arrest through autophagy induction in breast cancer cells. Anti-Cancer Drugs 2014, 25, 652–662. [Google Scholar] [CrossRef]
- Wojcik, S. Crosstalk between autophagy and proteasome protein degradation systems: Possible implications for cancer therapy. Folia Histochem. Cyto. 2013, 51, 249–264. [Google Scholar] [CrossRef]
- Yang, Z.F.; Klionsky, D.J. Eaten alive: A history of macroautophagy. Nat. Cell Biol. 2010, 12, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.X.; Ni, H.M.; Gao, W.T.; Yoshimori, T.; Stolz, D.B.; Ron, D.; Yin, X.M. Linking of autophagy to ubiquitin-proteasome system is important for the regulation of endoplasmic reticulum stress and cell viability. Am. J. Pathol. 2007, 171, 513–524. [Google Scholar] [CrossRef]
- Zhang, X.D.; Li, W.M.; Wang, C.L.; Leng, X.Y.; Lian, S.L.; Feng, J.B.; Li, J.L.; Wang, H.L. Inhibition of autophagy enhances apoptosis induced by proteasome inhibitor bortezomib in human glioblastoma U87 and U251 cells. Mol. Cell Biochem. 2014, 385, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Daido, S.; Kanzawa, T.; Kondo, S.; Kondo, Y. Radiation-induced autophagy is associated with LC3 and its inhibition sensitizes malignant glioma cells. Int. J. Oncol. 2005, 26, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, B.; Hu, F.L.; Jiang, X.; Xu, J.; Zhao, D.L.; Liu, B.; Pan, S.H.; Dong, X.S.; Tan, G.; Wei, Z.; et al. Inhibition of Akt Reverses the Acquired Resistance to Sorafenib by Switching Protective Autophagy to Autophagic Cell Death in Hepatocellular Carcinoma. Mol. Cancer Ther. 2014, 13, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Lee, S.; Lee, K.; Shin, Y.S.; Kang, H.; Cho, H. Anti-cancer effect of Cordyceps militaris in human colorectal carcinoma RKO cells via cell cycle arrest and mitochondrial apoptosis. Daru 2015, 23, 35. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S. Molecular steps of death receptor and mitochondrial pathways of apoptosis. Life Sci. 2001, 69, 2957–2964. [Google Scholar] [CrossRef]
- Boulares, A.H.; Yakovlev, A.G.; Ivanova, V.; Stoica, B.A.; Wang, G.P.; Iyer, S.; Smulson, M. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis: Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J. Biol. Chem. 1999, 274, 22932–22940. [Google Scholar] [CrossRef] [PubMed]
- Cryns, V.; Yuan, J.Y. Proteases to die for. Gene Dev. 1998, 12, 1551–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieger, A.M.; Nelson, K.L.; Konowalchuk, J.D.; Barreda, D.R. Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. J. Vis. Exp. 2011, e2597. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T. How to interpret LC3 immunoblotting. Autophagy 2007, 3, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 and Autophagy. Methods Mol. Biol. 2008, 445, 77–88. [Google Scholar] [PubMed]
- Sarkar, S.; Ravikumar, B.; Rubinsztein, D.C. Autophagic clearance of aggregate-prone proteins associated with neurodegeneration. Methods Enzymol. 2009, 453, 83–110. [Google Scholar]
- Morgan, D.O. Principles of Cdk Regulation. Nature 1995, 374, 131–134. [Google Scholar] [CrossRef]
- Sherr, C.J.; Roberts, J.M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995, 9, 1149–1163. [Google Scholar] [CrossRef]
- Neganova, I.; Vilella, F.; Atkinson, S.P.; Lloret, M.; Passos, J.F.; von Zglinicki, T.; O’Connor, J.E.; Burks, D.; Jones, R.; Armstrong, L.; et al. An important role for CDK2 in G1 to S checkpoint activation and DNA damage response in human embryonic stem cells. Stem Cells 2011, 29, 651–659. [Google Scholar] [CrossRef]
- Nigg, E.A. Cyclin-dependent protein kinases: Key regulators of the eukaryotic cell cycle. Bioessays 1995, 17, 471–480. [Google Scholar] [CrossRef]
- Stark, G.R.; Taylor, W.R. Control of the G2/M transition. Mol. Biotechnol. 2006, 32, 227–248. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Wang, J.; Ye, Z.; Li, J.C. Oridonin up-regulates expression of P21 and induces autophagy and apoptosis in human prostate cancer cells. Int. J. Biol. Sci. 2012, 8, 901–912. [Google Scholar] [CrossRef]
- Ouyang, D.Y.; Zeng, L.H.; Pan, H.; Xu, L.H.; Wang, Y.; Liu, K.P.; He, X.H. Piperine inhibits the proliferation of human prostate cancer cells via induction of cell cycle arrest and autophagy. Food Chem. Toxicol. 2013, 60, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Mascitti, M.; Rubini, C.; De Michele, F.; Balercia, P.; Girotto, R.; Troiano, G.; Lo Muzio, L.; Santarelli, A. American Joint Committee on Cancer staging system 7th edition versus 8th edition: Any improvement for patients with squamous cell carcinoma of the tongue? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.W.; Yeh, Y.L.; Wang, Y.C.; Huang, W.J.; Ho, S.Y.; Lin, P.; Wang, Y.J. Combination of the novel histone deacetylase inhibitor YCW1 and radiation induces autophagic cell death through the downregulation of BNIP3 in triple-negative breast cancer cells in vitro and in an orthotopic mouse model. Mol. Cancer 2016, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, A.; Mascitti, M.; Rubini, C.; Bambini, F.; Giannatempo, G.; Lo Russo, L.; Sartini, D.; Emanuelli, M.; Procaccini, M.; Lo Muzio, L. Nuclear Survivin as a Prognostic Factor in Squamous-Cell Carcinoma of the Oral Cavity. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 566–570. [Google Scholar] [CrossRef]
- Xin, Y.; Jiang, F.; Yang, C.; Yan, Q.; Guo, W.; Huang, Q.; Zhang, L.; Jiang, G. Role of autophagy in regulating the radiosensitivity of tumor cells. J. Cancer Res. Clin. Oncol. 2017, 143, 2147–2157. [Google Scholar] [CrossRef]
- Kroemer, G.; Levine, B. Autophagic cell death: The story of a misnomer. Nat. Rev. Mol. Cell Biol. 2008, 9, 1004–1010. [Google Scholar] [CrossRef]
- Liu, X.; Yang, W.T.; Zheng, P.S. Msi1 promotes tumor growth and cell proliferation by targeting cell cycle checkpoint proteins p21, p27 and p53 in cervical carcinomas. Oncotarget 2014, 5, 10870–10885. [Google Scholar] [CrossRef] [Green Version]
- Baker, S.J.; Reddy, E.P. CDK4: A Key Player in the Cell Cycle, Development, and Cancer. Genes Cancer 2012, 3, 658–669. [Google Scholar] [CrossRef] [Green Version]
- Du, Z.; Tong, X.; Ye, X. Cyclin D1 promotes cell cycle progression through enhancing NDR1/2 kinase activity independent of cyclin-dependent kinase 4. J. Biol. Chem. 2013, 288, 26678–26687. [Google Scholar] [CrossRef]
- Torricelli, C.; Salvadori, S.; Valacchi, G.; Soucek, K.; Slabakova, E.; Muscettola, M.; Volpi, N.; Maioli, E. Alternative Pathways of Cancer Cell Death by Rottlerin: Apoptosis versus Autophagy. Evid.-Based Compl. Alt. 2012, 2012, 980658. [Google Scholar] [CrossRef]
- Carmichael, J.; Degraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a Tetrazolium-Based Semiautomated Colorimetric Assay-Assessment of Chemosensitivity Testing. Cancer Res. 1987, 47, 936–942. [Google Scholar] [PubMed]
- Siu, K.T.; Rosner, M.R.; Minella, A.C. An integrated view of cyclin E function and regulation. Cell Cycle 2012, 11, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capparelli, C.; Chiavarina, B.; Whitaker-Menezes, D.; Pestell, T.G.; Pestell, R.G.; Hulit, J.; Ando, S.; Howell, A.; Martinez-Outschoorn, U.E.; Sotgia, F.; et al. CDK inhibitors (p16/p19/p21) induce senescence and autophagy in cancer-associated fibroblasts, “fueling” tumor growth via paracrine interactions, without an increase in neo-angiogenesis. Cell Cycle 2012, 11, 3599–3610. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Chiu, H.W.; Lin, J.H.; Chen, Y.A.; Ho, S.Y.; Wang, Y.J. Combination treatment with arsenic trioxide and irradiation enhances cell-killing effects in human fibrosarcoma cells in vitro and in vivo through induction of both autophagy and apoptosis. Autophagy 2010, 6, 353–365. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.D.; Wang, Y.J.; Chen, C.H.; Yu, C.F.; Chen, L.C.; Lin, J.K.; Liang, Y.C.; Lin, S.Y.; Ho, Y.S. Molecular mechanisms of G0/G1 cell-cycle arrest and apoptosis induced by terfenadine in human cancer cells. Mol. Carcinog. 2003, 37, 39–50. [Google Scholar] [CrossRef]
- Kanzawa, T.; Kondo, Y.; Ito, H.; Kondo, S.; Germano, I. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. 2003, 63, 2103–2108. [Google Scholar]
- Traganos, F.; Darzynkiewicz, Z. Lysosomal proton pump activity: Supravital cell staining with acridine orange differentiates leukocyte subpopulations. Methods Cell Biol. 1994, 41, 185–194. [Google Scholar]
- van Engeland, M.; Ramaekers, F.C.; Schutte, B.; Reutelingsperger, C.P. A novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in culture. Cytometry 1996, 24, 131–139. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, S.-Y.; Wu, W.-S.; Lin, L.-C.; Wu, Y.-H.; Chiu, H.-W.; Yeh, Y.-L.; Huang, B.-M.; Wang, Y.-J. Cordycepin Enhances Radiosensitivity in Oral Squamous Carcinoma Cells by Inducing Autophagy and Apoptosis Through Cell Cycle Arrest. Int. J. Mol. Sci. 2019, 20, 5366. https://doi.org/10.3390/ijms20215366
Ho S-Y, Wu W-S, Lin L-C, Wu Y-H, Chiu H-W, Yeh Y-L, Huang B-M, Wang Y-J. Cordycepin Enhances Radiosensitivity in Oral Squamous Carcinoma Cells by Inducing Autophagy and Apoptosis Through Cell Cycle Arrest. International Journal of Molecular Sciences. 2019; 20(21):5366. https://doi.org/10.3390/ijms20215366
Chicago/Turabian StyleHo, Sheng-Yow, Wun-Syuan Wu, Li-Ching Lin, Yuan-Hua Wu, Hui-Wen Chiu, Ya-Ling Yeh, Bu-Miin Huang, and Ying-Jan Wang. 2019. "Cordycepin Enhances Radiosensitivity in Oral Squamous Carcinoma Cells by Inducing Autophagy and Apoptosis Through Cell Cycle Arrest" International Journal of Molecular Sciences 20, no. 21: 5366. https://doi.org/10.3390/ijms20215366
APA StyleHo, S. -Y., Wu, W. -S., Lin, L. -C., Wu, Y. -H., Chiu, H. -W., Yeh, Y. -L., Huang, B. -M., & Wang, Y. -J. (2019). Cordycepin Enhances Radiosensitivity in Oral Squamous Carcinoma Cells by Inducing Autophagy and Apoptosis Through Cell Cycle Arrest. International Journal of Molecular Sciences, 20(21), 5366. https://doi.org/10.3390/ijms20215366