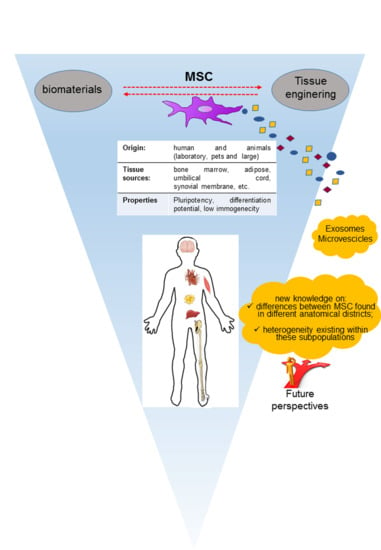
Substantial Overview on Mesenchymal Stem Cell Biological and Physical Properties as an Opportunity in Translational Medicine
Abstract
:1. Introduction
2. Current Aspects of Animal-Derived MSC as Experimental Models for Therapeutic Protocols
3. MSC-Dependent Immunomodulation and Interaction with the Vicinity: Autocrine, Paracrine and Remote Effects
4. Challenges Facing Angiogenesis, Bone Healing/Regeneration and other Regenerative Prospectives
5. MSC Plasticity in Their Interaction with Physical Cues
6. Challenges in MSC Translation into Clinical Practice: The Bone Disease Framework
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ad-MSC | adipose-MSC |
| BDNF | Brain-derived neurotrophic factor |
| BM | bone marrow |
| BMA | bone marrow aspirate |
| BMC | bone marrow concentrate |
| CFU | colony-forming unit |
| cMSC | canine MSC |
| ECM | extracellular matrix |
| FGF | fibroblast growth factor |
| HGF | Hepatocyte Growth Factor |
| HIF-1α | Hypoxia-inducible factor 1-alpha, |
| INF | Interferon |
| hMSC | human MSC |
| IL | Interleukine |
| MCP-1 | Macrophage chemotactic factor 1 |
| MMP-14 | Matrix metalloproteinase-14 |
| MSC | mesenchymal stem cells |
| MSCM | mesenchymal stem cells derived from synovial membrane |
| oMSC | ovine MSC |
| NGF | Nerve growth factor |
| PDGF | Platelet derived Growth Factor |
| pMSC | pig MSC |
| TGF-β | Transforming Growth Factor Beta |
| VEGF | Vascular endothelial growth factor |
| vMSC | Vertebra-derived MSC |
References
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A. International Society for Cellular, T. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Rustad, K.C.; Gurtner, G.C. Mesenchymal Stem Cells Home to Sites of Injury and Inflammation. Adv. Wound Care 2012, 1, 147–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marofi, F.; Vahedi, G.; Biglari, A.; Esmaeilzadeh, A.; Athari, S.S. Mesenchymal Stromal/Stem Cells: A New Era in the Cell-Based Targeted Gene Therapy of Cancer. Front. Immunol. 2017, 8, 1770. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.B.; Moncivais, K.; Caplan, A.I. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, e54. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Pelagalli, A.; Nardelli, A.; Lucarelli, E.; Zannetti, A.; Brunetti, A. Autocrine signals increase ovine mesenchymal stem cells migration through Aquaporin-1 and CXCR4 overexpression. J. Cell Physiol. 2018, 233, 6241–6249. [Google Scholar] [CrossRef]
- Bearden, R.N.; Huggins, S.S.; Cummings, K.J.; Smith, R.; Gregory, C.A.; Saunders, W.B. In-Vitro characterization of canine multipotent stromal cells isolated from synovium, bone marrow, and adipose tissue: A donor-matched comparative study. Stem Cell Res. Ther. 2017, 8, 218. [Google Scholar] [CrossRef]
- Devireddy, L.R.; Myers, M.; Screven, R.; Liu, Z.; Boxer, L. A serum-free medium formulation efficiently supports isolation and propagation of canine adipose-derived mesenchymal stem/stromal cells. PLoS ONE 2019, 14, e0210250. [Google Scholar] [CrossRef]
- Lin, H.Y.; Fujita, N.; Endo, K.; Morita, M.; Takeda, T.; Nakagawa, T.; Nishimura, R. Isolation and Characterization of Multipotent Mesenchymal Stem Cells Adhering to Adipocytes in Canine Bone Marrow. Stem Cells Dev. 2017, 26, 431–440. [Google Scholar] [CrossRef]
- Mellado-Lopez, M.; Griffeth, R.J.; Meseguer-Ripolles, J.; Cugat, R.; Garcia, M.; Moreno-Manzano, V. Plasma Rich in Growth Factors Induces Cell Proliferation, Migration, Differentiation, and Cell Survival of Adipose-Derived Stem Cells. Stem Cells Int. 2017, 2017, 5946527. [Google Scholar] [CrossRef] [PubMed]
- Endo, K.; Fujita, N.; Nakagawa, T.; Nishimura, R. Comparison of the effect of growth factors on chondrogenesis of canine mesenchymal stem cells. J. Vet. Med. Sci. 2019, 81, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Durgam, S.S.; Stewart, A.A.; Pondenis, H.C.; Gutierrez-Nibeyro, S.M.; Evans, R.B.; Stewart, M.C. Comparison of equine tendon- and bone marrow-derived cells cultured on tendon matrix with or without insulin-like growth factor-I supplementation. Am. J. Vet. Res. 2012, 73, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.P.; Schubert, S.; Scheibe, P.; Gross, C.; Brehm, W.; Burk, J. Growth Factor-Mediated Tenogenic Induction of Multipotent Mesenchymal Stromal Cells Is Altered by the Microenvironment of Tendon Matrix. Cell Transpl. 2018, 27, 1434–1450. [Google Scholar] [CrossRef] [Green Version]
- Bussche, L.; Van de Walle, G.R. Peripheral Blood-Derived Mesenchymal Stromal Cells Promote Angiogenesis via Paracrine Stimulation of Vascular Endothelial Growth Factor Secretion in the Equine Model. Stem Cells Transl. Med. 2014, 3, 1514–1525. [Google Scholar] [CrossRef]
- Somers, P.; Cornelissen, R.; Thierens, H.; Van Nooten, G. An optimized growth factor cocktail for ovine mesenchymal stem cells. Growth Factors 2012, 30, 37–48. [Google Scholar] [CrossRef]
- Endo, K.; Fujita, N.; Nakagawa, T.; Nishimura, R. Effect of Fibroblast Growth Factor-2 and Serum on Canine Mesenchymal Stem Cell Chondrogenesis. Tissue Eng. Part A 2019, 25, 901–910. [Google Scholar] [CrossRef]
- Trindade, A.B.; Therrien, J.; Garcia, J.M.; Smith, L.C. Mesenchymal-like stem cells in canine ovary show high differentiation potential. Cell Prolif. 2017, 50. [Google Scholar] [CrossRef]
- Wheeler, D.L.; Fredericks, D.C.; Dryer, R.F.; Bae, H.W. Allogeneic mesenchymal precursor cells (MPCs) combined with an osteoconductive scaffold to promote lumbar interbody spine fusion in an ovine model. Spine J. 2016, 16, 389–399. [Google Scholar] [CrossRef]
- Kira, T.; Akahane, M.; Omokawa, S.; Shimizu, T.; Kawate, K.; Onishi, T.; Tanaka, Y. Bone regeneration with osteogenic matrix cell sheet and tricalcium phosphate: An experimental study in sheep. World J. Orthop. 2017, 8, 754–760. [Google Scholar] [CrossRef]
- Chandran, S.; Shenoy, S.J.; Babu, S.S.; Nair, R.P.; Varma, H.K.; John, A. Strontium Hydroxyapatite scaffolds engineered with stem cells aid osteointegration and osteogenesis in osteoporotic sheep model. Colloids Surf. B Biointerfaces 2018, 163, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Castro-Malaspina, H.; Gay, R.E.; Resnick, G.; Kapoor, N.; Meyers, P.; Chiarieri, D.; McKenzie, S.; Broxmeyer, H.E.; Moore, M.A. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood 1980, 56, 289–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colosimo, A.; Russo, V.; Mauro, A.; Curini, V.; Marchisio, M.; Bernabo, N.; Alfonsi, M.; Mattioli, M.; Barboni, B. Prolonged in vitro expansion partially affects phenotypic features and osteogenic potential of ovine amniotic fluid-derived mesenchymal stromal cells. Cytotherapy 2013, 15, 930–950. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Tao, L.; Zhao, S.; Tai, D.; Liu, D.; Liu, P. Isolation and morphological characterization of ovine amniotic fluid mesenchymal stem cells. Exp. Anim. 2016, 65, 125–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, M.; Bai, C.; Li, L.; Fan, Y.; Ma, C.; Li, X.; Guan, W. Biological characterization of sheep kidney-derived mesenchymal stem cells. Exp. Ther. Med. 2016, 12, 3963–3971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vahedi, P.; Soleimanirad, J.; Roshangar, L.; Shafaei, H.; Jarolmasjed, S.; Nozad Charoudeh, H. Advantages of Sheep Infrapatellar Fat Pad Adipose Tissue Derived Stem Cells in Tissue Engineering. Adv. Pharm. Bull. 2016, 6, 105–110. [Google Scholar] [CrossRef]
- Gugjoo, M.B.; Amarpal; Makhdoomi, D.M.; Sharma, G.T. Equine Mesenchymal Stem Cells: Properties, Sources, Characterization and Potential Therapeutic Applications. J. Equine Vet. Sci. 2019, 72, 16–27. [Google Scholar] [CrossRef]
- Rhodes, N.P.; Srivastava, J.K.; Smith, R.F.; Longinotti, C. Heterogeneity in proliferative potential of ovine mesenchymal stem cell colonies. J. Mater. Sci. Mater. Med. 2004, 15, 397–402. [Google Scholar] [CrossRef] [Green Version]
- Martinello, T.; Gomiero, C.; Perazzi, A.; Iacopetti, I.; Gemignani, F.; DeBenedictis, G.M.; Ferro, S.; Zuin, M.; Martines, E.; Brun, P.; et al. Allogeneic mesenchymal stem cells improve the wound healing process of sheep skin. BMC Vet. Res. 2018, 14, 202. [Google Scholar] [CrossRef]
- Bosch, P.; Pratt, S.L.; Stice, S.L. Isolation, characterization, gene modification, and nuclear reprogramming of porcine mesenchymal stem cells. Biol. Reprod. 2006, 74, 46–57. [Google Scholar] [CrossRef]
- McDaniel, J.S.; Antebi, B.; Pilia, M.; Hurtgen, B.J.; Belenkiy, S.; Necsoiu, C.; Cancio, L.C.; Rathbone, C.R.; Batchinsky, A.I. Quantitative Assessment of Optimal Bone Marrow Site for the Isolation of Porcine Mesenchymal Stem Cells. Stem Cells Int. 2017, 2017, 1836960. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Yin, Y.; Zhou, H.; Song, G.; Fan, A.; Tang, B.; Shi, W.; Li, Z. Proliferative capacity and pluripotent characteristics of porcine adult stem cells derived from adipose tissue and bone marrow. Cell Reprogram. 2012, 14, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Miernik, K.; Karasinski, J. Porcine uterus contains a population of mesenchymal stem cells. Reproduction 2012, 143, 203–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardini, C.; Bertocchi, M.; Zannoni, A.; Salaroli, R.; Tubon, I.; Dothel, G.; Fernandez, M.; Bacci, M.L.; Calza, L.; Forni, M. Constitutive and LPS-stimulated secretome of porcine Vascular Wall-Mesenchymal Stem Cells exerts effects on in vitro endothelial angiogenesis. BMC Vet. Res. 2019, 15, 123. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.H.; Pfeifer, J.P.H.; de Souza, J.B.; Milani, B.H.G.; de Oliveira, R.A.; Assis, M.G.; Deffune, E.; Moroz, A.; Alves, A.L.G. Correction to: Culture of mesenchymal stem cells derived from equine synovial membrane in alginate hydrogel microcapsules. Stem Cell Res. Ther. 2018, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.H.; Pfeifer, J.P.H.; de Souza, J.B.; Milani, B.H.G.; de Oliveira, R.A.; Assis, M.G.; Deffune, E.; Moroz, A.; Alves, A.L.G. Culture of mesenchymal stem cells derived from equine synovial membrane in alginate hydrogel microcapsules. BMC Vet. Res. 2018, 14, 114. [Google Scholar] [CrossRef] [PubMed]
- Desance, M.; Contentin, R.; Bertoni, L.; Gomez-Leduc, T.; Branly, T.; Jacquet, S.; Betsch, J.M.; Batho, A.; Legendre, F.; Audigie, F.; et al. Chondrogenic Differentiation of Defined Equine Mesenchymal Stem Cells Derived from Umbilical Cord Blood for Use in Cartilage Repair Therapy. Int. J. Mol. Sci. 2018, 19, 537. [Google Scholar] [CrossRef]
- Arevalo-Turrubiarte, M.; Olmeo, C.; Accornero, P.; Baratta, M.; Martignani, E. Analysis of mesenchymal cells (MSCs) from bone marrow, synovial fluid and mesenteric, neck and tail adipose tissue sources from equines. Stem Cell Res. 2019, 37, 101442. [Google Scholar] [CrossRef]
- Longhini, A.L.F.; Salazar, T.E.; Vieira, C.; Trinh, T.; Duan, Y.; Pay, L.M.; Li Calzi, S.; Losh, M.; Johnston, N.A.; Xie, H.; et al. Peripheral blood-derived mesenchymal stem cells demonstrate immunomodulatory potential for therapeutic use in horses. PLoS ONE 2019, 14, e0212642. [Google Scholar] [CrossRef]
- Martin, D.R.; Cox, N.R.; Hathcock, T.L.; Niemeyer, G.P.; Baker, H.J. Isolation and characterization of multipotential mesenchymal stem cells from feline bone marrow. Exp. Hematol. 2002, 30, 879–886. [Google Scholar] [CrossRef]
- Zajic, L.B.; Webb, T.L.; Webb, P.; Coy, J.W.; Dow, S.W.; Quimby, J.M. Comparison of proliferative and immunomodulatory potential of adipose-derived mesenchymal stem cells from young and geriatric cats. J. Feline Med. Surg. 2017, 19, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Panasophonkul, S.; Samart, P.; Kongon, K.; Sathanawongs, A. Phenotypic characteristics of feline adipose-derived stem cells affected by cell passage number. Pol. J. Vet. Sci. 2017, 20, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Daems, R.; Van Hecke, L.; Schwarzkopf, I.; Depuydt, E.; Broeckx, S.Y.; David, M.; Beerts, C.; Vandekerckhove, P.; Spaas, J.H. A Feasibility Study on the Use of Equine Chondrogenic Induced Mesenchymal Stem Cells as a Treatment for Natural Occurring Osteoarthritis in Dogs. Stem Cells Int. 2019, 2019, 4587594. [Google Scholar] [CrossRef] [PubMed]
- Penha, E.M.; Aguiar, P.H.; Barrouin-Melo, S.M.; de Lima, R.S.; da Silveira, A.C.; Otelo, A.R.; Pinheiro, C.M.; Ribeiro-Dos-Santos, R.; Soares, M.B. Clinical neurofunctional rehabilitation of a cat with spinal cord injury after hemilaminectomy and autologous stem cell transplantation. Int. J. Stem Cells 2012, 5, 146–150. [Google Scholar] [CrossRef]
- Quimby, J.M.; Webb, T.L.; Randall, E.; Marolf, A.; Valdes-Martinez, A.; Dow, S.W. Assessment of intravenous adipose-derived allogeneic mesenchymal stem cells for the treatment of feline chronic kidney disease: A randomized, placebo-controlled clinical trial in eight cats. J. Feline Med. Surg. 2016, 18, 165–171. [Google Scholar] [CrossRef]
- Zeira, O.; Scaccia, S.; Pettinari, L.; Ghezzi, E.; Asiag, N.; Martinelli, L.; Zahirpour, D.; Dumas, M.P.; Konar, M.; Lupi, D.M.; et al. Intra-Articular Administration of Autologous Micro-Fragmented Adipose Tissue in Dogs with Spontaneous Osteoarthritis: Safety, Feasibility, and Clinical Outcomes. Stem Cells Transl. Med. 2018, 7, 819–828. [Google Scholar] [CrossRef] [Green Version]
- McIlwraith, C.W.; Frisbie, D.D.; Rodkey, W.G.; Kisiday, J.D.; Werpy, N.M.; Kawcak, C.E.; Steadman, J.R. Evaluation of intra-articular mesenchymal stem cells to augment healing of microfractured chondral defects. Arthroscopy 2011, 27, 1552–1561. [Google Scholar] [CrossRef]
- Ferris, D.J.; Frisbie, D.D.; Kisiday, J.D.; McIlwraith, C.W.; Hague, B.A.; Major, M.D.; Schneider, R.K.; Zubrod, C.J.; Kawcak, C.E.; Goodrich, L.R. Clinical outcome after intra-articular administration of bone marrow derived mesenchymal stem cells in 33 horses with stifle injury. Vet. Surg. 2014, 43, 255–265. [Google Scholar] [CrossRef]
- Barczewska, M.; Jezierska-Wozniak, K.; Habich, A.; Lipinski, S.; Holak, P.; Maksymowicz, W.; Wojtkiewicz, J. Evaluation of regenerative processes in the pig model of intervertebral disc degeneration after transplantation of bone marrow-derived mesenchymal stem cells. Folia Neuropathol. 2018, 56, 124–132. [Google Scholar] [CrossRef] [Green Version]
- La Francesca, S.; Aho, J.M.; Barron, M.R.; Blanco, E.W.; Soliman, S.; Kalenjian, L.; Hanson, A.D.; Todorova, E.; Marsh, M.; Burnette, K.; et al. Long-term regeneration and remodeling of the pig esophagus after circumferential resection using a retrievable synthetic scaffold carrying autologous cells. Sci. Rep. 2018, 8, 4123. [Google Scholar] [CrossRef]
- Ochiai, H.; Kishi, K.; Kubota, Y.; Oka, A.; Hirata, E.; Yabuki, H.; Iso, Y.; Suzuki, H.; Umezawa, A. Transplanted mesenchymal stem cells are effective for skin regeneration in acute cutaneous wounds of pigs. Regen. Ther. 2017, 7, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Caminal, M.; Fonseca, C.; Peris, D.; Moll, X.; Rabanal, R.M.; Barrachina, J.; Codina, D.; Garcia, F.; Cairo, J.J.; Godia, F.; et al. Use of a chronic model of articular cartilage and meniscal injury for the assessment of long-term effects after autologous mesenchymal stromal cell treatment in sheep. New Biotechnol. 2014, 31, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Ude, C.C.; Sulaiman, S.B.; Min-Hwei, N.; Hui-Cheng, C.; Ahmad, J.; Yahaya, N.M.; Saim, A.B.; Idrus, R.B. Cartilage regeneration by chondrogenic induced adult stem cells in osteoarthritic sheep model. PLoS ONE 2014, 9, e98770. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, I.; van der Meulen-de Jong, A.E.; Verspaget, H.W.; Veenendaal, R.A.; Hommes, D.W.; Bonsing, B.A.; Peeters, K. Standardization of mesenchymal stromal cell therapy for perianal fistulizing Crohn’s disease. Eur. J. Gastroenterol. Hepatol. 2018, 30, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Galleu, A.; Milojkovic, D.; Deplano, S.; Szydlo, R.; Loaiza, S.; Wynn, R.; Marks, D.I.; Richardson, D.; Orchard, K.; Kanfer, E.; et al. Mesenchymal stromal cells for acute graft-versus-host disease: Response at 1 week predicts probability of survival. Br. J. Haematol. 2019, 185, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Sahraian, M.A.; Mohyeddin Bonab, M.; Baghbanian, S.M.; Owji, M.; Naser Moghadasi, A. Therapeutic Use of Intrathecal Mesenchymal Stem Cells in patients with Multiple Sclerosis: A Pilot Study with Booster Injection. Immunol. Invest. 2019, 48, 160–168. [Google Scholar] [CrossRef]
- Keyhanmanesh, R.; Rahbarghazi, R.; Ahmadi, M. Systemic Transplantation of Mesenchymal Stem Cells Modulates Endothelial Cell Adhesion Molecules Induced by Ovalbumin in Rat Model of Asthma. Inflammation 2018, 41, 2236–2245. [Google Scholar] [CrossRef]
- Yin, J.Q.; Zhu, J.; Ankrum, J.A. Manufacturing of primed mesenchymal stromal cells for therapy. Nat. Biomed. Eng. 2019, 3, 90–104. [Google Scholar] [CrossRef]
- Barry, F.; Murphy, M. Mesenchymal stem cells in joint disease and repair. Nat. Rev. Rheumatol. 2013, 9, 584–594. [Google Scholar] [CrossRef]
- Al-Maawali, A.K.; Nguyen, P.; Phang, P.T. Modern Treatments and Stem Cell Therapies for Perianal Crohn’s Fistulas. Can. J. Gastroenterol. Hepatol. 2016, 2016, 1651570. [Google Scholar] [CrossRef]
- Spaggiari, G.M.; Capobianco, A.; Abdelrazik, H.; Becchetti, F.; Mingari, M.C.; Moretta, L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: Role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 2008, 111, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, G.M.; Abdelrazik, H.; Becchetti, F.; Moretta, L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: Central role of MSC-derived prostaglandin E2. Blood 2009, 113, 6576–6583. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yamamoto, Y.; Xiao, Z.; Ochiya, T. The Immunomodulatory Functions of Mesenchymal Stromal/Stem Cells Mediated via Paracrine Activity. J. Clin. Med. 2019, 8, 1025. [Google Scholar] [CrossRef] [PubMed]
- Mougiakakos, D.; Jitschin, R.; Johansson, C.C.; Okita, R.; Kiessling, R.; Le Blanc, K. The impact of inflammatory licensing on heme oxygenase-1-mediated induction of regulatory T cells by human mesenchymal stem cells. Blood 2011, 117, 4826–4835. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef]
- de Castro, L.L.; Lopes-Pacheco, M.; Weiss, D.J.; Cruz, F.F.; Rocco, P.R.M. Current understanding of the immunosuppressive properties of mesenchymal stromal cells. J. Mol. Med. 2019, 97, 605–618. [Google Scholar] [CrossRef]
- Li, D.; Cheng, P.; Jiang, H.; Cao, T.; Wang, J.; Gao, Y.; Lin, Y.; Wang, C.; Zhang, S.; Li, J.; et al. Vascularization converts the lineage fate of bone mesenchymal stem cells to endothelial cells in tissue-engineered bone grafts by modulating FGF2-RhoA/ROCK signaling. Cell Death Dis. 2018, 9, 959. [Google Scholar] [CrossRef]
- Allan, D.S.; Tieu, A.; Lalu, M.; Burger, D. Concise Review: Mesenchymal Stromal Cell-Derived Extracellular Vesicles for Regenerative Therapy and Immune Modulation: Progress and Challenges Toward Clinical Application. Stem Cells Transl. Med. 2019. [Google Scholar] [CrossRef]
- Jiang, D.; Muschhammer, J.; Qi, Y.; Kugler, A.; de Vries, J.C.; Saffarzadeh, M.; Sindrilaru, A.; Beken, S.V.; Wlaschek, M.; Kluth, M.A.; et al. Suppression of Neutrophil-Mediated Tissue Damage-A Novel Skill of Mesenchymal Stem Cells. Stem Cells 2016, 34, 2393–2406. [Google Scholar] [CrossRef]
- Griffin, M.D.; Ryan, A.E.; Alagesan, S.; Lohan, P.; Treacy, O.; Ritter, T. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: What have we learned so far? Immunol. Cell Biol. 2013, 91, 40–51. [Google Scholar] [CrossRef]
- Berglund, A.K.; Fortier, L.A.; Antczak, D.F.; Schnabel, L.V. Immunoprivileged no more: Measuring the immunogenicity of allogeneic adult mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 288. [Google Scholar] [CrossRef] [PubMed]
- Abu-El-Rub, E.; Sequiera, G.L.; Sareen, N.; Yan, W.; Moudgil, M.; Sabbir, M.G.; Dhingra, S. Hypoxia-induced 26S proteasome dysfunction increases immunogenicity of mesenchymal stem cells. Cell Death Dis. 2019, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Abdelrazik, H.; Spaggiari, G.M.; Chiossone, L.; Moretta, L. Mesenchymal stem cells expanded in human platelet lysate display a decreased inhibitory capacity on T- and NK-cell proliferation and function. Eur. J. Immunol. 2011, 41, 3281–3290. [Google Scholar] [CrossRef] [PubMed]
- Robb, K.P.; Fitzgerald, J.C.; Barry, F.; Viswanathan, S. Mesenchymal stromal cell therapy: Progress in manufacturing and assessments of potency. Cytotherapy 2019, 21, 289–306. [Google Scholar] [CrossRef]
- Karantalis, V.; Hare, J.M. Use of mesenchymal stem cells for therapy of cardiac disease. Circ. Res. 2015, 116, 1413–1430. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.R.; Hare, J.M. Mesenchymal stem cells: Biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ. Res. 2011, 109, 923–940. [Google Scholar] [CrossRef]
- Siciliano, C.; Bordin, A.; Ibrahim, M.; Chimenti, I.; Cassiano, F.; Gatto, I.; Mangino, G.; Coccia, A.; Miglietta, S.; Bastianelli, D.; et al. The adipose tissue of origin influences the biological potential of human adipose stromal cells isolated from mediastinal and subcutaneous fat depots. Stem Cell Res. 2016, 17, 342–351. [Google Scholar] [CrossRef] [Green Version]
- DeLisser, H.M.; Christofidou-Solomidou, M.; Strieter, R.M.; Burdick, M.D.; Robinson, C.S.; Wexler, R.S.; Kerr, J.S.; Garlanda, C.; Merwin, J.R.; Madri, J.A.; et al. Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am. J. Pathol. 1997, 151, 671–677. [Google Scholar]
- Liu, L.; Shi, G.P. CD31: Beyond a marker for endothelial cells. Cardiovasc Res. 2012, 94, 3–5. [Google Scholar] [CrossRef]
- Privratsky, J.R.; Newman, P.J. PECAM-1: Regulator of endothelial junctional integrity. Cell Tissue Res. 2014, 355, 607–619. [Google Scholar] [CrossRef]
- Bareeqa, S.B.; Bibi, F.; Ahmed, S.I.; Samar, S.S. Advancement in Stem Cell Therapy for Ischemic Myocardial Cell: A Systematic Review. Int. J. Hematol. Oncol. Stem Cell Res. 2018, 12, 282–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selmani, Z.; Naji, A.; Zidi, I.; Favier, B.; Gaiffe, E.; Obert, L.; Borg, C.; Saas, P.; Tiberghien, P.; Rouas-Freiss, N.; et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells 2008, 26, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Magaki, T.; Takeda, M.; Kajiwara, Y.; Hanaya, R.; Sugiyama, K.; Arita, K.; Nishimura, M.; Kato, Y.; Kurisu, K. Intravenous administration of bone marrow stromal cells increases survivin and Bcl-2 protein expression and improves sensorimotor function following ischemia in rats. Neurosci. Lett. 2008, 430, 109–114. [Google Scholar] [CrossRef]
- Siciliano, C.; Chimenti, I.; Bordin, A.; Ponti, D.; Iudicone, P.; Peruzzi, M.; Rendina, E.A.; Calogero, A.; Pierelli, L.; Ibrahim, M.; et al. The potential of GMP-compliant platelet lysate to induce a permissive state for cardiovascular transdifferentiation in human mediastinal adipose tissue-derived mesenchymal stem cells. Biomed. Res. Int. 2015, 2015, 162439. [Google Scholar] [CrossRef]
- Siciliano, C.; Chimenti, I.; Ibrahim, M.; Napoletano, C.; Mangino, G.; Scafetta, G.; Zoccai, G.B.; Rendina, E.A.; Calogero, A.; Frati, G.; et al. Cardiosphere conditioned media influence the plasticity of human mediastinal adipose tissue-derived mesenchymal stem cells. Cell Transpl. 2015, 24, 2307–2322. [Google Scholar] [CrossRef]
- Aboutaleb, N.; Faezi, M.; Nasseri Maleki, S.; Nazarinia, D.; Razavi Tousi, S.M.T.; Hashemirad, N. Conditioned medium obtained from mesenchymal stem cells attenuates focal cerebral ischemia reperfusion injury through activation of ERK1/ERK2-BDNF signaling pathway. J. Chem. Neuroanat. 2019, 97, 87–98. [Google Scholar] [CrossRef]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef]
- Ren, G.; Rezaee, M.; Razavi, M.; Taysir, A.; Wang, J.; Thakor, A.S. Adipose tissue-derived mesenchymal stem cells rescue the function of islets transplanted in sub-therapeutic numbers via their angiogenic properties. Cell Tissue Res. 2019, 376, 343–364. [Google Scholar] [CrossRef]
- Salazar-Noratto, G.E.; Luo, G.; Denoeud, C.; Padrona, M.; Moya, A.; Bensidhoum, M.; Bizios, R.; Potier, E.; Logeart-Avramoglou, D.; Petite, H. Concise Review: Understanding and leveraging cell metabolism to Enhance Mesenchymal Stem Cell Transplantation Survival in Tissue Engineering and Regenerative Medicine Applications. Stem Cells 2019. [Google Scholar] [CrossRef]
- Rowlands, A.S.; George, P.A.; Cooper-White, J.J. Directing osteogenic and myogenic differentiation of MSCs: Interplay of stiffness and adhesive ligand presentation. Am. J. Physiol. Cell Physiol. 2008, 295, C1037–C1044. [Google Scholar] [CrossRef]
- De Falco, E.; Porcelli, D.; Torella, A.R.; Straino, S.; Iachininoto, M.G.; Orlandi, A.; Truffa, S.; Biglioli, P.; Napolitano, M.; Capogrossi, M.C.; et al. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood 2004, 104, 3472–3482. [Google Scholar] [CrossRef] [PubMed]
- De Falco, E.; Avitabile, D.; Totta, P.; Straino, S.; Spallotta, F.; Cencioni, C.; Torella, A.R.; Rizzi, R.; Porcelli, D.; Zacheo, A.; et al. Altered SDF-1-mediated differentiation of bone marrow-derived endothelial progenitor cells in diabetes mellitus. J. Cell Mol. Med. 2009, 13, 3405–3414. [Google Scholar] [CrossRef] [PubMed]
- Haider, H.; Jiang, S.; Idris, N.M.; Ashraf, M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ. Res. 2008, 103, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.J.; Mestas, J.; Gharaee-Kermani, M.; Burdick, M.D.; Sica, A.; Belperio, J.A.; Keane, M.P.; Strieter, R.M. Epidermal growth factor and hypoxia-induced expression of CXC chemokine receptor 4 on non-small cell lung cancer cells is regulated by the phosphatidylinositol 3-kinase/PTEN/AKT/mammalian target of rapamycin signaling pathway and activation of hypoxia inducible factor-1alpha. J. Biol. Chem. 2005, 280, 22473–22481. [Google Scholar] [CrossRef] [PubMed]
- Burchfield, J.S.; Iwasaki, M.; Koyanagi, M.; Urbich, C.; Rosenthal, N.; Zeiher, A.M.; Dimmeler, S. Interleukin-10 from transplanted bone marrow mononuclear cells contributes to cardiac protection after myocardial infarction. Circ. Res. 2008, 103, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, A.A.; Weiss, J.B.; Lee, J.; Kilian, K.A. Matrix composition and mechanics direct proangiogenic signaling from mesenchymal stem cells. Tissue Eng. Part A 2014, 20, 2737–2745. [Google Scholar] [CrossRef]
- Neve, A.; Cantatore, F.P.; Maruotti, N.; Corrado, A.; Ribatti, D. Extracellular matrix modulates angiogenesis in physiological and pathological conditions. Biomed. Res. Int. 2014, 2014, 756078. [Google Scholar] [CrossRef]
- Watt, S.M.; Gullo, F.; van der Garde, M.; Markeson, D.; Camicia, R.; Khoo, C.P.; Zwaginga, J.J. The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential. Br. Med. Bull. 2013, 108, 25–53. [Google Scholar] [CrossRef]
- Ranganath, S.H.; Levy, O.; Inamdar, M.S.; Karp, J.M. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell 2012, 10, 244–258. [Google Scholar] [CrossRef]
- Businaro, R.; Corsi, M.; Di Raimo, T.; Marasco, S.; Laskin, D.L.; Salvati, B.; Capoano, R.; Ricci, S.; Siciliano, C.; Frati, G.; et al. Multidisciplinary approaches to stimulate wound healing. Ann. N. Y. Acad. Sci. 2016, 1378, 137–142. [Google Scholar] [CrossRef]
- Businaro, R.; Scaccia, E.; Bordin, A.; Pagano, F.; Corsi, M.; Siciliano, C.; Capoano, R.; Procaccini, E.; Salvati, B.; Petrozza, V.; et al. Platelet Lysate-Derived Neuropeptide y Influences Migration and Angiogenesis of Human Adipose Tissue-Derived Stromal Cells. Sci. Rep. 2018, 8, 14365. [Google Scholar] [CrossRef] [PubMed]
- Miranville, A.; Heeschen, C.; Sengenes, C.; Curat, C.A.; Busse, R.; Bouloumie, A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation 2004, 110, 349–355. [Google Scholar] [CrossRef] [PubMed]
- De Falco, E.; Carnevale, R.; Pagano, F.; Chimenti, I.; Fianchini, L.; Bordin, A.; Siciliano, C.; Monticolo, R.; Equitani, F.; Carrizzo, A.; et al. Role of NOX2 in mediating doxorubicin-induced senescence in human endothelial progenitor cells. Mech. Ageing Dev. 2016, 159, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.; El-Badri, N. Pericytes: The Role of Multipotent Stem Cells in Vascular Maintenance and Regenerative Medicine. Adv. Exp. Med. Biol 2018, 1079, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Bianco, P.; Robey, P.G.; Simmons, P.J. Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell Stem Cell 2008, 2, 313–319. [Google Scholar] [CrossRef] [PubMed]
- McFadden, T.M.; Duffy, G.P.; Allen, A.B.; Stevens, H.Y.; Schwarzmaier, S.M.; Plesnila, N.; Murphy, J.M.; Barry, F.P.; Guldberg, R.E.; O’Brien, F.J. The delayed addition of human mesenchymal stem cells to pre-formed endothelial cell networks results in functional vascularization of a collagen-glycosaminoglycan scaffold in vivo. Acta Biomater. 2013, 9, 9303–9316. [Google Scholar] [CrossRef]
- Leroux, L.; Descamps, B.; Tojais, N.F.; Seguy, B.; Oses, P.; Moreau, C.; Daret, D.; Ivanovic, Z.; Boiron, J.M.; Lamaziere, J.M.; et al. Hypoxia preconditioned mesenchymal stem cells improve vascular and skeletal muscle fiber regeneration after ischemia through a Wnt4-dependent pathway. Mol. Ther. J. Am. Soc. Gene Ther. 2010, 18, 1545–1552. [Google Scholar] [CrossRef]
- Dufourcq, P.; Descamps, B.; Tojais, N.F.; Leroux, L.; Oses, P.; Daret, D.; Moreau, C.; Lamaziere, J.M.; Couffinhal, T.; Duplaa, C. Secreted frizzled-related protein-1 enhances mesenchymal stem cell function in angiogenesis and contributes to neovessel maturation. Stem Cells 2008, 26, 2991–3001. [Google Scholar] [CrossRef]
- Ljung, K.; Gronlund, A.; Felldin, U.; Rodin, S.; Corbascio, M.; Osterholm, C.; Grinnemo, K.H. Human Fetal Cardiac Mesenchymal Stromal Cells Differentiate In Vivo into Endothelial Cells and Contribute to Vasculogenesis in Immunocompetent Mice. Stem Cells Dev. 2019, 28, 310–318. [Google Scholar] [CrossRef]
- Gong, M.; Yu, B.; Wang, J.; Wang, Y.; Liu, M.; Paul, C.; Millard, R.W.; Xiao, D.S.; Ashraf, M.; Xu, M. Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget 2017, 8, 45200–45212. [Google Scholar] [CrossRef]
- Allameh, A.; Jazayeri, M.; Adelipour, M. In Vivo Vascularization of Endothelial Cells Derived from Bone Marrow Mesenchymal Stem Cells in SCID Mouse Model. Cell J. 2016, 18, 179–188. [Google Scholar] [PubMed]
- Oswald, J.; Boxberger, S.; Jorgensen, B.; Feldmann, S.; Ehninger, G.; Bornhauser, M.; Werner, C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 2004, 22, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Sun, Z.; Liao, L.; Meng, Y.; Han, Q.; Zhao, R.C. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem. Biophys. Res. Commun. 2005, 332, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.J.; McIlhenny, S.; Tulenko, T.; Golesorkhi, N.; Zhang, P.; Larson, R.; Lombardi, J.; Shapiro, I.; DiMuzio, P.J. Endothelial differentiation of adipose-derived stem cells: Effects of endothelial cell growth supplement and shear force. J. Surg. Res. 2009, 152, 157–166. [Google Scholar] [CrossRef]
- Silva, G.V.; Litovsky, S.; Assad, J.A.; Sousa, A.L.; Martin, B.J.; Vela, D.; Coulter, S.C.; Lin, J.; Ober, J.; Vaughn, W.K.; et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation 2005, 111, 150–156. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Yang, M.; Zou, Y.; Liu, H.; Liang, Z.; Yin, Y.; Niu, G.; Yan, Z.; Zhang, B. Efficient Differentiation of Bone Marrow Mesenchymal Stem Cells into Endothelial Cells in Vitro. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Janeczek Portalska, K.; Leferink, A.; Groen, N.; Fernandes, H.; Moroni, L.; van Blitterswijk, C.; de Boer, J. Endothelial differentiation of mesenchymal stromal cells. PLoS ONE 2012, 7, e46842. [Google Scholar] [CrossRef]
- Ball, S.G.; Shuttleworth, C.A.; Kielty, C.M. Mesenchymal stem cells and neovascularization: Role of platelet-derived growth factor receptors. J. Cell Mol. Med. 2007, 11, 1012–1030. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, R.; Wang, S.J.; Zhang, C.L.; Mao, L.B.; Zhuang, C.Y.; Tang, Y.Y.; Luo, X.G.; Zhou, H.; Zhang, T.C. Vascular endothelial growth factor stimulates endothelial differentiation from mesenchymal stem cells via Rho/myocardin-related transcription factor--a signaling pathway. Int. J. Biochem. Cell Biol. 2013, 45, 1447–1456. [Google Scholar] [CrossRef]
- Dong, J.D.; Gu, Y.Q.; Li, C.M.; Wang, C.R.; Feng, Z.G.; Qiu, R.X.; Chen, B.; Li, J.X.; Zhang, S.W.; Wang, Z.G.; et al. Response of mesenchymal stem cells to shear stress in tissue-engineered vascular grafts. Acta Pharm. Sin. 2009, 30, 530–536. [Google Scholar] [CrossRef] [Green Version]
- Potter, C.M.; Lao, K.H.; Zeng, L.; Xu, Q. Role of biomechanical forces in stem cell vascular lineage differentiation. Arter. Thromb. Vasc. Biol. 2014, 34, 2184–2190. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Dunoyer-Geindre, S.; Serre-Beinier, V.; Mai, G.; Lambert, J.F.; Fish, R.J.; Pernod, G.; Buehler, L.; Bounameaux, H.; Kruithof, E.K. Characterization of endothelial-like cells derived from human mesenchymal stem cells. J. Thromb. Haemost. 2007, 5, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Lilly, B. Endothelial cells direct mesenchymal stem cells toward a smooth muscle cell fate. Stem Cells Dev. 2014, 23, 2581–2590. [Google Scholar] [CrossRef]
- Kurpinski, K.; Lam, H.; Chu, J.; Wang, A.; Kim, A.; Tsay, E.; Agrawal, S.; Schaffer, D.V.; Li, S. Transforming growth factor-beta and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells 2010, 28, 734–742. [Google Scholar] [CrossRef]
- Ai, W.J.; Li, J.; Lin, S.M.; Li, W.; Liu, C.Z.; Lv, W.M. R-Smad signaling-mediated VEGF expression coordinately regulates endothelial cell differentiation of rat mesenchymal stem cells. Stem Cells Dev. 2015, 24, 1320–1331. [Google Scholar] [CrossRef]
- Jian, H.; Shen, X.; Liu, I.; Semenov, M.; He, X.; Wang, X.F. Smad3-dependent nuclear translocation of beta-catenin is required for TGF-beta1-induced proliferation of bone marrow-derived adult human mesenchymal stem cells. Genes Dev. 2006, 20, 666–674. [Google Scholar] [CrossRef]
- Liao, J.; Wei, Q.; Zou, Y.; Fan, J.; Song, D.; Cui, J.; Zhang, W.; Zhu, Y.; Ma, C.; Hu, X.; et al. Notch Signaling Augments BMP9-Induced Bone Formation by Promoting the Osteogenesis-Angiogenesis Coupling Process in Mesenchymal Stem Cells (MSCs). Cell. Physiol. Biochem. 2017, 41, 1905–1923. [Google Scholar] [CrossRef]
- Jin, H.J.; Bae, Y.K.; Kim, M.; Kwon, S.J.; Jeon, H.B.; Choi, S.J.; Kim, S.W.; Yang, Y.S.; Oh, W.; Chang, J.W. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int. J. Mol. Sci. 2013, 14, 17986–18001. [Google Scholar] [CrossRef]
- Ryan, J.M.; Barry, F.; Murphy, J.M.; Mahon, B.P. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin. Exp. Immunol. 2007, 149, 353–363. [Google Scholar] [CrossRef]
- Lu, H.; Wang, F.; Mei, H.; Wang, S.; Cheng, L. Human Adipose Mesenchymal Stem Cells Show More Efficient Angiogenesis Promotion on Endothelial Colony-Forming Cells than Umbilical Cord and Endometrium. Stem Cells Int. 2018, 2018, 7537589. [Google Scholar] [CrossRef]
- Pennings, I.; van Dijk, L.A.; van Huuksloot, J.; Fledderus, J.O.; Schepers, K.; Braat, A.K.; Hsiao, E.C.; Barruet, E.; Morales, B.M.; Verhaar, M.C.; et al. Effect of donor variation on osteogenesis and vasculogenesis in hydrogel cocultures. J. Tissue Eng. Regen. Med. 2019, 13, 433–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vailhe, B.; Vittet, D.; Feige, J.J. In Vitro models of vasculogenesis and angiogenesis. Lab. Investig. 2001, 81, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Roubelakis, M.G.; Tsaknakis, G.; Pappa, K.I.; Anagnou, N.P.; Watt, S.M. Spindle shaped human mesenchymal stem/stromal cells from amniotic fluid promote neovascularization. PLoS ONE 2013, 8, e54747. [Google Scholar] [CrossRef] [PubMed]
- Roura, S.; Bago, J.R.; Soler-Botija, C.; Pujal, J.M.; Galvez-Monton, C.; Prat-Vidal, C.; Llucia-Valldeperas, A.; Blanco, J.; Bayes-Genis, A. Human umbilical cord blood-derived mesenchymal stem cells promote vascular growth in vivo. PLoS ONE 2012, 7, e49447. [Google Scholar] [CrossRef]
- Au, P.; Tam, J.; Fukumura, D.; Jain, R.K. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood 2008, 111, 4551–4558. [Google Scholar] [CrossRef]
- Melero-Martin, J.M.; De Obaldia, M.E.; Kang, S.Y.; Khan, Z.A.; Yuan, L.; Oettgen, P.; Bischoff, J. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ. Res. 2008, 103, 194–202. [Google Scholar] [CrossRef]
- Traktuev, D.O.; Prater, D.N.; Merfeld-Clauss, S.; Sanjeevaiah, A.R.; Saadatzadeh, M.R.; Murphy, M.; Johnstone, B.H.; Ingram, D.A.; March, K.L. Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ. Res. 2009, 104, 1410–1420. [Google Scholar] [CrossRef]
- Hashi, C.K.; Zhu, Y.; Yang, G.Y.; Young, W.L.; Hsiao, B.S.; Wang, K.; Chu, B.; Li, S. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc. Natl. Acad. Sci. USA 2007, 104, 11915–11920. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhang, S.; Zhou, J.; Wang, J.; Zhen, M.; Liu, Y.; Chen, J.; Qi, Z. The development of a tissue-engineered artery using decellularized scaffold and autologous ovine mesenchymal stem cells. Biomaterials 2010, 31, 296–307. [Google Scholar] [CrossRef]
- Zhang, J.; Yi, Y.; Yang, S.; Zhu, Y.; Hu, X. Effects of adipose-derived stem cell released exosomes on proliferation, migration, and tube-like differentiation of human umbilical vein endothelial cells. Chin. J. Reparative Reconstr. Surg. 2018, 32, 1351–1357. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, Z.; Wang, P.; Xia, Y.; Wu, J.; Xia, D.; Fang, S.; Xu, S. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1alpha-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2019, 52, e12570. [Google Scholar] [CrossRef] [PubMed]
- Angelini, F.; Pagano, F.; Bordin, A.; Milan, M.; Chimenti, I.; Peruzzi, M.; Valenti, V.; Marullo, A.; Schirone, L.; Palmerio, S.; et al. The Impact of Environmental Factors in Influencing Epigenetics Related to Oxidative States in the Cardiovascular System. Oxidative Med. Cell. Longev. 2017, 2017, 2712751. [Google Scholar] [CrossRef] [PubMed]
- Angelini, F.; Pagano, F.; Bordin, A.; Picchio, V.; De Falco, E.; Chimenti, I. Getting Old through the Blood: Circulating Molecules in Aging and Senescence of Cardiovascular Regenerative Cells. Front. Cardiovasc. Med. 2017, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Ferguson, S.W.; Wang, J.; Lee, C.J.; Liu, M.; Neelamegham, S.; Canty, J.M.; Nguyen, J. The microRNA regulatory landscape of MSC-derived exosomes: A systems view. Sci. Rep. 2018, 8, 1419. [Google Scholar] [CrossRef]
- Baglio, S.R.; Rooijers, K.; Koppers-Lalic, D.; Verweij, F.J.; Perez Lanzon, M.; Zini, N.; Naaijkens, B.; Perut, F.; Niessen, H.W.; Baldini, N.; et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res. Ther. 2015, 6, 127. [Google Scholar] [CrossRef]
- Anand, S. A brief primer on microRNAs and their roles in angiogenesis. Vasc. Cell 2013, 5, 2. [Google Scholar] [CrossRef]
- Katare, R.; Riu, F.; Mitchell, K.; Gubernator, M.; Campagnolo, P.; Cui, Y.; Fortunato, O.; Avolio, E.; Cesselli, D.; Beltrami, A.P.; et al. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circ. Res. 2011, 109, 894–906. [Google Scholar] [CrossRef]
- Wen, Z.; Zheng, S.; Zhou, C.; Wang, J.; Wang, T. Repair mechanisms of bone marrow mesenchymal stem cells in myocardial infarction. J. Cell Mol. Med. 2011, 15, 1032–1043. [Google Scholar] [CrossRef]
- Ma, T.; Chen, Y.; Chen, Y.; Meng, Q.; Sun, J.; Shao, L.; Yu, Y.; Huang, H.; Hu, Y.; Yang, Z.; et al. MicroRNA-132, Delivered by Mesenchymal Stem Cell-Derived Exosomes, Promote Angiogenesis in Myocardial Infarction. Stem Cells Int. 2018, 2018, 3290372. [Google Scholar] [CrossRef]
- Feng, Y.; Lu, S.H.; Wang, X.; Cui, J.J.; Li, X.; Du, W.J.; Wang, Y.; Li, J.J.; Song, B.Q.; Chen, F.; et al. Biological characteristics of exosomes secreted by human bone marrow mesenchymal stem cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2014, 22, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.; Raimondi, L.; Conigliaro, A.; Salamanna, F.; Carina, V.; De Luca, A.; Bellavia, D.; Alessandro, R.; Fini, M.; Giavaresi, G. Hypoxia-inducible factor 1Alpha may regulate the commitment of mesenchymal stromal cells toward angio-osteogenesis by mirna-675-5P. Cytotherapy 2017, 19, 1412–1425. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.F.; Liao, H.T. Platelet-rich plasma enhances adipose-derived stem cell-mediated angiogenesis in a mouse ischemic hindlimb model. World J. Stem Cells 2018, 10, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Teng, Y.; Ye, Z.; Zhou, Y.; Tan, W.S. The effect of gap junction-mediated transfer of miR-200b on osteogenesis and angiogenesis in a co-culture of MSCs and HUVECs. J. Cell Sci. 2018, 131, jcs216135. [Google Scholar] [CrossRef]
- Luther, K.M.; Haar, L.; McGuinness, M.; Wang, Y.; Lynch Iv, T.L.; Phan, A.; Song, Y.; Shen, Z.; Gardner, G.; Kuffel, G.; et al. Exosomal miR-21a-5p mediates cardioprotection by mesenchymal stem cells. J. Mol. Cell Cardiol. 2018, 119, 125–137. [Google Scholar] [CrossRef]
- Zhang, H.C.; Liu, X.B.; Huang, S.; Bi, X.Y.; Wang, H.X.; Xie, L.X.; Wang, Y.Q.; Cao, X.F.; Lv, J.; Xiao, F.J.; et al. Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev. 2012, 21, 3289–3297. [Google Scholar] [CrossRef]
- Wang, J.A.; Chen, T.L.; Jiang, J.; Shi, H.; Gui, C.; Luo, R.H.; Xie, X.J.; Xiang, M.X.; Zhang, X. Hypoxic preconditioning attenuates hypoxia/reoxygenation-induced apoptosis in mesenchymal stem cells. Acta Pharm. Sin. 2008, 29, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Chen, H.; Zeng, X.; Guo, W.; Jin, Y.; Wang, S.; Tian, R.; Han, Y.; Guo, L.; Han, J.; et al. Efficient lung cancer-targeted drug delivery via a nanoparticle/MSC system. Acta Pharm. Sin. B 2019, 9, 167–176. [Google Scholar] [CrossRef]
- Papaccio, F.; Paino, F.; Regad, T.; Papaccio, G.; Desiderio, V.; Tirino, V. Concise Review: Cancer Cells, Cancer Stem Cells, and Mesenchymal Stem Cells: Influence in Cancer Development. Stem Cells Transl. Med. 2017, 6, 2115–2125. [Google Scholar] [CrossRef]
- Hill, B.S.; Pelagalli, A.; Passaro, N.; Zannetti, A. Tumor-educated mesenchymal stem cells promote pro-metastatic phenotype. Oncotarget 2017, 8, 73296–73311. [Google Scholar] [CrossRef] [Green Version]
- Dostert, G.; Mesure, B.; Menu, P.; Velot, E. How do Mesenchymal Stem Cells Influence or are Influenced by Microenvironment through Extracellular Vesicles Communication? Front. Cell Dev. Biol. 2017, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Vallabhaneni, K.C.; Penfornis, P.; Dhule, S.; Guillonneau, F.; Adams, K.V.; Mo, Y.Y.; Xu, R.; Liu, Y.; Watabe, K.; Vemuri, M.C.; et al. Extracellular vesicles from bone marrow mesenchymal stem/stromal cells transport tumor regulatory microRNA, proteins, and metabolites. Oncotarget 2015, 6, 4953–4967. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ji, S.; Shao, G.; Zhang, J.; Zhao, K.; Wang, Z.; Wu, A. Effect of exosome biomarkers for diagnosis and prognosis of breast cancer patients. Clin. Transl. Oncol. 2018, 20, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Takahara, K.; Ii, M.; Inamoto, T.; Nakagawa, T.; Ibuki, N.; Yoshikawa, Y.; Tsujino, T.; Uchimoto, T.; Saito, K.; Takai, T.; et al. microRNA-145 Mediates the Inhibitory Effect of Adipose Tissue-Derived Stromal Cells on Prostate Cancer. Stem Cells Dev. 2016, 25, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, L.; Ezquer, M.; Lillo-Vera, F.; Pedraza, P.L.; Ortuzar, M.I.; Gonzalez, P.L.; Figueroa-Valdes, A.I.; Cuenca, J.; Ezquer, F.; Khoury, M.; et al. Stem cell exosomes inhibit angiogenesis and tumor growth of oral squamous cell carcinoma. Sci. Rep. 2019, 9, 663. [Google Scholar] [CrossRef]
- Luo, L.; Tang, J.; Nishi, K.; Yan, C.; Dinh, P.U.; Cores, J.; Kudo, T.; Zhang, J.; Li, T.S.; Cheng, K. Fabrication of Synthetic Mesenchymal Stem Cells for the Treatment of Acute Myocardial Infarction in Mice. Circ. Res. 2017, 120, 1768–1775. [Google Scholar] [CrossRef] [Green Version]
- van Dongen, J.A.; Getova, V.; Brouwer, L.A.; Liguori, G.R.; Sharma, P.; Stevens, H.P.; van der Lei, B.; Harmsen, M.C. Adipose tissue-derived extracellular matrix hydrogels as a release platform for secreted paracrine factors. J. Tissue Eng. Regen. Med. 2019, 13, 973–985. [Google Scholar] [CrossRef]
- Ajit, A.; Retnabai, S.T.; Krishnan, L.K. Engineered human adipose-derived stem cells inducing endothelial lineage and angiogenic response. Tissue Eng. Part C Methods 2019, 25, 148–159. [Google Scholar] [CrossRef]
- Afzal, M.R.; Haider, H.; Idris, N.M.; Jiang, S.; Ahmed, R.P.; Ashraf, M. Preconditioning promotes survival and angiomyogenic potential of mesenchymal stem cells in the infarcted heart via NF-kappaB signaling. Antioxid. Redox Signal. 2010, 12, 693–702. [Google Scholar] [CrossRef]
- Yang, F.; Cho, S.W.; Son, S.M.; Bogatyrev, S.R.; Singh, D.; Green, J.J.; Mei, Y.; Park, S.; Bhang, S.H.; Kim, B.S.; et al. Genetic engineering of human stem cells for enhanced angiogenesis using biodegradable polymeric nanoparticles. Proc. Natl. Acad. Sci. USA 2010, 107, 3317–3322. [Google Scholar] [CrossRef]
- Kim, Y.W.; Byzova, T.V. Oxidative stress in angiogenesis and vascular disease. Blood 2014, 123, 625–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, M.P.; Keaney, J.F., Jr. Epigenetic control of angiogenesis via DNA methylation. Circulation 2011, 123, 2916–2918. [Google Scholar] [CrossRef] [PubMed]
- Titushkin, I.A.; Cho, M.R. Controlling cellular biomechanics of human mesenchymal stem cells. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 2090–2093. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Tensegrity-based mechanosensing from macro to micro. Prog. Biophys. Mol. Biol. 2008, 97, 163–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stricker, J.; Falzone, T.; Gardel, M.L. Mechanics of the F-actin cytoskeleton. J. Biomech. 2010, 43, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darling, E.M.; Topel, M.; Zauscher, S.; Vail, T.P.; Guilak, F. Viscoelastic properties of human mesenchymally-derived stem cells and primary osteoblasts, chondrocytes, and adipocytes. J. Biomech. 2008, 41, 454–464. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.; Zhang, Y.; Jing, D.; Shen, Y.; Tang, G.; Huang, S.; Zhao, Z. Mechanobiology of mesenchymal stem cells: Perspective into mechanical induction of MSC fate. Acta Biomater. 2015, 20, 1–9. [Google Scholar] [CrossRef]
- Reilly, G.C.; Engler, A.J. Intrinsic extracellular matrix properties regulate stem cell differentiation. J. Biomech. 2010, 43, 55–62. [Google Scholar] [CrossRef]
- Yim, E.K.; Sheetz, M.P. Force-dependent cell signaling in stem cell differentiation. Stem Cell Res. Ther. 2012, 3, 41. [Google Scholar] [CrossRef]
- Di Cio, S.; Gautrot, J.E. Cell sensing of physical properties at the nanoscale: Mechanisms and control of cell adhesion and phenotype. Acta Biomater. 2016, 30, 26–48. [Google Scholar] [CrossRef]
- Arnsdorf, E.J.; Tummala, P.; Kwon, R.Y.; Jacobs, C.R. Mechanically induced osteogenic differentiation–the role of RhoA, ROCKII and cytoskeletal dynamics. J. Cell Sci. 2009, 122, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Paluch, E.K.; Nelson, C.M.; Biais, N.; Fabry, B.; Moeller, J.; Pruitt, B.L.; Wollnik, C.; Kudryasheva, G.; Rehfeldt, F.; Federle, W. Mechanotransduction: Use the force(s). BMC Biol. 2015, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tytell, J.D.; Ingber, D.E. Mechanotransduction at a distance: Mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 2009, 10, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zu, Y.; Li, J.; Du, S.; Xu, Y.; Zhang, L.; Jiang, L.; Wang, Z.; Chien, S.; Yang, C. Extracellular matrix stiffness dictates Wnt expression through integrin pathway. Sci. Rep. 2016, 6, 20395. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.W.; Swift, J.; Ivanovska, I.; Spinler, K.R.; Buxboim, A.; Discher, D.E. Mechanobiology of bone marrow stem cells: From myosin-II forces to compliance of matrix and nucleus in cell forms and fates. Differentiation 2013, 86, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Polte, T.R.; Eichler, G.S.; Wang, N.; Ingber, D.E. Extracellular matrix controls myosin light chain phosphorylation and cell contractility through modulation of cell shape and cytoskeletal prestress. Am. J. Physiol. Cell Physiol. 2004, 286, C518–C528. [Google Scholar] [CrossRef] [Green Version]
- Swift, J.; Ivanovska, I.L.; Buxboim, A.; Harada, T.; Dingal, P.C.; Pinter, J.; Pajerowski, J.D.; Spinler, K.R.; Shin, J.W.; Tewari, M.; et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 2013, 341, 1240104. [Google Scholar] [CrossRef]
- Plunkett, N.; O’Brien, F.J. Bioreactors in tissue engineering. Technol. Health Care 2011, 19, 55–69. [Google Scholar] [CrossRef] [Green Version]
- Govoni, M.; Berardi, A.C.; Muscari, C.; Campardelli, R.; Bonafe, F.; Guarnieri, C.; Reverchon, E.; Giordano, E.; Maffulli, N.; Della Porta, G. An Engineered Multiphase Three-Dimensional Microenvironment to Ensure the Controlled Delivery of Cyclic Strain and Human Growth Differentiation Factor 5 for the Tenogenic Commitment of Human Bone Marrow Mesenchymal Stem Cells. Tissue Eng. Part A 2017, 23, 811–822. [Google Scholar] [CrossRef]
- Govoni, M.; Lotti, F.; Biagiotti, L.; Lannocca, M.; Pasquinelli, G.; Valente, S.; Muscari, C.; Bonafe, F.; Caldarera, C.M.; Guarnieri, C.; et al. An innovative stand-alone bioreactor for the highly reproducible transfer of cyclic mechanical stretch to stem cells cultured in a 3D scaffold. J. Tissue Eng. Regen. Med. 2014, 8, 787–793. [Google Scholar] [CrossRef]
- Govoni, M.; Muscari, C.; Guarnieri, C.; Giordano, E. Mechanostimulation protocols for cardiac tissue engineering. Biomed. Res. Int. 2013, 2013, 918640. [Google Scholar] [CrossRef] [PubMed]
- Govoni, M.; Muscari, C.; Lovecchio, J.; Guarnieri, C.; Giordano, E. Mechanical Actuation Systems for the Phenotype Commitment of Stem Cell-Based Tendon and Ligament Tissue Substitutes. Stem Cell Rev. 2016, 12, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Pasini, A.; Lovecchio, J.; Ferretti, G.; Giordano, E. Medium Perfusion Flow Improves Osteogenic Commitment of Human Stromal Cells. Stem Cells Int. 2019, 2019, 1304194. [Google Scholar] [CrossRef]
- Govoni, M.; Giordano, E.; Muscari, C.; Caldarera, C.M.; Guarnieri, C. Dynamic Cell Culture in Cardiac Tissue Engineering and Regenerative Medicine. Adult Stem Cell Stand. 2011, 1, 135–147. [Google Scholar]
- Martin, V.; Bettencourt, A. Bone regeneration: Biomaterials as local delivery systems with improved osteoinductive properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 363–371. [Google Scholar] [CrossRef]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef]
- Iaquinta, M.R.; Mazzoni, E.; Manfrini, M.; D’Agostino, A.; Trevisiol, L.; Nocini, R.; Trombelli, L.; Barbanti-Brodano, G.; Martini, F.; Tognon, M. Innovative Biomaterials for Bone Regrowth. Int. J. Mol. Sci. 2019, 20, 618. [Google Scholar] [CrossRef]
- Stanovici, J.; Le Nail, L.R.; Brennan, M.A.; Vidal, L.; Trichet, V.; Rosset, P.; Layrolle, P. Bone regeneration strategies with bone marrow stromal cells in orthopaedic surgery. Curr. Res. Transl. Med. 2016, 64, 83–90. [Google Scholar] [CrossRef]
- Jager, M.; Hernigou, P.; Zilkens, C.; Herten, M.; Fischer, J.; Krauspe, R. Cell therapy in bone-healing disorders. Orthopade 2010, 2, e20. [Google Scholar] [CrossRef]
- Noth, U.; Reichert, J.; Reppenhagen, S.; Steinert, A.; Rackwitz, L.; Eulert, J.; Beckmann, J.; Tingart, M. Cell based therapy for the treatment of femoral head necrosis. Orthopade 2007, 36, 466–471. [Google Scholar] [CrossRef]
- Fortier, L.A.; Potter, H.G.; Rickey, E.J.; Schnabel, L.V.; Foo, L.F.; Chong, L.R.; Stokol, T.; Cheetham, J.; Nixon, A.J. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J. Bone Jt. Surg. Am. 2010, 92, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- Gangji, V.; De Maertelaer, V.; Hauzeur, J.P. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: Five year follow-up of a prospective controlled study. Bone 2011, 49, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, F.; Salamanna, F.; Tschon, M.; Maglio, M.; Nicoli Aldini, N.; Fini, M. Mesenchymal stem cells for tendon healing: What is on the horizon? J. Tissue Eng. Regen. Med. 2017, 11, 3202–3219. [Google Scholar] [CrossRef] [PubMed]
- Salamanna, F.; Sartori, M.; Brodano, G.B.; Griffoni, C.; Martini, L.; Boriani, S.; Fini, M. Mesenchymal Stem Cells for the Treatment of Spinal Arthrodesis: From Preclinical Research to Clinical Scenario. Stem Cells Int. 2017, 2017, 3537094. [Google Scholar] [CrossRef] [PubMed]
- Robbins, M.A.; Haudenschild, D.R.; Wegner, A.M.; Klineberg, E.O. Stem Cells in Spinal Fusion. Glob. Spine J. 2017, 7, 801–810. [Google Scholar] [CrossRef]
- Gan, Y.; Dai, K.; Zhang, P.; Tang, T.; Zhu, Z.; Lu, J. The clinical use of enriched bone marrow stem cells combined with porous beta-tricalcium phosphate in posterior spinal fusion. Biomaterials 2008, 29, 3973–3982. [Google Scholar] [CrossRef]
- Eastlack, R.K.; Garfin, S.R.; Brown, C.R.; Meyer, S.C. Osteocel Plus cellular allograft in anterior cervical discectomy and fusion: Evaluation of clinical and radiographic outcomes from a prospective multicenter study. Spine 2014, 39, E1331–E1337. [Google Scholar] [CrossRef]
- Khashan, M.; Inoue, S.; Berven, S.H. Cell based therapies as compared to autologous bone grafts for spinal arthrodesis. Spine 2013, 38, 1885–1891. [Google Scholar] [CrossRef]
- Ammerman, J.M.; Libricz, J.; Ammerman, M.D. The role of Osteocel Plus as a fusion substrate in minimally invasive instrumented transforaminal lumbar interbody fusion. Clin. Neurol. Neurosurg. 2013, 115, 991–994. [Google Scholar] [CrossRef]
- Kerr, E.J., 3rd; Jawahar, A.; Wooten, T.; Kay, S.; Cavanaugh, D.A.; Nunley, P.D. The use of osteo-conductive stem-cells allograft in lumbar interbody fusion procedures: An alternative to recombinant human bone morphogenetic protein. J. Surg. Orthop. Adv. 2011, 20, 193–197. [Google Scholar]
- Hustedt, J.W.; Jegede, K.A.; Badrinath, R.; Bohl, D.D.; Blizzard, D.J.; Grauer, J.N. Optimal aspiration volume of vertebral bone marrow for use in spinal fusion. Spine J. 2013, 13, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Chauhan, V.; Sharma, S.; Maheshwari, R.; Juyal, A.; Raghuvanshi, S. Evaluation of hydroxyapatite and beta-tricalcium phosphate mixed with bone marrow aspirate as a bone graft substitute for posterolateral spinal fusion. Indian J. Orthop. 2009, 43, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.C.; Tsai, T.T.; Fu, T.S.; Lai, P.L.; Chen, L.H.; Chen, W.J. A comparison of posterolateral lumbar fusion comparing autograft, autogenous laminectomy bone with bone marrow aspirate, and calcium sulphate with bone marrow aspirate: A prospective randomized study. Spine 2009, 34, 2715–2719. [Google Scholar] [CrossRef] [PubMed]
- Kitchel, S.H. A preliminary comparative study of radiographic results using mineralized collagen and bone marrow aspirate versus autologous bone in the same patients undergoing posterior lumbar interbody fusion with instrumented posterolateral lumbar fusion. Spine J. 2006, 6, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Picchi, J.; Trombi, L.; Spugnesi, L.; Barachini, S.; Maroni, G.; Brodano, G.B.; Boriani, S.; Valtieri, M.; Petrini, M.; Magli, M.C. HOX and TALE signatures specify human stromal stem cell populations from different sources. J. Cell Physiol. 2013, 228, 879–889. [Google Scholar] [CrossRef]
- Barbanti Brodano, G.; Terzi, S.; Trombi, L.; Griffoni, C.; Valtieri, M.; Boriani, S.; Magli, M.C. Mesenchymal stem cells derived from vertebrae (vMSCs) show best biological properties. Eur. Spine J. 2013, 22 (Suppl. 6), S979–S984. [Google Scholar] [CrossRef]
- Veronesi, F.; Giavaresi, G.; Tschon, M.; Borsari, V.; Nicoli Aldini, N.; Fini, M. Clinical use of bone marrow, bone marrow concentrate, and expanded bone marrow mesenchymal stem cells in cartilage disease. Stem Cells Dev. 2013, 22, 181–192. [Google Scholar] [CrossRef]
- Salamanna, F.; Contartese, D.; Nicoli Aldini, N.; Barbanti Brodano, G.; Griffoni, C.; Gasbarrini, A.; Fini, M. Bone marrow aspirate clot: A technical complication or a smart approach for musculoskeletal tissue regeneration? J. Cell Physiol. 2018, 233, 2723–2732. [Google Scholar] [CrossRef]
- Salamanna, F.; Cepollaro, S.; Contartese, D.; Giavaresi, G.; Brodano, G.B.; Griffoni, C.; Gasbarrini, A.; Fini, M. Biological Rationale for the Use of Vertebral Whole Bone Marrow in Spinal Surgery. Spine 2018, 43, 1401–1410. [Google Scholar] [CrossRef]
- Yang, D.C.; Yang, M.H.; Tsai, C.C.; Huang, T.F.; Chen, Y.H.; Hung, S.C. Hypoxia inhibits osteogenesis in human mesenchymal stem cells through direct regulation of RUNX2 by TWIST. PLoS ONE 2011, 6, e23965. [Google Scholar] [CrossRef]
- Hung, S.P.; Ho, J.H.; Shih, Y.R.; Lo, T.; Lee, O.K. Hypoxia promotes proliferation and osteogenic differentiation potentials of human mesenchymal stem cells. J. Orthop. Res. 2012, 30, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Della Bella, E.; Pagani, S.; Giavaresi, G.; Capelli, I.; Comai, G.; Donadei, C.; Cappuccilli, M.; La Manna, G.; Fini, M. Uremic Serum Impairs Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stromal Cells. J. Cell Physiol. 2017, 232, 2201–2209. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.J.; Ernst, P.; Wang, Y.; Dekens, M.P.; Kingsley, P.D.; Palis, J.; Korsmeyer, S.J.; Daley, G.Q.; Zon, L.I. cdx4 mutants fail to specify blood progenitors and can be rescued by multiple hox genes. Nature 2003, 425, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Holstege, J.C.; de Graaff, W.; Hossaini, M.; Cardona Cano, S.; Jaarsma, D.; van den Akker, E.; Deschamps, J. Loss of Hoxb8 alters spinal dorsal laminae and sensory responses in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 6338–6343. [Google Scholar] [CrossRef]
- Mallo, M.; Wellik, D.M.; Deschamps, J. Hox genes and regional patterning of the vertebrate body plan. Dev. Biol. 2010, 344, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.X.; Li, Z.Y.; Guo, Z.K.; Guo, Z.K. Easily-handled method to isolate mesenchymal stem cells from coagulated human bone marrow samples. World J. Stem Cells 2015, 7, 1137–1144. [Google Scholar] [CrossRef]
- Salamanna, F.; Giavaresi, G.; Contartese, D.; Bigi, A.; Boanini, E.; Parrilli, A.; Lolli, R.; Gasbarrini, A.; Barbanti Brodano, G.; Fini, M. Effect of strontium substituted ss-TCP associated to mesenchymal stem cells from bone marrow and adipose tissue on spinal fusion in healthy and ovariectomized rat. J. Cell Physiol. 2019, 234, 20046–20056. [Google Scholar] [CrossRef]
- Brown, C.; McKee, C.; Bakshi, S.; Walker, K.; Hakman, E.; Halassy, S.; Svinarich, D.; Dodds, R.; Govind, C.K.; Chaudhry, G.R. Mesenchymal stem cells: Cell therapy and regeneration potential. J. Tissue Eng. Regen. Med. 2019, 13, 1738–1755. [Google Scholar] [CrossRef]

| Animal Species | MSC Origin | Growth Factor | Role | Reference |
|---|---|---|---|---|
| Dog | Bone marrow | PDGF and bFGF | Growth factor and proliferation | [9] |
| Adipose tissue | PRGF, FGF-2 | Proliferation and chondrogenesis | [11,12] | |
| Horse | Bone marrow | IGF-1 | Cell proliferation and collagen and GAG synthesis | [13] |
| Adipose tissue | TGFβ3 | Tenogenic differentiation of equine MSC | [14] | |
| Pig | Bone marrow | VEGF | Angiogenesis | [15] |
| Sheep | Bone marrow | EGF + bFGF + TGFβ | Proliferation, migration and invasion | [16] |
| Animal | N. Subjects | MSC Type | Disease | Treatment | Effects | Ref |
|---|---|---|---|---|---|---|
| Cat | 1 | Spinal | Spinal cord injury | Autologous MSC (7 × 108) + collagen | Significant functional clinical improvement; long melioration | [44] |
| 6 | AdMSC | Chronic kidney | Allogenic MSC (2 × 106 cells; 2–6 weeks) | Long term melioration | [45] | |
| Dog | 130 | Micro fragmented AdMSC | Osteoarthritis | Intra-articular injection | Long-term pain control | [46] |
| Horse | 10 | BM | Cartilage defects | Intra-articular injection (2 × 106 cells) | Increase in repair tissue firmness | [47] |
| 33 | BM | Femorotibial lesions (meniscal, cartilage or ligamentous) | Intra-articular injection (1.5 × 107–2.0 × 107 cells) | Improvement | [48] | |
| Pig | 1 | BM | Model of intervertebral degeneration | Autologous (1 × 106 cells/mL) | Reduction of degenerative process | [49] |
| 8 | AdMSC | Esophagus | Cells implanted on scaffold | Regrowth of esophageal tissue | [50] | |
| 2 | BM | Cutaneous wound healing | Autologous MSC (1.5 × 107 cells) injected intradermally | Regeneration | [51] | |
| Sheep | 10 | BM | Osteoarthritis | Autologous MSC injected intra-articular | Improvement of articular cartilage | [52] |
| 6 | PB-MSCs | Cutaneous wound healing | Injection (1 × 106 cells) intradermally | Skin re-epithelialization | [29] | |
| 1 | AdMSC and BM | Osteoarthritis | Autologous chondrogenic induced Ad and BM cells | Improvement of articular cartilage within 6 weeks post-treatment | [53] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelrazik, H.; Giordano, E.; Barbanti Brodano, G.; Griffoni, C.; De Falco, E.; Pelagalli, A. Substantial Overview on Mesenchymal Stem Cell Biological and Physical Properties as an Opportunity in Translational Medicine. Int. J. Mol. Sci. 2019, 20, 5386. https://doi.org/10.3390/ijms20215386
Abdelrazik H, Giordano E, Barbanti Brodano G, Griffoni C, De Falco E, Pelagalli A. Substantial Overview on Mesenchymal Stem Cell Biological and Physical Properties as an Opportunity in Translational Medicine. International Journal of Molecular Sciences. 2019; 20(21):5386. https://doi.org/10.3390/ijms20215386
Chicago/Turabian StyleAbdelrazik, Heba, Emanuele Giordano, Giovanni Barbanti Brodano, Cristiana Griffoni, Elena De Falco, and Alessandra Pelagalli. 2019. "Substantial Overview on Mesenchymal Stem Cell Biological and Physical Properties as an Opportunity in Translational Medicine" International Journal of Molecular Sciences 20, no. 21: 5386. https://doi.org/10.3390/ijms20215386
APA StyleAbdelrazik, H., Giordano, E., Barbanti Brodano, G., Griffoni, C., De Falco, E., & Pelagalli, A. (2019). Substantial Overview on Mesenchymal Stem Cell Biological and Physical Properties as an Opportunity in Translational Medicine. International Journal of Molecular Sciences, 20(21), 5386. https://doi.org/10.3390/ijms20215386