Blood Platelet Adenosine Receptors as Potential Targets for Anti-Platelet Therapy
Abstract
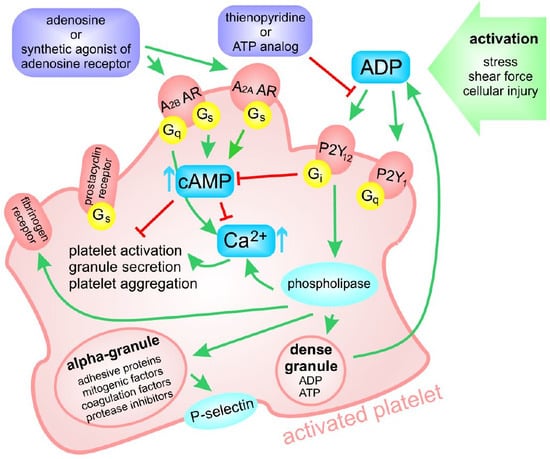
:1. Introduction
2. The Classification, Distribution, and Signaling of Adenosine Receptors
3. Adenosine Receptor Agonists—Structure, Chemical Properties, and Known Effects on Platelet Function
3.1. Adenosine Derivatives
3.1.1. Adenosine Derivatives with Substituents at C1 to C8 Positions
2-chloroadanosine
Regadenoson
Binodenoson
PSB Family
MRE0094
CV1808
AMP597
3.1.2. Adenosine Derivatives with Substituents at C1′ to C5′ Positions
NECA
3.1.3. Compounds with Substituents at C1 to C8 and C1′ to C5′ Positions
CGS 21680
HE-NECA
UK-432094
3.2. Non-Adenosine Compounds
3.2.1 BAY 60-6583
3.2.2 LUF5834 and LUF5835
4. Dual Therapy
5. Conclusions
Funding
Conflicts of Interest
Abbreviations
| ADP | Adenosine Diphosphate |
| AR | Adenosine Receptor |
| cAMP | Cyclic Adenosine Monophosphate |
| CHO | Chinese Hamster Ovary Cells |
| EC50 | Half Maximal Effective Concentration |
| IC50 | Half Maximal Inhibitory Concentration |
| KO | Knock-Out |
| FDA | Food and Drug Administration |
| HE-NECA | 2-Hexynyl-5′-(N-ethylcarboxamido)adenosine |
| IUPAC | International Union of Pure and Applied Chemistry |
| NECA | 5′-(N-ethylcarboxamido)adenosine |
| PRP | Platelet-Rich Plasma |
References
- Kaplan, Z.S.; Jackson, S.P. The role of platelets in atherothrombosis. Hematology Am. Soc. Hematol. Educ. Program 2011, 2011, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Kupka, D.; Sibbing, D. P2Y12 receptor inhibitors: An evolution in drug design to prevent arterial thrombosis. Expert Opin. Drug Metab. Toxicol. 2018, 14, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Patrono, C.; Morais, J.; Baigent, C.; Collet, J.P.; Fitzgerald, D.; Halvorsen, S.; Rocca, B.; Siegbahn, A.; Storey, R.F.; Vilahur, G. Antiplatelet Agents for the Treatment and Prevention of Coronary Atherothrombosis. J. Am. Coll. Cardiol. 2017, 70, 1760–1776. [Google Scholar] [CrossRef] [PubMed]
- Patrono, C.; Andreotti, F.; Arnesen, H.; Badimon, L.; Baigent, C.; Collet, J.P.; De Caterina, R.; Gulba, D.; Huber, K.; Husted, S.; et al. Antiplatelet agents for the treatment and prevention of atherothrombosis. Eur. Heart J. 2011, 32, 2922–2932. [Google Scholar] [CrossRef] [PubMed]
- van Giezen, J.J.; Humphries, R.G. Preclinical and clinical studies with selective reversible direct P2Y12 antagonists. Semin. Thromb. Hemost. 2005, 31, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Angiolillo, D.J.; Rollini, F.; Storey, R.F.; Bhatt, D.L.; James, S.; Schneider, D.J.; Sibbing, D.; So, D.Y.F.; Trenk, D.; Alexopoulos, D.; et al. International Expert Consensus on Switching Platelet P2Y12 Receptor-Inhibiting Therapies. Circulation 2017, 136, 1955–1975. [Google Scholar] [CrossRef]
- Tyler, J.M.; Burris, R.J.; Seto, A.H. Why we need intravenous antiplatelet agents. Future Cardiology 2016, 12, 553–561. [Google Scholar] [CrossRef]
- Delaney, M.K.; Kim, K.; Estevez, B.; Xu, Z.; Stojanovic-Terpo, A.; Shen, B.; Ushio-Fukai, M.; Cho, J.; Du, X. Differential Roles of the NADPH-Oxidase 1 and 2 in Platelet Activation and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 846–854. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.I.; Siddiqui, F.M.; Goldstein, J.N.; Cox, M.; Xian, Y.; Matsouaka, R.A.; Heidenreich, P.A.; Peterson, E.D.; Bhatt, D.L.; Fonarow, G.C.; et al. Association Between Previous Use of Antiplatelet Therapy and Intracerebral Hemorrhage Outcomes. Stroke 2017, 48, 1810–1817. [Google Scholar] [CrossRef]
- Rozalski, M.; Boncler, M.; Luzak, B.; Watala, C. Genetic factors underlying differential blood platelet sensitivity to inhibitors. Pharmacol. Rep. 2005, 57, 1–13. [Google Scholar]
- Chen, J.F.; Eltzschig, H.K.; Fredholm, B.B. Adenosine receptors as drug targets--what are the challenges? Nat. Rev. Drug Discov. 2013, 12, 265–286. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B. Adenosine receptors as drug targets. Exp. Cell Res. 2010, 316, 1284–1288. [Google Scholar] [CrossRef] [Green Version]
- Johnston-Cox, H.A.; Ravid, K. Adenosine and blood platelets. Purinergic Signal 2011, 7, 357–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston-Cox, H.; Koupenova, M.; Yang, D.; Corkey, B.; Gokce, N.; Farb, M.G.; LeBrasseur, N.; Ravid, K. The A2b adenosine receptor modulates glucose homeostasis and obesity. PLoS ONE 2012, 7, e40584. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.; Brito, R.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Adenosine Receptors: Expression, Function and Regulation. Int. J. Mol. Sci. 2014, 15, 2024–2052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gessi, S.; Varani, K.; Merighi, S.; Ongini, E.; Borea, P.A. A(2A) adenosine receptors in human peripheral blood cells. Br. J. Pharmacol. 2000, 129, 2–11. [Google Scholar] [CrossRef]
- Wang, J.; Miao, Y. Mechanistic Insights into Specific G Protein Interactions with Adenosine Receptors. J. Phys. Chem. B 2019, 123, 6462–6473. [Google Scholar] [CrossRef]
- Koupenova, M.; Ravid, K. Biology of Platelet Purinergic Receptors and Implications for Platelet Heterogeneity. Front. Pharmacol. 2018, 9, 37. [Google Scholar] [CrossRef] [Green Version]
- Poulsen, S.A.; Quinn, R.J. Adenosine receptors: New opportunities for future drugs. Bioorg. Med. Chem. 1998, 6, 619–641. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Cunha, R.A.; Svenningsson, P. Pharmacology of adenosine A2A receptors and therapeutic applications. Curr. Top. Med. Chem. 2003, 3, 413–426. [Google Scholar] [CrossRef]
- Cacciari, B.; Pastorin, G.; Bolcato, C.; Spalluto, G.; Bacilieri, M.; Moro, S. A2B adenosine receptor antagonists: Recent developments. Mini Rev. Med. Chem. 2005, 5, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Amisten, S.; Braun, O.O.; Bengtsson, A.; Erlinge, D. Gene expression profiling for the identification of G-protein coupled receptors in human platelets. Thromb. Res. 2007, 122, 47–57. [Google Scholar] [CrossRef] [PubMed]
- .Yang, D.; Chen, H.; Koupenova, M.; Carroll, S.H.; Eliades, A.; Freedman, J.E.; Toselli, P.; Ravid, K. A new role for the A2b adenosine receptor in regulating platelet function. J. Thromb. Haemost. 2010, 8, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Cristalli, G.; Lambertucci, C.; Taffi, S.; Vittori, S.; Volpini, R. Medicinal chemistry of adenosine A2A receptor agonists. Curr. Top. Med. Chem. 2003, 3, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Federico, S.; Spalluto, G. Therapeutic potential of A2 and A3 adenosine receptor: A review of novel patented ligands. Expert Opin. Ther. Pat. 2012, 22, 369–390. [Google Scholar] [CrossRef] [PubMed]
- .Ongini, E.; Fredholm, B.B. Pharmacology of adenosine A2A receptors. Trends Pharmacol. Sci. 1996, 17, 364–372. [Google Scholar] [CrossRef]
- Parkman, R.; Gelfand, E.W.; Rosen, F.S.; Sanderson, A.; Hirschhorn, R. Severe Combined Immunodeficiency and Adenosine Deaminase Deficiency. New Engl. J. Med. 1975, 292, 714–719. [Google Scholar] [CrossRef]
- Farrukh, S.; Safiah, S.; Hasan, H.; Agha, M.; Rehan, K.; Sulaiman Al, G.; Hasan, A.; Ghuzayel, A.-D.; Riad El, F.; Rand, A. Thrombocytopenia in a Patient with Severe Combined Immune Deficiency: An Unusual Cause. Int. J. Allergy Medicat. 2018, 4. [Google Scholar] [CrossRef]
- Lee, C.H.; Evans, S.P.; Rozenberg, M.C.; Bagnara, A.S.; Ziegler, J.B.; Van der Weyden, M.B. In vitro platelet abnormality in adenosine deaminase deficiency and severe combined immunodeficiency. Blood 1979, 53, 465–471. [Google Scholar] [CrossRef] [Green Version]
- Wijten, P.; van Holten, T.; Woo, L.L.; Bleijerveld, O.B.; Roest, M.; Heck, A.J.; Scholten, A. High precision platelet releasate definition by quantitative reversed protein profiling--brief report. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1635–1638. [Google Scholar] [CrossRef]
- Burkhart, J.M.; Vaudel, M.; Gambaryan, S.; Radau, S.; Walter, U.; Martens, L.; Geiger, J.; Sickmann, A.; Zahedi, R.P. The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood 2012, 120, e73–e82. [Google Scholar] [CrossRef] [Green Version]
- Ohlmann, P.; Lecchi, A.; El-Tayeb, A.; Muller, C.E.; Cattaneo, M.; Gachet, C. The platelet P2Y(12) receptor under normal and pathological conditions. Assessment with the radiolabeled selective antagonist [(3)H]PSB-0413. Purinergic Signal. 2013, 9, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.E.; Scior, T. Adenosine receptors and their modulators. Pharm. Acta Helv. 1993, 68, 77–111. [Google Scholar] [CrossRef]
- Paul, S.; Feoktistov, I.; Hollister, A.S.; Robertson, D.; Biaggioni, I. Adenosine inhibits the rise in intracellular calcium and platelet aggregation produced by thrombin: Evidence that both effects are coupled to adenylate cyclase. Mol. Pharmacol. 1990, 37, 870–875. [Google Scholar] [PubMed]
- Ledent, C.; Vaugeois, J.M.; Schiffmann, S.N.; Pedrazzini, T.; El Yacoubi, M.; Vanderhaeghen, J.J.; Costentin, J.; Heath, J.K.; Vassart, G.; Parmentier, M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature 1997, 388, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Livingston, M.; Heaney, L.G.; Ennis, M. Adenosine, inflammation and asthma—a review. Inflamm. Res. 2004, 53, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.E.; Jacobson, K.A. Recent developments in adenosine receptor ligands and their potential as novel drugs. Biochim. Biophys. Acta 2011, 1808, 1290–1308. [Google Scholar] [CrossRef] [Green Version]
- Born, G.V. Strong Inhibition by 2-Chloroadenosine of the Aggregation of Blood Platelets by Adenosine Diphosphate. Nature 1964, 202, 95–96. [Google Scholar] [CrossRef]
- Born, G.V.; Honour, A.J.; Mitchell, J.R. Inhibition by Adenosine and by 2-Chloroadenosine of the Formation and Embolization of Platelet Thrombi. Nature 1964, 202, 761–765. [Google Scholar] [CrossRef]
- Born, G.V. Observations on the change in shape of blood platelets brought about by adenosine diphosphate. J. Physiol. 1970, 209, 487–511. [Google Scholar] [CrossRef] [Green Version]
- Born, G.V.; Dearnley, R.; Foulks, J.G.; Sharp, D.E. Quantification of the morphological reaction of platelets to aggregating agents and of its reversal by aggregation inhibitors. J. Physiol. 1978, 280, 193–212. [Google Scholar] [CrossRef] [PubMed]
- Quattrin, S.; Genovese, A.; Cirillo, R.; Formisano, S.; Marone, G. Functional and biochemical evidence of a specific adenosine A2/Ra receptor on human platelets. Ric. Clin. Lab. 1988, 18, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Milton, J.G.; Frojmovic, M.M.; Tang, S.S.; White, J.G. Spontaneous platelet aggregation in a hereditary giant platelet syndrome (MPS). Am. J. Pathol. 1984, 114, 336–345. [Google Scholar] [PubMed]
- Saniabadi, A.R.; Takeich, S.; Yukawa, N.; Nakajima, Y.; Umemura, K.; Nakashima, M. Apo E4/3-rich remnant lipoproteins and platelet aggregation: A case report. Thromb. Haemost. 1998, 79, 878–879. [Google Scholar]
- Haslam, R.J.; Lynham, J.A. Activation and inhibition of blood platelet adenylate cyclase by adenosine or by 2-chloroadenosine. Life Sci. II 1972, 11, 1143–1154. [Google Scholar] [CrossRef]
- Ukena, D.; Bohme, E.; Schwabe, U. Effects of several 5’-carboxamide derivatives of adenosine on adenosine receptors of human platelets and rat fat cells. N-S Arch. Pharmacol. 1984, 327, 36–42. [Google Scholar] [CrossRef]
- Dawicki, D.D.; Agarwal, K.C.; Parks, R.E., Jr. Potentiation of the antiplatelet action of adenosine in whole blood by dipyridamole or dilazep and the cAMP phosphodiesterase inhibitor, RA 233. Thromb. Res. 1986, 43, 161–175. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Tandon, N.N.; Greco, N.J.; Cusack, N.J.; Jamieson, G.A. Evaluation of the binding to fixed platelets of agonists and antagonists of ADP-induced aggregation. Thromb. Haemost. 1989, 62, 1103–1106. [Google Scholar] [CrossRef]
- Fein, T.; Schulze, E.; Bar, J.; Schwabe, U. Purification and characterization of an adenotin-like adenosine binding protein from human platelets. N-S Arch. Pharmacol. 1994, 349, 374–380. [Google Scholar] [CrossRef]
- Mares, P. Anticonvulsant action of 2-chloroadenosine against pentetrazol-induced seizures in immature rats is due to activation of A1 adenosine receptors. J. Neural Transm. (Vienna) 2010, 117, 1269–1277. [Google Scholar] [CrossRef]
- Saze, Z.; Schuler, P.J.; Hong, C.S.; Cheng, D.; Jackson, E.K.; Whiteside, T.L. Adenosine production by human B cells and B cell-mediated suppression of activated T cells. Blood 2013, 122, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Patinha, D.; Afonso, J.; Sousa, T.; Morato, M.; Albino-Teixeira, A. Activation of adenosine receptors improves renal antioxidant status in diabetic Wistar but not SHR rats. Ups. J. Med. Sci. 2014, 119, 10–18. [Google Scholar] [CrossRef] [PubMed]
- .Dubey, R.K.; Fingerle, J.; Gillespie, D.G.; Mi, Z.; Rosselli, M.; Imthurn, B.; Jackson, E.K. Adenosine Attenuates Human Coronary Artery Smooth Muscle Cell Proliferation by Inhibiting Multiple Signaling Pathways That Converge on Cyclin D. Hypertension 2015, 66, 1207–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gough, G.; Maguire, M.H.; Michal, F. 2-chloroadenosine 5’-phosphate and 2-chloroadenosine 5’-diphosphate, pharmacologically active nucleotide analogs. J. Med. Chem. 1969, 12, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Li, Z.; Baker, S.P.; Lasley, R.D.; Meyer, S.; Elzein, E.; Palle, V.; Zablocki, J.A.; Blackburn, B.; Belardinelli, L. Novel short-acting A2A adenosine receptor agonists for coronary vasodilation: Inverse relationship between affinity and duration of action of A2A agonists. J. Pharmacol. Exp. Ther. 2001, 298, 209–218. [Google Scholar]
- Lieu, H.D.; Shryock, J.C.; von Mering, G.O.; Gordi, T.; Blackburn, B.; Olmsted, A.W.; Belardinelli, L.; Kerensky, R.A. Regadenoson, a selective A2A adenosine receptor agonist, causes dose-dependent increases in coronary blood flow velocity in humans. J. Nucl. Cardiol. 2007, 14, 514–520. [Google Scholar] [CrossRef]
- Glover, D.K.; Ruiz, M.; Yang, J.Y.; Koplan, B.A.; Allen, T.R.; Smith, W.H.; Watson, D.D.; Barrett, R.J.; Beller, G.A. Pharmacological stress thallium scintigraphy with 2-cyclohexylmethylidenehydrazinoadenosine (WRC-0470). A novel, short-acting adenosine A2A receptor agonist. Circulation 1996, 94, 1726–1732. [Google Scholar] [CrossRef]
- Firschke, C.; Lindner, J.R.; Goodman, N.C.; Skyba, D.M.; Wei, K.; Kaul, S. Myocardial contrast echocardiography in acute myocardial infarction using aortic root injections of microbubbles in conjunction with harmonic imaging: Potential application in the cardiac catheterization laboratory. J. Am. Coll. Cardiol. 1997, 29, 207–216. [Google Scholar] [CrossRef]
- Barrett, R.J.; Lamson, M.J.; Johnson, J.; Smith, W.B. Pharmacokinetics and safety of binodenoson after intravenous dose escalation in healthy volunteers. J. Nucl. Cardiol. 2005, 12, 166–171. [Google Scholar] [CrossRef]
- Udelson, J.E.; Heller, G.V.; Wackers, F.J.; Chai, A.; Hinchman, D.; Coleman, P.S.; Dilsizian, V.; DiCarli, M.; Hachamovitch, R.; Johnson, J.R.; et al. Randomized, controlled dose-ranging study of the selective adenosine A2A receptor agonist binodenoson for pharmacological stress as an adjunct to myocardial perfusion imaging. Circulation 2004, 109, 457–464. [Google Scholar] [CrossRef]
- Murray, J.J.; Weiler, J.M.; Schwartz, L.B.; Busse, W.W.; Katial, R.K.; Lockey, R.F.; McFadden, E.R., Jr.; Pixton, G.C.; Barrett, R.J. Safety of binodenoson, a selective adenosine A2A receptor agonist vasodilator pharmacological stress agent, in healthy subjects with mild intermittent asthma. Circ. Cardiovasc. Imaging 2009, 2, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Atta-ur-Rahman; Choudhary, M.I. Advancement in Developing New Adenosine Receptors Ligands. In Frontiers in Cardiovascular Drug Discovery: Volume 4; Bentham Science Publishers: Sharjah, UAE, 2019; pp. 16–64. [Google Scholar] [CrossRef]
- El-Tayeb, A.; Michael, S.; Abdelrahman, A.; Behrenswerth, A.; Gollos, S.; Nieber, K.; Muller, C.E. Development of Polar Adenosine A2A Receptor Agonists for Inflammatory Bowel Disease: Synergism with A2B Antagonists. ACS Med. Chem. Lett. 2011, 2, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Deuther, C.; Peter, B.; Martin, E.L.; Matthias, J.B.; Sven, E.; Jürgen, S.; Gennady, G.Y.; Christa, E.M.; Alexander Pfeifer, T.G.; Saskia, S.; et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 2014, 516, 395–399. [Google Scholar]
- Fuentes, E.; Badimon, L.; Caballero, J.; Padro, T.; Vilahur, G.; Alarcon, M.; Perez, P.; Palomo, I. Protective mechanisms of adenosine 5’-monophosphate in platelet activation and thrombus formation. Thromb. Haemost. 2014, 111, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Volpini, R.; Costanzi, S.; Lambertucci, C.; Portino, F.R.; Taffi, S.; Vittori, S.; Zablocki, J.A.; Klotz, K.N.; Cristalli, G. Adenosine receptor agonists: Synthesis and binding affinity of 2-(aryl)alkylthioadenosine derivatives. Arkivoc 2004, 2004, 301–311. [Google Scholar]
- .El-Tayeb, A.; Iqbal, J.; Behrenswerth, A.; Romio, M.; Schneider, M.; Zimmermann, H.; Schrader, J.; Muller, C.E. Nucleoside-5’-monophosphates as prodrugs of adenosine A2A receptor agonists activated by ecto-5’-nucleotidase. J. Med. Chem. 2009, 52, 7669–7677. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Fuentes, M.; Caballero, J.; Palomo, I.; Hinz, S.; El-Tayeb, A.; Muller, C.E. Adenosine A2A receptor agonists with potent antiplatelet activity. Platelets 2018, 29, 292–300. [Google Scholar] [CrossRef]
- .Ueeda, M.; Thompson, R.D.; Arroyo, L.H.; Olsson, R.A. 2-aralkoxyadenosines: Potent and selective agonists at the coronary artery A2 adenosine receptor. J. Med. Chem. 1991, 34, 1340–1344. [Google Scholar] [CrossRef]
- Nguyen, D.K.; Montesinos, M.C.; Williams, A.J.; Kelly, M.; Cronstein, B.N. Th1 cytokines regulate adenosine receptors and their downstream signaling elements in human microvascular endothelial cells. J. Immunol. 2003, 171, 3991–3998. [Google Scholar]
- Reiss, A.B.; Rahman, M.M.; Chan, E.S.; Montesinos, M.C.; Awadallah, N.W.; Cronstein, B.N. Adenosine A2A receptor occupancy stimulates expression of proteins involved in reverse cholesterol transport and inhibits foam cell formation in macrophages. J. Leukoc. Biol. 2004, 76, 727–734. [Google Scholar] [CrossRef]
- Desai, A.; Victor-Vega, C.; Gadangi, S.; Montesinos, M.C.; Chu, C.C.; Cronstein, B.N. Adenosine A2A receptor stimulation increases angiogenesis by down-regulating production of the antiangiogenic matrix protein thrombospondin 1. Mol. Pharmacol. 2005, 67, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Victor-Vega, C.; Desai, A.; Montesinos, M.C.; Cronstein, B.N. Adenosine A2A receptor agonists promote more rapid wound healing than recombinant human platelet-derived growth factor (Becaplermin gel). Inflammation 2002, 26, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Valls, M.D.; Cronstein, B.N.; Montesinos, M.C. Adenosine receptor agonists for promotion of dermal wound healing. Biochem. Pharmacol. 2009, 77, 1117–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ukena, D.; Olsson, R.A.; Daly, J.W. Definition of subclasses of adenosine receptors associated with adenylate cyclase: Interaction of adenosine analogs with inhibitory A1 receptors and stimulatory A2 receptors. Can. J. Physiol. Pharmacol. 1987, 65, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Gurden, M.F.; Coates, J.; Ellis, F.; Evans, B.; Foster, M.; Hornby, E.; Kennedy, I.; Martin, D.P.; Strong, P.; Vardey, C.J.; et al. Functional characterization of three adenosine receptor types. Br. J. Pharmacol. 1993, 109, 693–698. [Google Scholar] [CrossRef] [Green Version]
- Brackett, L.E.; Daly, J.W. Functional characterization of the A2b adenosine receptor in NIH 3T3 fibroblasts. Biochem. Pharmacol. 1994, 47, 801–814. [Google Scholar] [CrossRef]
- Cunha, R.A.; Johansson, B.; Constantino, M.D.; Sebastiao, A.M.; Fredholm, B.B. Evidence for high-affinity binding sites for the adenosine A2A receptor agonist [3H] CGS 21680 in the rat hippocampus and cerebral cortex that are different from striatal A2A receptors. N-S Arch. Pharmacol. 1996, 353, 261–271. [Google Scholar] [CrossRef]
- Ravyn, V.; Bostwick, J.R. Functional coupling of the Galpha(olf) variant XLGalpha(olf) with the human adenosine A2A receptor. J. Recept. Signal. Transduct. Res. 2006, 26, 241–258. [Google Scholar] [CrossRef]
- White, P.J.; Rose’Meyer, R.B.; Hope, W. Functional characterization of adenosine receptors in the nucleus tractus solitarius mediating hypotensive responses in the rat. Br. J. Pharmacol. 1996, 117, 305–308. [Google Scholar] [CrossRef] [Green Version]
- Tostes, R.C.; Giachini, F.R.; Carneiro, F.S.; Leite, R.; Inscho, E.W.; Webb, R.C. Determination of adenosine effects and adenosine receptors in murine corpus cavernosum. J. Pharmacol. Exp. Ther. 2007, 322, 678–685. [Google Scholar] [CrossRef]
- Nalos, M.; Huang, S.; Sluyter, R.; Khan, A.; Santner-Nanan, B.; Nanan, R.; McLean, A.S. “Host tissue damage” signal ATP impairs IL-12 and IFNgamma secretion in LPS stimulated whole human blood. Intensive Care Med. 2008, 34, 1891–1897. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Mokelke, E.A.; Neeb, Z.P.; Alloosh, M.; Edwards, J.M.; Sturek, M. Adenosine receptor regulation of coronary blood flow in Ossabaw miniature swine. J. Pharmacol. Exp. Ther. 2010, 335, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Yip, K.H.; Lau, H.Y.; Wise, H. Reciprocal modulation of anti-IgE induced histamine release from human mast cells by A(1) and A(2B) adenosine receptors. Br. J. Pharmacol. 2011, 164, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Smits, G.J.; McVey, M.; Cox, B.F.; Perrone, M.H.; Clark, K.L. Cardioprotective effects of the novel adenosine A1/A2 receptor agonist AMP 579 in a porcine model of myocardial infarction. J. Pharmacol. Exp. Ther. 1998, 286, 611–618. [Google Scholar] [PubMed]
- Clark, K.L.; Merkel, L.; Zannikos, P.; Kelley, M.F.; Boutouyrie, B.; Perrone, M.H. AMP 579, a Novel Adenosine Agonist for the Treatment of Acute Myocardial Infarction. Cardiovasc. Drug Rev. 2000, 18, 183–210. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Yang, X.M.; Walker, S.; Forster, K.; Cohen, M.V.; Krieg, T.; Downey, J.M. AMP579 is revealed to be a potent A2b-adenosine receptor agonist in human 293 cells and rabbit hearts. Basic Res. Cardiol. 2010, 105, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Cusack, N.J.; Hourani, S.M. 5’-N-ethylcarboxamidoadenosine: A potent inhibitor of human platelet aggregation. Br. J. Pharmacol. 1981, 72, 443–447. [Google Scholar] [CrossRef]
- Huttemann, E.; Ukena, D.; Lenschow, V.; Schwabe, U. Ra adenosine receptors in human platelets. Characterization by 5′-N-ethylcarboxamido[3 H]adenosine binding in relation to adenylate cyclase activity. N-S Arch. Pharmacol. 1984, 325, 226–233. [Google Scholar]
- Lohse, M.J.; Klotz, K.N.; Schwabe, U. Mechanism of A2 adenosine receptor activation. I. Blockade of A2 adenosine receptors by photoaffinity labeling. Mol. Pharmacol. 1991, 39, 517–523. [Google Scholar]
- Cristalli, G.; Vittori, S.; Thompson, R.D.; Padgett, W.L.; Shi, D.; Daly, J.W.; Olsson, R.A. Inhibition of platelet aggregation by adenosine receptor agonists. N-S Arch. Pharmacol. 1994, 349, 644–650. [Google Scholar] [CrossRef]
- Volpini, R.; Dal Ben, D.; Lambertucci, C.; Taffi, S.; Vittori, S.; Klotz, K.-N.; Cristalli, G. N 6 -Methoxy-2-alkynyladenosine Derivatives as Highly Potent and Selective Ligands at the Human A 3 Adenosine Receptor. J. Med. Chem. 2007, 50, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- de Zwart, M.; Link, R.; von Frijtag Drabbe Kunzel, J.K.; Cristalli, G.; Jacobson, K.A.; Townsend-Nicholson, A.; AP, I.J. A functional screening of adenosine analogues at the adenosine A2B receptor: A search for potent agonists. Nucleos Nucleot 1998, 17, 969–985. [Google Scholar] [CrossRef] [PubMed]
- Feoktistov, I.; Biaggioni, I. Characterization of adenosine receptors in human erythroleukemia cells and platelets: Further evidence for heterogeneity of adenosine A2 receptor subtypes. Mol. Pharmacol. 1993, 43, 909–914. [Google Scholar] [PubMed]
- van Calker, D.; Muller, M.; Hamprecht, B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J. Neurochem. 1979, 33, 999–1005. [Google Scholar] [CrossRef]
- Berk, M.; Plein, H.; Ferreira, D.; Jersky, B. Blunted adenosine A2a receptor function in platelets in patients with major depression. Eur. Neuropsychopharmacol. 2001, 11, 183–186. [Google Scholar] [CrossRef]
- Linden, M.D.; Barnard, M.R.; Frelinger, A.L.; Michelson, A.D.; Przyklenk, K. Effect of adenosine A2 receptor stimulation on platelet activation-aggregation: Differences between canine and human models. Thromb. Res. 2008, 121, 689–698. [Google Scholar] [CrossRef]
- .Darbousset, R.; Delierneux, C.; Mezouar, S.; Hego, A.; Lecut, C.; Guillaumat, I.; Riederer, M.A.; Evans, R.J.; Dignat-George, F.; Panicot-Dubois, L.; et al. P2X1 expressed on polymorphonuclear neutrophils and platelets is required for thrombosis in mice. Blood 2014, 124, 2575–2585. [Google Scholar] [CrossRef] [Green Version]
- Cristalli, G.; Volpini, R.; Vittori, S.; Camaioni, E.; Monopoli, A.; Conti, A.; Dionisotti, S.; Zocchi, C.; Ongini, E. 2-Alkynyl derivatives of adenosine-5’-N-ethyluronamide: Selective A2 adenosine receptor agonists with potent inhibitory activity on platelet aggregation. J. Med. Chem. 1994, 37, 1720–1726. [Google Scholar] [CrossRef]
- Sandoli, D.; Chiu, P.J.; Chintala, M.; Dionisotti, S.; Ongini, E. In vivo and ex vivo effects of adenosine A1 and A2 receptor agonists on platelet aggregation in the rabbit. Eur. J. Pharmacol. 1994, 259, 43–49. [Google Scholar] [CrossRef]
- Varani, K.; Portaluppi, F.; Merighi, S.; Ongini, E.; Belardinelli, L.; Borea, P.A. Caffeine alters A2A adenosine receptors and their function in human platelets. Circulation 1999, 99, 2499–2502. [Google Scholar] [CrossRef]
- Varani, K.; Portaluppi, F.; Gessi, S.; Merighi, S.; Ongini, E.; Belardinelli, L.; Borea, P.A. Dose and time effects of caffeine intake on human platelet adenosine A(2A) receptors: Functional and biochemical aspects. Circulation 2000, 102, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Przygodzki, T.; Wolska, N.; Talar, M.; Polak, D.; Gapinska, M.; Watala, C. Comparison of different microscopy approaches to quantification of inhibitory effect on thrombus formation under flow conditions by the example of adenosine receptor agonist HE-NECA. J. Pharmacol. Toxicol. Methods 2018, 94, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Panzacchi, G.; Demarchi, B.; Busca, G.; Protasoni, G.; Golin, R.; Stella, A. Effects of adenosine receptor agonists on renal function in anaesthetized rats. J. Hypertens. 1997, 15, 1785–1789. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.A.; Constantino, M.D.; Ribeiro, J.A. ZM241385 is an antagonist of the facilitatory responses produced by the A2A adenosine receptor agonists CGS21680 and HENECA in the rat hippocampus. Br. J. Pharmacol. 1997, 122, 1279–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagpure, B.V.; Bian, J.S. Hydrogen sulfide inhibits A2A adenosine receptor agonist induced beta-amyloid production in SH-SY5Y neuroblastoma cells via a cAMP dependent pathway. Plos ONE 2014, 9, e88508. [Google Scholar] [CrossRef]
- Varani, K.; Gessi, S.; Merighi, S.; Iannotta, V.; Cattabriga, E.; Spisani, S.; Cadossi, R.; Borea, P.A. Effect of low frequency electromagnetic fields on A2A adenosine receptors in human neutrophils. Br. J. Pharmacol. 2002, 136, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wu, H.; Katritch, V.; Han, G.W.; Jacobson, K.A.; Gao, Z.G.; Cherezov, V.; Stevens, R.C. Structure of an agonist-bound human A2A adenosine receptor. Science 2011, 332, 322–327. [Google Scholar] [CrossRef]
- Astrand, A.B.; Lamm Bergstrom, E.; Zhang, H.; Borjesson, L.; Soderdahl, T.; Wingren, C.; Jansson, A.H.; Smailagic, A.; Johansson, C.; Bladh, H.; et al. The discovery of a selective and potent A2a agonist with extended lung retention. Pharmacol Res. Perspect 2015, 3, e00134. [Google Scholar] [CrossRef]
- Boncler, M.; Wzorek, J.; Wolska, N.; Polak, D.; Watala, C.; Rozalski, M. Adenosine receptor agonists deepen the inhibition of platelet aggregation by P2Y12 antagonists. Vascul. Pharmacol. 2019, 113, 47–56. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lyman, E. Agonist dynamics and conformational selection during microsecond simulations of the A(2A) adenosine receptor. Biophys. J. 2012, 102, 2114–2120. [Google Scholar] [CrossRef]
- Lebon, G.; Edwards, P.C.; Leslie, A.G.; Tate, C.G. Molecular Determinants of CGS21680 Binding to the Human Adenosine A2A Receptor. Mol. Pharmacol. 2015, 87, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Van Eps, N.; Zimmer, M.; Ernst, O.P.; Prosser, R.S. Activation of the A2A adenosine G-protein-coupled receptor by conformational selection. Nature 2016, 533, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Patel, R.; Lyman, E. Ligand-dependent cholesterol interactions with the human A(2A) adenosine receptor. Chem. Phys. Lipids 2013, 169, 39–45. [Google Scholar] [CrossRef] [PubMed]
- van der Hoeven, D.; Wan, T.C.; Gizewski, E.T.; Kreckler, L.M.; Maas, J.E.; Van Orman, J.; Ravid, K.; Auchampach, J.A. A role for the low-affinity A2B adenosine receptor in regulating superoxide generation by murine neutrophils. J. Pharmacol. Exp. Ther. 2011, 338, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Eckle, T.; Krahn, T.; Grenz, A.; Kohler, D.; Mittelbronn, M.; Ledent, C.; Jacobson, M.A.; Osswald, H.; Thompson, L.F.; Unertl, K.; et al. Cardioprotection by ecto-5’-nucleotidase (CD73) and A2B adenosine receptors. Circulation 2007, 115, 1581–1590. [Google Scholar] [CrossRef]
- Xi, J.; McIntosh, R.; Shen, X.; Lee, S.; Chanoit, G.; Criswell, H.; Zvara, D.A.; Xu, Z. Adenosine A2A and A2B receptors work in concert to induce a strong protection against reperfusion injury in rat hearts. J. Mol. Cell. Cardiol. 2009, 47, 684–690. [Google Scholar] [CrossRef] [Green Version]
- Sorrentino, C.; Miele, L.; Porta, A.; Pinto, A.; Morello, S. Myeloid-derived suppressor cells contribute to A2B adenosine receptor-induced VEGF production and angiogenesis in a mouse melanoma model. Oncotarget 2015, 6, 27478–27489. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.M.; Lorimer, E.; Tyburski, M.D.; Williams, C.L. beta-Adrenergic receptors suppress Rap1B prenylation and promote the metastatic phenotype in breast cancer cells. Cancer Biol. Ther. 2015, 16, 1364–1374. [Google Scholar] [CrossRef]
- Schingnitz, U.; Hartmann, K.; Macmanus, C.F.; Eckle, T.; Zug, S.; Colgan, S.P.; Eltzschig, H.K. Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J. Immunol. 2010, 184, 5271–5279. [Google Scholar] [CrossRef]
- Koscso, B.; Trepakov, A.; Csoka, B.; Nemeth, Z.H.; Pacher, P.; Eltzschig, H.K.; Hasko, G. Stimulation of A2B adenosine receptors protects against trauma-hemorrhagic shock-induced lung injury. Purinergic Signal. 2013, 9, 427–432. [Google Scholar] [CrossRef]
- Tak, E.; Ridyard, D.; Kim, J.H.; Zimmerman, M.; Werner, T.; Wang, X.X.; Shabeka, U.; Seo, S.W.; Christians, U.; Klawitter, J.; et al. CD73-dependent generation of adenosine and endothelial Adora2b signaling attenuate diabetic nephropathy. J. Am. Soc. Nephrol. 2014, 25, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, P.G.; Tabrizi, M.A.; Fruttarolo, F.; Romagnoli, R.; Preti, D. Recent improvements in the development of A(2B) adenosine receptor agonists. Purinergic Signal. 2008, 4, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Bot, I.; de Vries, H.; Korporaal, S.J.; Foks, A.C.; Bot, M.; van Veldhoven, J.; Ter Borg, M.N.; van Santbrink, P.J.; van Berkel, T.J.; Kuiper, J.; et al. Adenosine A(2)B receptor agonism inhibits neointimal lesion development after arterial injury in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Beukers, M.W.; Chang, L.C.; von Frijtag Drabbe Kunzel, J.K.; Mulder-Krieger, T.; Spanjersberg, R.F.; Brussee, J.; AP, I.J. New, non-adenosine, high-potency agonists for the human adenosine A2B receptor with an improved selectivity profile compared to the reference agonist N-ethylcarboxamidoadenosine. J. Med. Chem. 2004, 47, 3707–3709. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.R.; Klein Herenbrink, C.; van Westen, G.J.; Spoorendonk, J.A.; Hoffmann, C.; AP, I.J. A novel nonribose agonist, LUF5834, engages residues that are distinct from those of adenosine-like ligands to activate the adenosine A(2a) receptor. Mol. Pharmacol. 2012, 81, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Urmaliya, V.B.; Church, J.E.; Coupar, I.M.; Rose’Meyer, R.B.; Pouton, C.W.; White, P.J. Cardioprotection induced by adenosine A1 receptor agonists in a cardiac cell ischemia model involves cooperative activation of adenosine A2A and A2B receptors by endogenous adenosine. J. Cardiovasc. Pharmacol. 2009, 53, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Koda, K.; Salazar-Rodriguez, M.; Corti, F.; Chan, N.Y.; Estephan, R.; Silver, R.B.; Mochly-Rosen, D.; Levi, R. Aldehyde dehydrogenase activation prevents reperfusion arrhythmias by inhibiting local renin release from cardiac mast cells. Circulation 2010, 122, 771–781. [Google Scholar] [CrossRef]
- Wang, Y.; Johnston, S.C.; Bath, P.M.; Grotta, J.C.; Pan, Y.; Amarenco, P.; Wang, Y.; Simon, T.; Kim, J.S.; Jeng, J.-S.; et al. Acute dual antiplatelet therapy for minor ischaemic stroke or transient ischaemic attack. BMJ 2019, 364, l895. [Google Scholar] [CrossRef] [Green Version]
- Serebruany, V.L.; Pokov, A.N.; Fortmann, S.D.; DiNicolantonio, J.J. Disbalance between mortality and non-fatal vascular events in the CHAMPION-PHOENIX trial: The cangrelor efficacy challenge. Thromb. Haemost. 2014, 111, 3–7. [Google Scholar]
- Watala, C.; Ulicna, O.; Golanski, J.; Nocun, M.; Waczulikova, I.; Markuszewski, L.; Drzewoski, J. High glucose contributes to aspirin insensitivity in streptozotocin-diabetic rats: A multiparametric aggregation study. Blood Coagul. Fibrinolysis 2006, 17, 113–124. [Google Scholar] [CrossRef]
| Receptor Subtype | High Expression | Intermediary Expression | Low Expression |
|---|---|---|---|
| A1 [19] | brain (cortex, hippocampus, cerebellum); spinal cord; adrenal gland; atria; eyes | brain (excluding cortex, hippocampus, and cerebellum); skeletal muscles; adipose tissue; liver; kidneys | lungs; pancreas |
| A2A [20] | blood platelets; leukocytes; spleen; thymus | heart; lungs; blood vessels; peripheral nerves | brain |
| A2B [21,22,23] | cecum; bladder | lungs; blood vessels; mast cells; eyes | brain; adipose tissue; blood platelets; adrenal gland; kidneys |
| A3 [19] | testis; mast cells | brain (hippocampus, cerebellum) | brain (excluding hippocampus and cerebellum); heart; thyroid; adrenal gland; spleen; liver; kidneys |
| Name | Other Names | IUPAC Name | Structure |
|---|---|---|---|
| 2-chloroadenosine | 2-Chloro Adenosine, Cl-Ado, 2 ClAdo, 2-CADO | (2R,3R,4S,5R)-2-(6-amino-2-chloropurin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol |  |
| Regadenoson | CVT 3146, CVT-3146, CVT3146, Lexiscan, Rapiscan | 1-[6-amino-9-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]purin-2-yl]-N-methylpyrazole-4-carboxamide |  |
| Binodenoson | 2-((Cyclohexylmethylene) hydrazino)adenosine | (2R,3R,4S,5R)-2-{6-amino-2-[(E)-2-(cyclohexylmethylidene)hydrazin-1-yl]-9H-purin-9-yl}-5-(hydroxymethyl)oxolane-3,4-diol |  |
| PSB-0777 | PSB0777 | 4-[2-[(6-Amino-9-b-D-ribofuranosyl-9H-purin-2-yl) thio]ethyl]benzenesulfonic acid ammonium salt |  |
| PSB-15826 | - | (2S,3S,4R,5R)-5-(6-Amino-2-((2-(4-(4-fluorophenyl)piperazin-1-yl) ethyl)thio)-9H-purin-9-yl)tetrahydrofuran-2,3,4-triol |  |
| PSB-12404 | - | (2R,3R,4S,5R)-2-(6-Amino-2-(2-cyclohexylethylthio)-9Hpurin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol |  |
| PSB-16301 | - | (2S,3S,4R,5R)-5-(6-amino-2-(phenethylthio)-9H-purin-9-yl)tetrahydrofuran-2,3,4-triol |  |
| MRE0094 | Sonedenoson, 2-[2-(4-Chlorophenyl)ethoxy]adenosine | (2R,3R,4S,5R)-2-[6-amino-2-[2-(4-chlorophenyl)ethoxy]purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol |  |
| CV1808 | 2-phenylaminoadenosine, CV-1808 | (2R,3R,4S,5R)-2-(6-amino-2-anilinopurin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol |  |
| AMP597 | RPR 100579 | (1S,2R,3S,4R)-4-(4-(((R)-1-(3-chlorothiophen-2-yl)butan-2-yl)amino)-7H-pyrrolo [2,3-d]pyrimidin-7-yl)-N-ethyl-2,3-dihydroxycyclopentane-1-carboxamide |  |
| NECA | N-Ethyl-5’-Carboxamido Adenosine, 5’-ethylcarboxamidoadenosine | (2S,3S,4R,5R)-5-(6-aminopurin-9-yl)-N-ethyl-3,4-dihydroxyoxolane-2-carboxamide |  |
| CGS21680 | CGS-21680, Cgs 21680, 2-(4-(2-carboxyethyl)phenethylamino)-5’-N-ethylcarboxamidoadenosine | 3-[4-[2-[[6-amino-9-[(2R,3R,4S,5S)-5-(ethylcarbamoyl)-3,4-dihydroxyoxolan-2-yl]purin-2-yl]amino]ethyl]phenyl]propanoic acid |  |
| HE-NECA | HENECA, Heneca, 2-hexynyl-NECA, 2-hexynyladenosine-5’-N-ethylcarboxamide | (2S,3S,4R,5R)-5-(6-amino-2-hex-1-ynylpurin-9-yl)-N-ethyl-3,4-dihydroxyoxolane-2-carboxamide |  |
| UK-432097 | UK-432,097 | 6-(2,2-diphenylethylamino)-9-[(2R,3R,4S,5S)-5-(ethylcarbamoyl)-3,4-dihydroxyoxolan-2-yl]-N-[2-[(1-pyridin-2-ylpiperidin-4-yl)carbamoylamino]ethyl]purine-2-carboxamide |  |
| BAY 60-6583 | BAY-60-6583, BAY60-6583, 2-((6-amino-3,5-dicyano-4-(4-(cyclopropylmethoxy)phenyl)pyridin-2 yl) sulfanyl)acetamide | 2-[6-amino-3,5-dicyano-4-[4-(cyclopropylmethoxy)phenyl]pyridin -2-yl]sulfanylacetamide |  |
| LUF5834 | LUF 5834, LUF-5834, 2-Amino-4-(4-hydroxy-phenyl)-6-(1H-imidazol-2-ylmethylsulfanyl)-pyridine-3,5-dicarbonitrile | 2-amino-6-(1H-imidazol-2-ylmethylsulfanyl)-4-(4-oxocyclohexa-2,5-dien-1-ylidene)-1H-pyridine-3,5-dicarbonitrile |  |
| LUF5835 | LUF 5835, LUF-5835 | 2-amino-6-(1H-imidazol-2-ylmethylsulfanyl)--4-(3-hydroxy-phenyl) pyridine-3,5dicarbonitrile |  |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolska, N.; Rozalski, M. Blood Platelet Adenosine Receptors as Potential Targets for Anti-Platelet Therapy. Int. J. Mol. Sci. 2019, 20, 5475. https://doi.org/10.3390/ijms20215475
Wolska N, Rozalski M. Blood Platelet Adenosine Receptors as Potential Targets for Anti-Platelet Therapy. International Journal of Molecular Sciences. 2019; 20(21):5475. https://doi.org/10.3390/ijms20215475
Chicago/Turabian StyleWolska, Nina, and Marcin Rozalski. 2019. "Blood Platelet Adenosine Receptors as Potential Targets for Anti-Platelet Therapy" International Journal of Molecular Sciences 20, no. 21: 5475. https://doi.org/10.3390/ijms20215475
APA StyleWolska, N., & Rozalski, M. (2019). Blood Platelet Adenosine Receptors as Potential Targets for Anti-Platelet Therapy. International Journal of Molecular Sciences, 20(21), 5475. https://doi.org/10.3390/ijms20215475