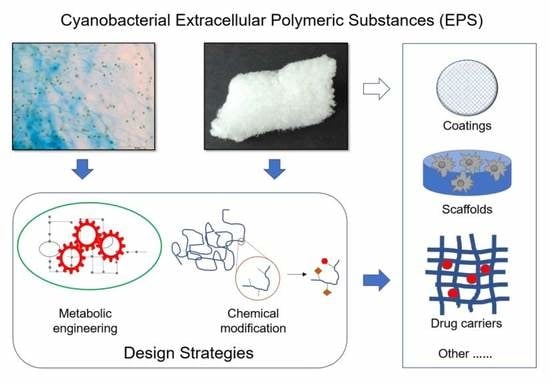
Strategies to Obtain Designer Polymers Based on Cyanobacterial Extracellular Polymeric Substances (EPS)
Abstract
:1. Introduction
2. Cyanobacterial Extracellular Polymeric Substances (EPS)
2.1. Polymer Characteristics
2.2. Relevant Biological Activities
3. Strategies to Optimize Cyanobacterial EPS Production and/or Polymer Characteristics
3.1. Metabolic Engineering of EPS-Producing Strains
3.1.1. Carbon Availability
3.1.2. Synthesis of Sugar Nucleotide Precursors
3.1.3. Assembly of the Repeating Unit
3.1.4. Polymerization and Export of the Polymer
4. Isolation, Purification, and Functionalization of Cyanobacterial EPS
5. Development and Possible Applications of Cyanobacterial EPS-Based Biomaterials
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| EDTA | Ethylenediaminetetraacetic acid |
| EPS | Extracellular polymeric substances |
| FDA | Food and drug administration |
| hMSCs | Human mesenchymal stem cells |
| ISO | International organization for standardization |
| LPS | Lipopolysaccharides |
| RPS | Released polysaccharides |
| UDP | Uridine diphosphate |
| UTP | Uridine triphosphate |
References
- Ates, O. Systems Biology of Microbial Exopolysaccharides Production. Front. Bioeng. Biotechnol. 2015, 3, 200. [Google Scholar] [CrossRef]
- Yadav, P.; Yadav, H.; Shah, V.G.; Shah, G.; Dhaka, G. Biomedical Biopolymers, their Origin and Evolution in Biomedical Sciences: A Systematic Review. J. Clin. Diagn. Res. 2015, 9, ZE21–ZE25. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H.M.N. Naturally-derived biopolymers: Potential platforms for enzyme immobilization. Int. J. Biol. Macromol. 2019, 130, 462–482. [Google Scholar] [CrossRef] [PubMed]
- Schmid, J. Recent insights in microbial exopolysaccharide biosynthesis and engineering strategies. Curr. Opin. Biotechnol. 2018, 53, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gao, Y.; Wang, L.; Chen, H.; Yan, N. Effect of Treatment Methods on Chitin Structure and Its Transformation into Nitrogen-Containing Chemicals. Chempluschem 2015, 80, 1565–1572. [Google Scholar] [CrossRef]
- Dassanayake, R.; Acharya, S.; Abidi, N. Biopolymer-Based Materials from Polysaccharides: Properties, Processing, Characterization and Sorption Applications. In Advanced Sorption Process Applications; Edebali, S., Ed.; IntechOpen: London, UK, 2018; pp. 1–24. [Google Scholar]
- Roca, C.; Alves, V.D.; Freitas, F.; Reis, M.A. Polysaccharides enriched in rare sugars: Bacterial sources, production and applications. Front. Microbiol. 2015, 6, 288. [Google Scholar] [CrossRef] [PubMed]
- Freitas, F.; Torres, C.A.V.; Reis, M.A.M. Engineering aspects of microbial exopolysaccharide production. Bioresour. Technol. 2017, 245, 1674–1683. [Google Scholar] [CrossRef]
- Anderson, L.A.; Islam, M.A.; Prather, K.L.J. Synthetic biology strategies for improving microbial synthesis of “green” biopolymers. J. Biol. Chem. 2018, 293, 5053–5061. [Google Scholar] [CrossRef]
- Freitas, F.; Alves, V.D.; Reis, M.A.M. Advances in bacterial exopolysaccharides: From production to biotechnological applications. Trends Biotechnol. 2011, 29, 388–398. [Google Scholar] [CrossRef]
- Pereira, S.; Zille, A.; Micheletti, E.; Moradas-Ferreira, P.; De Philippis, R.; Tamagnini, P. Complexity of cyanobacterial exopolysaccharides: Composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol. Rev. 2009, 33, 917–941. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; De Philippis, R. Exocellular Polysaccharides in Microalgae and Cyanobacteria: Chemical Features, Role and Enzymes and Genes Involved in Their Biosynthesis. In The Physiology of Microalgae; Borowitzka, M.A., Beardall, J., Raven, J.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 565–590. [Google Scholar]
- Mota, R.; Guimaraes, R.; Buttel, Z.; Rossi, F.; Colica, G.; Silva, C.J.; Santos, C.; Gales, L.; Zille, A.; De Philippis, R.; et al. Production and characterization of extracellular carbohydrate polymer from Cyanothece sp. CCY 0110. Carbohydr. Polym. 2013, 92, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, B.; Prasad Uday, U.S.; Oinam, G.; Mondal, A.; Bandyopadhyay, T.K.; Tiwari, O.N. Characterization, genetic regulation and production of cyanobacterial exopolysaccharides and its applicability for heavy metal removal. Carbohydr. Polym. 2018, 179, 228–243. [Google Scholar] [CrossRef]
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P. Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Micheletti, E.; Zille, A.; Santos, A.; Moradas-Ferreira, P.; Tamagnini, P.; De Philippis, R. Using extracellular polymeric substances (EPS)-producing cyanobacteria for the bioremediation of heavy metals: Do cations compete for the EPS functional groups and also accumulate inside the cell? Microbiology 2011, 157, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Mota, R.; Rossi, F.; Andrenelli, L.; Pereira, S.B.; De Philippis, R.; Tamagnini, P. Released polysaccharides (RPS) from Cyanothece sp. CCY 0110 as biosorbent for heavy metals bioremediation: Interactions between metals and RPS binding sites. Appl. Microbiol. Biotechnol. 2016, 100, 7765–7775. [Google Scholar] [CrossRef] [PubMed]
- De Philippis, R.; Colica, G.; Micheletti, E. Exopolysaccharide-producing cyanobacteria in heavy metal removal from water: Molecular basis and practical applicability of the biosorption process. Appl. Microbiol. Biotechnol. 2011, 92, 697–708. [Google Scholar] [CrossRef]
- Gupta, P.; Diwan, B. Bacterial Exopolysaccharide mediated heavy metal removal: A Review on biosynthesis, mechanism and remediation strategies. Biotechnol. Rep. 2017, 13, 58–71. [Google Scholar] [CrossRef]
- Ozturk, S.; Aslim, B.; Suludere, Z.; Tan, S. Metal removal of cyanobacterial exopolysaccharides by uronic acid content and monosaccharide composition. Carbohydr. Polym. 2014, 101, 265–271. [Google Scholar] [CrossRef]
- Han, P.-p.; Sun, Y.; Wu, X.-y.; Yuan, Y.-j.; Dai, Y.-j.; Jia, S.-r. Emulsifying, Flocculating, and Physicochemical Properties of Exopolysaccharide Produced by Cyanobacterium Nostoc flagelliforme. Appl. Biochem. Biotechnol. 2014, 172, 36–49. [Google Scholar] [CrossRef]
- Jindal, N.; Singh, D.P.; Khattar, J.I.S. Kinetics and physico-chemical characterization of exopolysaccharides produced by the cyanobacterium Oscillatoria formosa. World J. Microbiol. Biotechnol. 2011, 27, 2139–2146. [Google Scholar] [CrossRef]
- Paniagua-Michel, J.d.J.; Olmos-Soto, J.; Morales-Guerrero, E.R. Chapter Eleven-Algal and Microbial Exopolysaccharides: New Insights as Biosurfactants and Bioemulsifiers. In Advances in Food and Nutrition Research, 1st ed.; Kim, S.-K., Ed.; Academic Press: Amsterdam, The Netherlands, 2014; pp. 221–257. [Google Scholar]
- Bhatnagar, M.; Bhatnagar, A. Diversity of Polysaccharides in Cyanobacteria. In Microbial Diversity in Ecosystem Sustainability and Biotechnological Applications: Volume 1. Microbial Diversity in Normal & Extreme Environments; Satyanarayana, T., Johri, B.N., Das, S.K., Eds.; Springer Singapore: Singapore, 2019; pp. 447–496. [Google Scholar]
- Hussein, M.H.; Abou-ElWafa, G.S.; Shaaban-Dessuuki, S.A.; Hassan, N.I. Characterization and Antioxidant Activity of Exopolysaccharide Secreted by Nostoc carneum. Int. J. Pharmacol. 2015, 11, 432–439. [Google Scholar] [CrossRef]
- Li, H.; Su, L.; Chen, S.; Zhao, L.; Wang, H.; Ding, F.; Chen, H.; Shi, R.; Wang, Y.; Huang, Z. Physicochemical Characterization and Functional Analysis of the Polysaccharide from the Edible Microalga Nostoc sphaeroides. Molecules 2018, 23, 508. [Google Scholar] [CrossRef] [PubMed]
- Dewi, I.C.; Falaise, C.; Hellio, C.; Bourgougnon, N.; Mouget, J.-L. Chapter 12 - Anticancer, Antiviral, Antibacterial, and Antifungal Properties in Microalgae. In Microalgae in Health and Disease Prevention; Levine, I.A., Fleurence, J., Eds.; Academic Press: Amsterdam, The Netherlands, 2018; pp. 235–261. [Google Scholar]
- Kanekiyo, K.; Hayashi, K.; Takenaka, H.; Lee, J.B.; Hayashi, T. Anti-herpes simplex virus target of an acidic polysaccharide, nostoflan, from the edible blue-green alga Nostoc flagelliforme. Biol. Pharm. Bull. 2007, 30, 1573–1575. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Moghadamtousi, S.Z.; Abubakar, S.; Zandi, K. Antiviral Potential of Algae Polysaccharides Isolated from Marine Sources: A Review. Biomed. Res. Int. 2015, 825203. [Google Scholar] [CrossRef] [PubMed]
- Najdenski, H.M.; Gigova, L.G.; Iliev, I.I.; Pilarski, P.S.; Lukavsky, J.; Tsvetkova, I.V.; Ninova, M.S.; Kussovski, V.K. Antibacterial and antifungal activities of selected microalgae and cyanobacteria. Int. J. Food Sci. Technol. 2013, 48, 1533–1540. [Google Scholar] [CrossRef]
- Parwani, L.; Bhatnagar, M.; Bhatnagar, A.; Sharma, V. Antioxidant and iron-chelating activities of cyanobacterial exopolymers with potential for wound healing. J. Appl. Phycol. 2014, 26, 1473–1482. [Google Scholar] [CrossRef]
- Majdoub, H.; Ben Mansour, M.; Chaubet, F.; Roudesli, M.S.; Maaroufi, R.M. Anticoagulant activity of a sulfated polysaccharide from the green alga Arthrospira platensis. Biochim. Biophys. Acta 2009, 1790, 1377–1381. [Google Scholar] [CrossRef]
- Flores, C.; Lima, R.T.; Adessi, A.; Sousa, A.; Pereira, S.B.; Granja, P.L.; De Philippis, R.; Soares, P.; Tamagnini, P. Characterization and antitumor activity of the extracellular carbohydrate polymer from the cyanobacterium Synechocystis ΔsigF mutant. Int. J. Biol. Macromol. 2019, 136, 1219–1227. [Google Scholar] [CrossRef]
- Bellini, E.; Ciocci, M.; Savio, S.; Antonaroli, S.; Seliktar, D.; Melino, S.; Congestri, R. Trichormus variabilis (Cyanobacteria) Biomass: From the Nutraceutical Products to Novel EPS-Cell/Protein Carrier Systems. Mar. Drugs 2018, 16, 298. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Mota, R.; Leite, J.P.; Tamagnini, P.; Gales, L.; Rocha, F. Application of a cyanobacterial extracellular polymeric substance in the microencapsulation of vitamin B12. Powder Technol. 2019, 343, 644–651. [Google Scholar] [CrossRef]
- Leite, J.P.; Mota, R.; Durão, J.; Neves, S.C.; Barrias, C.C.; Tamagnini, P.; Gales, L. Cyanobacterium-Derived Extracellular Carbohydrate Polymer for the Controlled Delivery of Functional Proteins. Macromol. Biosci. 2017, 17, 1600206. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, S.; Torres, F.G.; López, D. Preparation and Characterization of Polysaccharide Films from the Cyanobacteria Nostoc commune. Polym. Renew. Resour. 2017, 8, 133–150. [Google Scholar] [CrossRef]
- Costa, B.; Mota, R.; Parreira, P.; Tamagnini, P. ; L.; Martins, M.C.; Costa, F. Broad-Spectrum Anti-Adhesive Coating Based on an Extracellular Polymer from a Marine Cyanobacterium. Mar. Drugs 2019, 17, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Harding, S.E.; Liu, Z. Cyanobacterial exopolysaccharides: Their nature and potential biotechnological applications. Biotechnol. Genet. Eng. Rev. 2001, 18, 375–404. [Google Scholar] [CrossRef]
- Forni, C.; Telo, F.R.; Caiola, M.G. Comparative analysis of the polysaccharides produced by different species of Microcystis (Chroococcales, Cyanophyta). Phycologia 1997, 36, 181–185. [Google Scholar] [CrossRef]
- Chi, Z.; Su, C.D.; Lu, W.D. A new exopolysaccharide produced by marine Cyanothece sp 113. Bioresour. Technol. 2007, 98, 1329–1332. [Google Scholar] [CrossRef]
- Kehr, J.-C.; Dittmann, E. Biosynthesis and Function of Extracellular Glycans in Cyanobacteria. Life 2015, 5, 164–180. [Google Scholar] [CrossRef] [Green Version]
- Nobles, D.R.; Romanovicz, D.K.; Brown, R.M. Cellulose in Cyanobacteria. Origin of Vascular Plant Cellulose Synthase? Plant. Physiol. 2001, 127, 529–542. [Google Scholar] [CrossRef]
- Colica, G.; De Philippis, R. Exopolysaccharides from cyanobacteria and their possible industrial applications. In Cyanobacteria; Sharma, N.K., Rai, A.K., Stal, L.J., Eds.; John Wiley & Sons, Ltd.: New York, NY, USA, 2013; pp. 197–207. [Google Scholar]
- Li, H.; Zhao, Q.Y.; Huang, H. Current states and challenges of salt-affected soil remediation by cyanobacteria. Sci. Total Environ. 2019, 669, 258–272. [Google Scholar] [CrossRef]
- Anahas, A.M.P.; Muralitharan, G. Characterization of heterocystous cyanobacterial strains for biodiesel production based on fatty acid content analysis and hydrocarbon production. Energ. Convers. Manage. 2018, 157, 423–437. [Google Scholar] [CrossRef]
- De Philippis, R.; Micheletti, E. Heavy Metal Removal with Exopolysaccharide-Producing Cyanobacteria. In Heavy Metals in the Environment; Wang, L.K., Chen, J.P., Hung, Y.T., Shammas, N.K., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 89–122. [Google Scholar]
- Radonić, A.; Thulke, S.; Achenbach, J.; Kurth, A.; Vreemann, A.; König, T.; Walter, C.; Possinger, K.; Nitsche, A. Anionic Polysaccharides From Phototrophic Microorganisms Exhibit Antiviral Activities to Vaccinia Virus. J. Antivir. Antiretrovir. 2011, 2, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, K.; Hayashi, T.; Kojima, I. A Natural Sulfated Polysaccharide, Calcium Spirulan, Isolated from Spirulina platensis: In Vitro and ex Vivo Evaluation of Anti-Herpes Simplex Virus and Anti-Human Immunodeficiency Virus Activities. AIDS Res. Hum. Retrovir. 1996, 12, 1463–1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, T.; Hayashi, K.; Maeda, M.; Kojima, I. Calcium spirulan, an inhibitor of enveloped virus replication, from a blue-green alga Spirulina platensis. J. Nat. Prod. 1996, 59, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Challouf, R.; Trabelsi, L.; Ben Dhieb, R.; El Abed, O.; Yahia, A.; Ghozzi, K.; Ben Ammar, J.; Omran, H.; Ben Ouada, H. Evaluation of cytotoxicity and biological activities in extracellular polysaccharides released by cyanobacterium Arthrospira platensis. Braz. Arch. Biol. Technol. 2011, 54, 831–838. [Google Scholar] [CrossRef]
- Volk, R.-B.; Venzke, K.; Blaschek, W.; Alban, S. Complement Modulating and Anticoagulant Effects of a Sulfated Exopolysaccharide Released by the Cyanobacterium Synechocystis aquatilis. Planta Med. 2006, 72, 1424–1427. [Google Scholar] [CrossRef]
- Gudmundsdottir, A.B.; Omarsdottir, S.; Brynjolfsdottir, A.; Paulsen, B.S.; Olafsdottir, E.S.; Freysdottir, J. Exopolysaccharides from Cyanobacterium aponinum from the Blue Lagoon in Iceland increase IL-10 secretion by human dendritic cells and their ability to reduce the IL-17(+)ROR gamma t(+)/IL-10(+)FoxP3(+) ratio in CD4(+) T cells. Immunol. Lett. 2015, 163, 157–162. [Google Scholar] [CrossRef]
- Mishima, T.; Murata, J.; Toyoshima, M.; Fujii, H.; Nakajima, M.; Hayashi, T.; Kato, T.; Saiki, I. Inhibition of tumor invasion and metastasis by calcium spirulan (Ca-SP), a novel sulfated polysaccharide derived from a blue-green alga, Spirulina platensis. Clin. Exp. Metastasis. 1998, 16, 541–550. [Google Scholar] [CrossRef]
- Gigova, L.; Toshkova, R.; Gardeva, E.; Gacheva, G.J.; Ivanova, N.; Yossifova, L.; Petkov, G. Growth inhibitory activity of selected microalgae and cyanobacteria towards human cervical carcinoma cells (HeLa). J. Pharm. Res. 2011, 4, 4702–4707. [Google Scholar]
- Xue, X.; Lv, Y.; Liu, Q.; Zhang, X.; Zhao, Y.; Zhang, L.; Xu, S. Extracellular polymeric substance from Aphanizomenon flos-aquae induces apoptosis via the mitochondrial pathway in A431 human epidermoid carcinoma cells. Exp. Ther. Med. 2015, 10, 927–932. [Google Scholar] [CrossRef] [Green Version]
- Ou, Y.; Xu, S.; Zhu, D.; Yang, X. Molecular mechanisms of exopolysaccharide from Aphanothece halaphytica (EPSAH) induced apoptosis in HeLa cells. PLoS ONE 2014, 9, e87223. [Google Scholar] [CrossRef] [PubMed]
- Kanekiyo, K.; Lee, J.B.; Hayashi, K.; Takenaka, H.; Hayakawa, Y.; Endo, S.; Hayashi, T. Isolation of an antiviral polysaccharide, nostoflan, from a terrestrial cyanobacterium, Nostoc flagelliforme. J. Nat. Prod. 2005, 68, 1037–1041. [Google Scholar] [CrossRef]
- Lüscher-Mattii, M. Polyanions—A Lost Chance in the Fight against HIV and other Virus Diseases? Antivir. Chem. Chemother. 2000, 11, 249–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witvrouw, M.; DeClercq, E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen. Pharmacol. 1997, 29, 497–511. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Menakha, M. Pharmaceutical applications of cyanobacteria-A review. J. Acute Med. 2015, 5, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Nunnery, J.K.; Mevers, E.; Gerwick, W.H. Biologically active secondary metabolites from marine cyanobacteria. Curr. Opin. Biotechnol. 2010, 21, 787–793. [Google Scholar] [CrossRef] [Green Version]
- Pathak, J.; Rajneesh; Maurya, P.K.; Singh, S.P.; Häder, D.-P.; Sinha, R.P. Cyanobacterial Farming for Environment Friendly Sustainable Agriculture Practices: Innovations and Perspectives. Front. Environ. Sci. 2018, 6, 7. [Google Scholar] [CrossRef]
- De Philippis, R.; Vincenzini, M. Exocellular polysaccharides from cyanobacteria and their possible applications. FEMS Microbiol. Rev. 1998, 22, 151–175. [Google Scholar] [CrossRef]
- Ruffing, A.; Chen, R.R. Metabolic Engineering of Microorganisms for Oligosaccharide and Polysaccharide Production. In Microbial Production of Biopolymers and Polymer Precursors: Applications and Perspectives; Rehm, B.H.A., Ed.; Caister Academic Press: Norfolk, UK, 2009; pp. 197–228. [Google Scholar]
- Jittawuttipoka, T.; Planchon, M.; Spalla, O.; Benzerara, K.; Guyot, F.; Cassier-Chauvat, C.; Chauvat, F. Multidisciplinary Evidences that Synechocystis PCC6803 Exopolysaccharides Operate in Cell Sedimentation and Protection against Salt and Metal Stresses. PLoS ONE 2013, 8, e55564. [Google Scholar] [CrossRef] [Green Version]
- Fisher, M.L.; Allen, R.; Luo, Y.; Curtiss, R. ; III. Export of Extracellular Polysaccharides Modulates Adherence of the Cyanobacterium Synechocystis. PLoS ONE 2013, 8, e74514. [Google Scholar] [CrossRef] [Green Version]
- Pereira, S.B.; Mota, R.; Vieira, C.P.; Vieira, J.; Tamagnini, P. Phylum-wide analysis of genes/proteins related to the last steps of assembly and export of extracellular polymeric substances (EPS) in cyanobacteria. Sci. Rep. 2015, 5, 14835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, S.B.; Santos, M.; Leite, J.P.; Flores, C.; Eisfeld, C.; Büttel, Z.; Mota, R.; Rossi, F.; De Philippis, R.; Gales, L.; et al. The role of the tyrosine kinase Wzc (Sll0923) and the phosphatase Wzb (Slr0328) in the production of extracellular polymeric substances (EPS) by Synechocystis PCC 6803. Microbiologyopen 2019, 8, e753. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.; Rittmann, B.E.; Curtiss, R. Axenic Biofilm Formation and Aggregation by Synechocystis PCC 6803 is Induced by Changes in Nutrient Concentration, and Requires Cell Surface Structures. Appl. Environ. Microbiol. 2019, 85, e02192-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitfield, C.; Larue, K. Stop and go: Regulation of chain length in the biosynthesis of bacterial polysaccharides. Nat. Struct. Mol. Biol. 2008, 15, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.R.; Hobbs, M.; Valvano, M.A.; Skurnik, M.; Whitfield, C.; Coplin, D.; Kido, N.; Klena, J.; Maskell, D.; Raetz, C.R.; et al. Bacterial polysaccharide synthesis and gene nomenclature. Trends Microbiol. 1996, 4, 495–503. [Google Scholar] [CrossRef]
- Wang, J.; Salem, D.R.; Sani, R.K. Extremophilic exopolysaccharides: A review and new perspectives on engineering strategies and applications. Carbohydr. Polym. 2019, 205, 8–26. [Google Scholar] [CrossRef]
- Kamennaya, N.A.; Eun Ahn, S.; Park, H.; Bartal, R.; Sasaki, K.A.; Holman, H.-Y.; Jansson, C. Installing extra bicarbonate transporters in the cyanobacterium Synechocystis sp. PCC6803 enhances biomass production. Metab. Eng. 2015, 29, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Kamennaya, N.A.; Zemla, M.; Mahoney, L.; Chen, L.; Holman, E.; Holman, H.-Y.; Auer, M.; Ajo-Franklin, C.M.; Jansson, C. High pCO2-induced exopolysaccharide-rich ballasted aggregates of planktonic cyanobacteria could explain Paleoproterozoic carbon burial. Nat. Commun. 2018, 9, 2116. [Google Scholar] [CrossRef] [Green Version]
- Zilliges, Y. Glycogen: A Dynamic Cellular Sink and Reservoir for Carbon. In The Cell Biology of Cyanobacteria; Flores, E., Herrero, A., Eds.; Caister Academic Press: Norfolk, UK, 2014; pp. 189–210. [Google Scholar]
- Cano, M.; Holland, S.C.; Artier, J.; Burnap, R.L.; Ghirardi, M.; Morgan, J.A.; Yu, J. Glycogen Synthesis and Metabolite Overflow Contribute to Energy Balancing in Cyanobacteria. Cell Rep. 2018, 23, 667–672. [Google Scholar] [CrossRef]
- Pade, N.; Mikkat, S.; Hagemann, M. Ethanol, glycogen and glucosylglycerol represent competing carbon pools in ethanol-producing cells of Synechocystis sp. PCC 6803 under high-salt conditions. Microbiology 2017, 163, 300–307. [Google Scholar] [CrossRef]
- Du, W.; Liang, F.; Duan, Y.; Tan, X.; Lu, X. Exploring the photosynthetic production capacity of sucrose by cyanobacteria. Metab. Eng. 2013, 19, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, S.; Aslim, B. Modification of exopolysaccharide composition and production by three cyanobacterial isolates under salt stress. Environ. Sci. Pollut. Res. Int. 2010, 17, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, F.; Pade, N.; Klähn, S.; Hess, W.R.; Hagemann, M. The glucosylglycerol-degrading enzyme GghA is involved in acclimation to fluctuating salinities by the cyanobacterium Synechocystis sp. strain PCC 6803. Microbiology 2017, 163, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Osanai, T.; Azuma, M.; Tanaka, K. Sugar catabolism regulated by light- and nitrogen-status in the cyanobacterium Synechocystis sp. PCC 6803. Photochem. Photobiol. Sci. 2007, 6, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Narikawa, R.; Ikeuchi, M. CugP Is a Novel Ubiquitous Non-GalU-Type Bacterial UDP-Glucose Pyrophosphorylase Found in Cyanobacteria. J. Bacteriol. 2014, 196, 2348–2354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitfield, C.; Trent, M.S. Biosynthesis and Export of Bacterial Lipopolysaccharides. Annu. Rev. Biochem. 2014, 83, 99–128. [Google Scholar] [CrossRef]
- Ristl, R.; Steiner, K.; Zarschler, K.; Zayni, S.; Messner, P.; Schaffer, C. The S-Layer Glycome-Adding to the Sugar Coat of Bacteria. Int. J. Microbiol. 2011, 2011, 127870. [Google Scholar] [CrossRef] [Green Version]
- Angermayr, S.A.; Gorchs Rovira, A.; Hellingwerf, K.J. Metabolic engineering of cyanobacteria for the synthesis of commodity products. Trends Biotechnol. 2015, 33, 352–361. [Google Scholar] [CrossRef]
- Schmid, J.; Sieber, V.; Rehm, B. Bacterial exopolysaccharides: Biosynthesis pathways and engineering strategies. Front. Microbiol. 2015, 6, 496. [Google Scholar] [CrossRef] [Green Version]
- Rehm, B.H. Bacterial polymers: Biosynthesis, modifications and applications. Nat. Rev. Microbiol. 2010, 8, 578–592. [Google Scholar] [CrossRef]
- Galvan, E.M.; Ielmini, M.V.; Patel, Y.N.; Bianco, M.I.; Franceschini, E.A.; Schneider, J.C.; Ielpi, L. Xanthan chain length is modulated by increasing the availability of the polysaccharide copolymerase protein GumC and the outer membrane polysaccharide export protein GumB. Glycobiology 2013, 23, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Barrera, A.; Soto, E.; Altamirano, C. Alginate production and alg8 gene expression by Azotobacter vinelandii in continuous cultures. J. Ind. Microbiol. Biotechnol. 2012, 39, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Miranda, H.; Immerzeel, P.; Gerber, L.; Hörnaeus, K.; Lind, S.B.; Pattanaik, B.; Lindberg, P.; Mamedov, F.; Lindblad, P. Sll1783, a monooxygenase associated with polysaccharide processing in the unicellular cyanobacterium Synechocystis PCC 6803. Physiol. Plant. 2017, 161, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Flores, C.; Tamagnini, P. Looking Outwards: Isolation of Cyanobacterial Released Carbohydrate Polymers and Proteins. JoVE 2019, 147, e59590. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.K.; Laroche, C.; Marcati, A.; Ursu, A.V.; Jubeau, S.; Marchal, L.; Petit, E.; Djelveh, G.; Michaud, P. Separation and fractionation of exopolysaccharides from Porphyridium cruentum. Bioresour. Technol. 2013, 145, 345–350. [Google Scholar] [CrossRef]
- Ghisalberti, E.L. Detection and Isolation of Bioactive Natural Products. In Bioactive Natural Products: Detection, Isolation, and Structural Determination, 2nd ed.; Colegate, S.M., Molyneux, R.J., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 11–76. [Google Scholar]
- Shi, L. Bioactivities, isolation and purification methods of polysaccharides from natural products: A review. Int. J. Biol. Macromol. 2016, 92, 37–48. [Google Scholar] [CrossRef]
- Chaplin, M.F.; Kennedy, J.F. Carbohydrate Analysis: A Practical Approach, 2nd ed.; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Silva, M.; Castellanos, L.; Ottens, M. Capture and Purification of Polyphenols Using Functionalized Hydrophobic Resins. Ind. Eng. Chem. Res. 2018, 57, 5359–5369. [Google Scholar] [CrossRef]
- Petsch, D.; Anspach, F.B. Endotoxin removal from protein solutions. J. Biotechnol. 2000, 76, 97–119. [Google Scholar] [CrossRef]
- Gao, B.; Tsan, M.-F. Endotoxin Contamination in Recombinant Human Heat Shock Protein 70 (Hsp70) Preparation Is Responsible for the Induction of Tumor Necrosis Factor α Release by Murine Macrophages. J. Biol. Chem. 2003, 278, 174–179. [Google Scholar] [CrossRef] [Green Version]
- Suri, R.M.; Austyn, J.M. Bacterial lipopolysaccharide contamination of commercial collagen preparations may mediate dendritic cell maturation in culture. J. Immunol. Methods 1998, 214, 149–163. [Google Scholar] [CrossRef]
- Commission, E.P. European Pharmacopoeia 7.0; Council Of Europe: European Directorate for the Quality of Medicines and Healthcare: Strasbourg, France, 2010; pp. 171–175, 520–523. [Google Scholar]
- Guidance for Industry Pyrogen and Endotoxins Testing: Questions and Answers; U.S. Department of Health and Human Services Food and Drug Administration: Washington, DC, USA, 2012.
- Gorbet, M.B.; Sefton, M.V. Endotoxin: The uninvited guest. Biomaterials 2005, 26, 6811–6817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-P.; Shen, C.-C.; Gao, F.-L.; Wei, H.; Ren, D.-F.; Lu, J. ; Isolation, Purification and Structural Characterization of Two Novel Water-Soluble Polysaccharides from Anredera cordifolia. Molecules 2017, 22, 1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Chase, H.A. Applications of membrane techniques for purification of natural products. Biotechnol. Lett. 2010, 32, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Azzam, T.; Eliyahu, H.; Makovitzki, A.; Linial, M.; Domb, A.J. Hydrophobized dextran-spermine conjugate as potential vector for in vitro gene transfection. J. Control. Release 2004, 96, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Chu, C.-C. Synthesis and characterization of dextran–methacrylate hydrogels and structural study by SEM. J. Biomed. Mater. Res. 2000, 49, 517–527. [Google Scholar] [CrossRef]
- Kumar, A. ; Deepak; Sharma, S.; Srivastava, A.; Kumar, R. Synthesis of xanthan gum graft copolymer and its application for controlled release of highly water soluble Levofloxacin drug in aqueous medium. Carbohydr. Polym. 2017, 171, 211–219. [Google Scholar] [CrossRef]
- Oudshoorn, M.H.M.; Rissmann, R.; Bouwstra, J.A.; Hennink, W.E. Synthesis of methacrylated hyaluronic acid with tailored degree of substitution. Polymer 2007, 48, 1915–1920. [Google Scholar] [CrossRef]
- Palma, S.I.C.J.; Rodrigues, C.A.V.; Carvalho, A.; Morales, M.D.; Freitas, F.; Fernandes, A.R.; Cabral, J.M.S.; Roque, A.C.A. A value-added exopolysaccharide as a coating agent for MRI nanoprobes. Nanoscale 2015, 7, 14272–14283. [Google Scholar] [CrossRef]
- Tang, Y.J.; Sun, J.; Fan, H.S.; Zhang, X.D. An improved complex gel of modified gellan gum and carboxymethyl chitosan for chondrocytes encapsulation. Carbohydr. Polym. 2012, 88, 46–53. [Google Scholar] [CrossRef]
- Theilacker, C.; Coleman, F.T.; Mueschenborn, S.; Llosa, N.; Grout, M.; Pier, G.B. Construction and characterization of a Pseudomonas aeruginosa mucoid exopolysaccharide-alginate conjugate vaccine. Infect. Immun. 2003, 71, 3875–3884. [Google Scholar] [CrossRef] [Green Version]
- van Dijk-Wolthuis, W.N.E.; Franssen, O.; Talsma, H.; van Steenbergen, M.J.; Kettenes-van den Bosch, J.J.; Hennink, W.E. ; Synthesis, Characterization, and Polymerization of Glycidyl Methacrylate Derivatized Dextran. Macromolecules 1995, 28, 6317–6322. [Google Scholar] [CrossRef]
- Ratner, B.D. The Biocompatibility Manifesto: Biocompatibility for the Twenty-first Century. J. Cardiovasc. Transl. Res. 2011, 4, 523–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, A.; Neves, S.C.; Gonçalves, I.C.; Barrias, C.C. cells. In Characterization of Polymeric Biomaterials; Tanzi, M.C., Farè, S., Eds.; Woodhead Publishing: Sawston, Cambridgeshire, England, 2017; pp. 285–315. [Google Scholar]
- Technical Committee ISO/TC 194. Part 5: Tests for in vitro cytotoxicity. In Biological Evaluation of Medical Devices, 3rd ed.; International Organization for Standardization: Geneva, Switzerland, 2009; ISO 10993-5; p. 34. [Google Scholar]
- Doak, S.H.; Manshian, B.; Jenkins, G.J.S.; Singh, N. In vitro genotoxicity testing strategy for nanomaterials and the adaptation of current OECD guidelines. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012, 745, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Cowie, H.; Magdolenova, Z.; Saunders, M.; Drlickova, M.; Carreira, S.C.; Kenzaoi, B.H.; Gombau, L.; Guadagnini, R.; Lorenzo, Y.; Walker, L.; et al. Suitability of human and mammalian cells of different origin for the assessment of genotoxicity of metal and polymeric engineered nanoparticles. Nanotoxicology 2015, 9, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Tchobanian, A.; Van Oosterwyck, H.; Fardim, P. Polysaccharides for tissue engineering: Current landscape and future prospects. Carbohydr. Polym. 2019, 205, 601–625. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, S.B.; Sousa, A.; Santos, M.; Araújo, M.; Serôdio, F.; Granja, P.; Tamagnini, P. Strategies to Obtain Designer Polymers Based on Cyanobacterial Extracellular Polymeric Substances (EPS). Int. J. Mol. Sci. 2019, 20, 5693. https://doi.org/10.3390/ijms20225693
Pereira SB, Sousa A, Santos M, Araújo M, Serôdio F, Granja P, Tamagnini P. Strategies to Obtain Designer Polymers Based on Cyanobacterial Extracellular Polymeric Substances (EPS). International Journal of Molecular Sciences. 2019; 20(22):5693. https://doi.org/10.3390/ijms20225693
Chicago/Turabian StylePereira, Sara B., Aureliana Sousa, Marina Santos, Marco Araújo, Filipa Serôdio, Pedro Granja, and Paula Tamagnini. 2019. "Strategies to Obtain Designer Polymers Based on Cyanobacterial Extracellular Polymeric Substances (EPS)" International Journal of Molecular Sciences 20, no. 22: 5693. https://doi.org/10.3390/ijms20225693
APA StylePereira, S. B., Sousa, A., Santos, M., Araújo, M., Serôdio, F., Granja, P., & Tamagnini, P. (2019). Strategies to Obtain Designer Polymers Based on Cyanobacterial Extracellular Polymeric Substances (EPS). International Journal of Molecular Sciences, 20(22), 5693. https://doi.org/10.3390/ijms20225693