Heterogeneous Pattern of Dependence on Anti-Apoptotic BCL-2 Family Proteins upon CHOP Treatment in Diffuse Large B-Cell Lymphoma
Abstract
:1. Introduction
2. Results
2.1. DLBCL Cells Are Dependent on BCL-2 or MCL-1, but Not BCL-XL
2.2. DLBCL Patients Show Simultaneous Expression of BCL-2, BCL-XL, and MCL-1
2.3. BCL-2 Expression Predicts Sensitivity to Venetoclax and CHOP
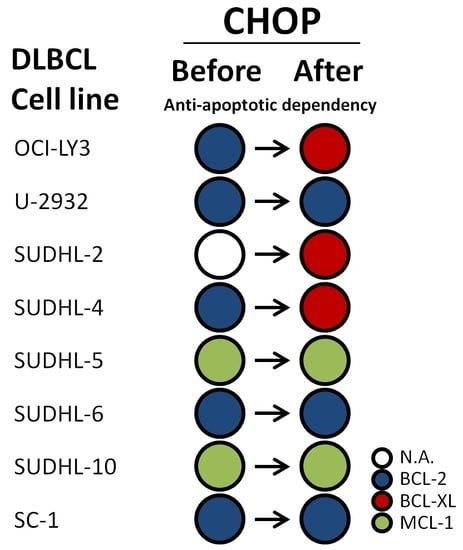
2.4. CHOP Chemotherapy Alters Dependency on Anti-Apoptotic Proteins
2.5. CHOP Chemotherapy Enhances Sensitivity to Anti-Apoptotic Inhibitors
2.6. Vincristine and Doxorubicin Change Dependency on Anti-Apoptotic Proteins
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture Conditions
4.2. Patient Material
4.3. Immunohistochemistry (IHC)
4.4. Compounds
4.5. BH3 Profiling-Plate-Based Assay
4.6. Flow Cytometry—Cell Viability for IC50
4.7. Resazurin—Metabolic Viability for Combination Therapies
4.8. Western Blot
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Maji, S.; Panda, S.; Samal, S.K.; Shriwas, O.; Rath, R.; Pellecchia, M.; Emdad, L.; Das, S.K.; Fisher, P.B.; Dash, R. Bcl-2 Antiapoptotic Family Proteins and Chemoresistance in Cancer. Adv. Cancer Res. 2018, 137, 37–75. [Google Scholar] [PubMed]
- Deng, J.; Carlson, N.; Takeyama, K.; Dal Cin, P.; Shipp, M.; Letai, A. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell 2007, 12, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Y.; Que, X.; Gao, X.; Gao, Q.; Yu, M.; Ma, K.; Xi, Y.; Wang, T. Prognostic significances of overexpression MYC and/or BCL2 in R-CHOP-treated diffuse large B-cell lymphoma: A Systematic review and meta-analysis. Sci. Rep. 2018, 8, 6267. [Google Scholar] [CrossRef] [PubMed]
- Rosenwald, A.; Wright, G.; Chan, W.C.; Connors, J.M.; Campo, E.; Fisher, R.I.; Gascoyne, R.D.; Muller-Hermelink, H.K.; Smeland, E.B.; Giltnane, J.M.; et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N. Engl. J. Med. 2002, 346, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Lenz, G.; Wright, G.W.; Emre, N.T.; Kohlhammer, H.; Dave, S.S.; Davis, R.E.; Carty, S.; Lam, L.T.; Shaffer, A.L.; Xiao, W.; et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 13520–13525. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.V.; Huang, S.; Zhang, H.; Zhang, J.; Bell, T.; Zhou, S.; Pogue, E.; Ding, Z.; Lam, L.; Westin, J.; et al. Strategic Therapeutic Targeting to Overcome Venetoclax Resistance in Aggressive B-cell Lymphomas. Clin. Cancer Res. 2018, 24, 3967–3980. [Google Scholar] [CrossRef] [PubMed]
- Davids, M.S.; Roberts, A.W.; Seymour, J.F.; Pagel, J.M.; Kahl, B.S.; Wierda, W.G.; Puvvada, S.; Kipps, T.J.; Anderson, M.A.; Salem, A.H.; et al. Phase I First-in-Human Study of Venetoclax in Patients With Relapsed or Refractory Non-Hodgkin Lymphoma. J. Clin. Oncol. 2017, 35, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Zelenetz, A.D.; Salles, G.; Mason, K.D.; Casulo, C.; Le Gouill, S.; Sehn, L.H.; Tilly, H.; Cartron, G.; Chamuleau, M.E.; Goy, A.; et al. Venetoclax plus R- or G-CHOP in non-Hodgkin lymphoma: Results from the CAVALLI phase 1b trial. Blood 2019, 133, 1964–1976. [Google Scholar] [CrossRef] [PubMed]
- Klanova, M.; Andera, L.; Brazina, J.; Svadlenka, J.; Benesova, S.; Soukup, J.; Prukova, D.; Vejmelkova, D.; Jaksa, R.; Helman, K.; et al. Targeting of BCL2 Family Proteins with ABT-199 and Homoharringtonine Reveals BCL2- and MCL1-Dependent Subgroups of Diffuse Large B-Cell Lymphoma. Clin. Cancer Res. 2016, 22, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Moore, V.D.G.; Letai, A. BH3 profiling—Measuring integrated function of the mitochondrial apoptotic pathway to predict cell fate decisions. Cancer Lett. 2013, 332, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Habermann, T.M.; Weller, E.A.; Morrison, V.A.; Gascoyne, R.D.; Cassileth, P.A.; Cohn, J.B.; Dakhil, S.R.; Woda, B.; Fisher, R.I.; Peterson, B.A.; et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J. Clin. Oncol. 2006, 24, 3121–3127. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Neppalli, V.T.; Wright, G.; Dave, B.J.; Horsman, D.E.; Rosenwald, A.; Lynch, J.; Hans, C.P.; Weisenburger, D.D.; Greiner, T.C.; et al. BCL2 expression is a prognostic marker for the activated B-cell-like type of diffuse large B-cell lymphoma. J. Clin. Oncol. 2006, 24, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Meyer, P.N.; Smith, L.M.; Johnson, N.A.; Vose, J.M.; Greiner, T.C.; Connors, J.M.; Staudt, L.M.; Rimsza, L.; Jaffe, E.; et al. BCL2 predicts survival in germinal center B-cell-like diffuse large B-cell lymphoma treated with CHOP-like therapy and rituximab. Clin. Cancer Res. 2011, 17, 7785–7795. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.M.; Dietz, A.; Henz, K.; Bruecher, D.; Jackson, R.; Kowald, L.; van Wijk, S.J.; Jayne, S.; Macip, S.; Fulda, S.; et al. Specific interactions of BCL-2 family proteins mediate sensitivity to BH3-mimetics in diffuse large B-cell lymphoma. Haematologica 2019. [Google Scholar] [CrossRef] [PubMed]
- Uchida, A.; Isobe, Y.; Asano, J.; Uemura, Y.; Hoshikawa, M.; Takagi, M.; Miura, I. Targeting BCL2 with venetoclax is a promising therapeutic strategy for “double-protein-expression” lymphoma with MYC and BCL2 rearrangements. Haematologica 2018, 104, 1417–1421. [Google Scholar] [CrossRef] [PubMed]
- Liston, D.R.; Davis, M. Clinically Relevant Concentrations of Anticancer Drugs: A Guide for Nonclinical Studies. Clin. Cancer Res. 2017, 23, 3489–3498. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.; Letai, A. BH3 profiling in whole cells by fluorimeter or FACS. Methods 2013, 61, 156–164. [Google Scholar] [CrossRef] [PubMed]
- De Jong, M.R.; Visser, L.; Huls, G.; Diepstra, A.; van Vugt, M.; Ammatuna, E.; van Rijn, R.S.; Vellenga, E.; van den Berg, A.; Fehrmann, R.S.; et al. Identification of relevant drugable targets in diffuse large B-cell lymphoma using a genome-wide unbiased CD20 guilt-by association approach. PLoS ONE 2018, 13, e0193098. [Google Scholar] [CrossRef] [PubMed] [Green Version]


 ) and cell lines with a ‘double hit’ status by closed symbols (
) and cell lines with a ‘double hit’ status by closed symbols (  ). Cell lines with the highest BCL-2 protein expression (U-2932 and SC-1) are represented by an asterisk (
). Cell lines with the highest BCL-2 protein expression (U-2932 and SC-1) are represented by an asterisk (  ).
).
 ) and cell lines with a ‘double hit’ status by closed symbols (
) and cell lines with a ‘double hit’ status by closed symbols (  ). Cell lines with the highest BCL-2 protein expression (U-2932 and SC-1) are represented by an asterisk (
). Cell lines with the highest BCL-2 protein expression (U-2932 and SC-1) are represented by an asterisk (  ).
).




| DLBCL Cases | BCL-2 Negative (n = 21) | BCL-2 Positive (n = 34) |
|---|---|---|
| BCL-XL negative | 0/21 (0%) | 1/34 (3%) |
| BCL-XL positive | 21/21 (100%) | 33/34 (97%) |
| MCL-1 negative | 1/21 (5%) | 2/34 (6%) |
| MCL-1 positive | 20/21 (95%) | 32/34 (94%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Jong, M.R.W.; Langendonk, M.; Reitsma, B.; Nijland, M.; van den Berg, A.; Ammatuna, E.; Visser, L.; van Meerten, T. Heterogeneous Pattern of Dependence on Anti-Apoptotic BCL-2 Family Proteins upon CHOP Treatment in Diffuse Large B-Cell Lymphoma. Int. J. Mol. Sci. 2019, 20, 6036. https://doi.org/10.3390/ijms20236036
de Jong MRW, Langendonk M, Reitsma B, Nijland M, van den Berg A, Ammatuna E, Visser L, van Meerten T. Heterogeneous Pattern of Dependence on Anti-Apoptotic BCL-2 Family Proteins upon CHOP Treatment in Diffuse Large B-Cell Lymphoma. International Journal of Molecular Sciences. 2019; 20(23):6036. https://doi.org/10.3390/ijms20236036
Chicago/Turabian Stylede Jong, Mathilde Rikje Willemijn, Myra Langendonk, Bart Reitsma, Marcel Nijland, Anke van den Berg, Emanuele Ammatuna, Lydia Visser, and Tom van Meerten. 2019. "Heterogeneous Pattern of Dependence on Anti-Apoptotic BCL-2 Family Proteins upon CHOP Treatment in Diffuse Large B-Cell Lymphoma" International Journal of Molecular Sciences 20, no. 23: 6036. https://doi.org/10.3390/ijms20236036
APA Stylede Jong, M. R. W., Langendonk, M., Reitsma, B., Nijland, M., van den Berg, A., Ammatuna, E., Visser, L., & van Meerten, T. (2019). Heterogeneous Pattern of Dependence on Anti-Apoptotic BCL-2 Family Proteins upon CHOP Treatment in Diffuse Large B-Cell Lymphoma. International Journal of Molecular Sciences, 20(23), 6036. https://doi.org/10.3390/ijms20236036