Functionalized Polymeric Materials with Bio-Derived Antimicrobial Peptides for “Active” Packaging
Abstract
:1. Introduction
2. Results and Discussion
2.1. Activation of PET Polymer by 1018K6
2.2. Immobilization Yield and Leakage of 1018K6 from PET Polymer
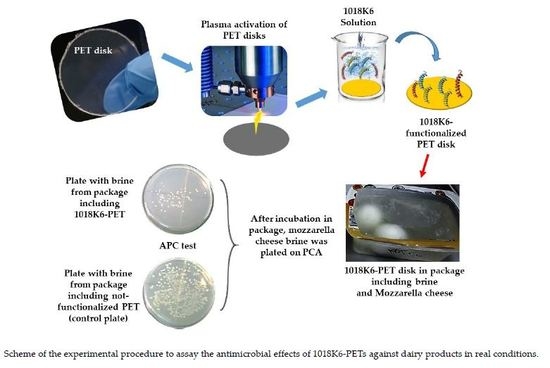
2.3. Effect of 1018K6 Functionalized PETs on Mozzarella Cheese
2.4. Inhibition of Listeria Biofilm Formation
3. Materials and Methods
3.1. Plasma Treatment
3.2. Water Contact Angle Measurements
3.3. Fourier Transform Infrared Spectroscopy
3.4. Peptide Bio-conjugation
3.5. Functionalization Yield Analysis of Polymers
3.6. Release Test
3.7. Shelf-life Testing on Mozzarella Cheese
3.8. Anti-adhesion Activity Assay
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Corradini, M.G. Shelf life of food products: From open labeling to real-time measurements. Annu. Rev. Food Sci. Technol. 2018, 9, 251–269. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.; Katzenberg, M.E. Information sharing to improve retail product freshness of perishables. Prod. Oper. Manag. 2006, 15, 57–73. [Google Scholar]
- Munoz-Bonilla, A.; Fernandez-Garcia, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Sobczak, M.; Dębek, C.; Olędzka, E.; Kozłowski, R. Polymeric systems of antimicrobial peptides—Strategies and potential applications. Molecules 2013, 18, 14122–14137. [Google Scholar] [CrossRef] [PubMed]
- Irkin, R.; Esmer, O.K. Novel food packaging systems with natural antimicrobial agents. J. Food Sci. Technol. 2015, 52, 6095–6111. [Google Scholar] [CrossRef] [Green Version]
- De Smet, K.; Contreras, R. Human antimicrobial peptides: Defensins, cathelicidins and histatins. Biotechnol. Lett. 2005, 27, 1337–1347. [Google Scholar] [CrossRef]
- Bals, R. Epithelial antimicrobial peptides in host defense against infection. Respir. Res. 2000, 1, 141–150. [Google Scholar] [CrossRef]
- Henzler Wildman, K.A.; Lee, D.K.; Ramamoorthy, A. Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry 2003, 42, 6545–6558. [Google Scholar] [CrossRef]
- Amer, L.S.; Bishop, B.M.; van Hoek, M.L. Antimicrobial and antibiofilm activity of cathelicidins and short, synthetic peptides against Francisella. Biochem. Biophys. Res. Commun. 2010, 396, 246–251. [Google Scholar] [CrossRef]
- De la Fuente-Núñez, C.; Korolik, V.; Bains, M.; Nguyen, U.; Breidenstein, E.B.; Horsman, S.; Lewenza, S.; Burrows, L.; Hancock, R.E. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob. Agents Chemother. 2012, 56, 2696–2704. [Google Scholar] [CrossRef]
- De la Fuente-Núñez, C.; Reffuveille, F.; Haney, E.F.; Straus, S.K.; Hancock, R.E. Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog. 2014, 10, e1004152. [Google Scholar] [CrossRef] [PubMed]
- Overhage, J.; Campisano, A.; Bains, M.; Torfs, E.C.; Rehm, B.H.; Hancock, R.E. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect. Immun. 2008, 76, 4176–4182. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [PubMed]
- Pompilio, A.; Scocchi, M.; Pomponio, S.; Guida, F.; Di Primio, A.; Fiscarelli, E.; Gennaro, R.; Di Bonaventura, G. Antibacterial and anti-biofilm effects of cathelicidin peptides against pathogens isolated from cystic fibrosis patients. Peptides 2011, 32, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Reffuveille, F.; de la Fuente-Núñez, C.; Mansour, S.; Hancock, R.E.W. A broad spectrum anti-biofilm peptide enhances antibiotic action against bacterial biofilms. Antimicrob. Agents Chemother. 2014, 58, 5363–5371. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, G.; Balestrieri, M.; Capuano, F.; Proroga, Y.T.R.; Pomilio, F.; Centorame, P.; Riccio, A.; Marrone, R.; Anastasio, A. Bactericidal and antibiofilm activity of bactenecin-derivative peptides against the food-pathogen Listeria monocytogenes: New perspectives for food processing industry. Int. J. Food. Microbiol. 2018, 279, 33–42. [Google Scholar] [CrossRef]
- Palmieri, G.; Balestrieri, M.; Proroga, Y.T.R.; Falcigno, L.; Facchiano, A.; Riccio, A.; Capuano, F.; Marrone, R.; Neglia, G.; Anastasio, A. New antimicrobial peptides against foodborne pathogens: From in silico design to experimental evidence. Food Chem. 2016, 211, 546–554. [Google Scholar] [CrossRef]
- Holmberg, K.V.; Abdolhosseini, M.; Li, Y.; Chen, X.; Gorr, S.-U.; Aparicio, C. Bio-inspired stable antimicrobial peptide coatings for dental applications. Acta Biomater. 2013, 9, 8224–8231. [Google Scholar] [CrossRef] [Green Version]
- Hamley, I.W. PEG−Peptide Conjugates. Biomacromolecules 2014, 15, 1543–1559. [Google Scholar] [CrossRef]
- Jordá-Vilaplana, A.; Fombuena, V.; García-García, D.; Samper, M.D.; Sánchez-Nácher, L. Surface modification of polylactic acid (PLA) by air atmospheric plasma treatment. Eur. Polym. J. 2014, 58, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Silvestre, C.; Duraccio, D.; Cimmino, S. Food packaging based on polymer nanomaterials. Prog. Polym. Sci. 2011, 36, 1766–1782. [Google Scholar] [CrossRef]
- Alavi, S.; Thomas, S.; Sandeep, K.P.; Kalarikkal, N.; Varghese, J.; Yaragalla, S. Polymers for Packaging Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2014; 486p. [Google Scholar]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; Milosavljevic, V.; O’Donnel, C.P.; Bourke, P.; Keener, K.M.; Cullen, P.J. Applications of cold plasma technology in food packaging. Trends Food Sci. Technol. 2014, 35, 5–17. [Google Scholar] [CrossRef]
- De Stefano, L.; Rotiroti, L.; De Tommasi, E.; Rea, I.; Rendina, I.; Canciello, M.; Maglio, G.; Palumbo, R. Hybrid polymer-porous silicon photonic crystals for optical sensing. J. App. Phys. 2009, 106, 023109. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies, 3rd ed.; John Wiley & Sons Ltd.: New York, NY, USA, 1994. [Google Scholar]
- De Stefano, L.; Oliviero, G.; Amato, J.; Borbone, N.; Piccialli, G.; Mayol, L.; Rendina, I.; Terracciano, M.; Rea, I. Aminosilane functionalizations of mesoporous oxidized silicon for oligonucleotide synthesis and detection. J. R. Soc. Interface 2013, 10, 20130160. [Google Scholar] [CrossRef] [PubMed]
- Holliday, R. Plant population and crop yield. Nature 1960, 186, 22–24. [Google Scholar] [CrossRef]
- Huis in‘t Veld, J.H.J. Microbial and biochemical spoilage of foods: An overview. Int. J. Food Microbiol. 1998, 33, 1–18. [Google Scholar] [CrossRef]
- Losito, F.; Arienzo, A.; Bottini, G.; Priolisi, F.R.; Mari, A.; Antonini, G. Microbiological safety and quality of Mozzarella cheese assessed by the microbiological survey method. J. Dairy Sci. 2014, 97, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Bondi, M.; Lauková, A.; de Niederhausern, S.; Messi, P.; Papadopoulou, C. Natural Preservatives to Improve Food Quality and Safety. J. Food Qual. 2017, 2017. [Google Scholar] [CrossRef]
- Beresford, M.R.; Andrew, P.W.; Shama, G. Listeria monocytogenes adheres to many materials found in food-processing environments. J. Appl. Microbiol. 2001, 90, 1000–1005. [Google Scholar] [CrossRef]
- Wong, A.C.L. Biofilms in Food Processing Environments. J. Dairy Sci. 1998, 81, 2765–2770. [Google Scholar] [CrossRef]







| Disk Diameter | Microorganisms | PET Disk in Brine + Mozzarella Cheese | 1018K6-PET Disk in Brine + Mozzarella Cheese | Inhibition of Growth (% Value) |
|---|---|---|---|---|
| 3 cm | APC | 311 ± 29 CFU/mL | 11 ± 2 CFU/mL | 97% |
| Yeasts and Molds | 700 ± 75 CFU/mL | 280 ± 25 CFU/mL | 60% | |
| 10 cm | APC | 173 ± 21 CFU/mL | 44 ± 7 CFU/mL | 75% |
| Yeasts and Molds | 406 ± 37 CFU/mL | 137 ± 23 CFU/mL | 67% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agrillo, B.; Balestrieri, M.; Gogliettino, M.; Palmieri, G.; Moretta, R.; Proroga, Y.T.R.; Rea, I.; Cornacchia, A.; Capuano, F.; Smaldone, G.; et al. Functionalized Polymeric Materials with Bio-Derived Antimicrobial Peptides for “Active” Packaging. Int. J. Mol. Sci. 2019, 20, 601. https://doi.org/10.3390/ijms20030601
Agrillo B, Balestrieri M, Gogliettino M, Palmieri G, Moretta R, Proroga YTR, Rea I, Cornacchia A, Capuano F, Smaldone G, et al. Functionalized Polymeric Materials with Bio-Derived Antimicrobial Peptides for “Active” Packaging. International Journal of Molecular Sciences. 2019; 20(3):601. https://doi.org/10.3390/ijms20030601
Chicago/Turabian StyleAgrillo, Bruna, Marco Balestrieri, Marta Gogliettino, Gianna Palmieri, Rosalba Moretta, Yolande T.R. Proroga, Ilaria Rea, Alessandra Cornacchia, Federico Capuano, Giorgio Smaldone, and et al. 2019. "Functionalized Polymeric Materials with Bio-Derived Antimicrobial Peptides for “Active” Packaging" International Journal of Molecular Sciences 20, no. 3: 601. https://doi.org/10.3390/ijms20030601
APA StyleAgrillo, B., Balestrieri, M., Gogliettino, M., Palmieri, G., Moretta, R., Proroga, Y. T. R., Rea, I., Cornacchia, A., Capuano, F., Smaldone, G., & De Stefano, L. (2019). Functionalized Polymeric Materials with Bio-Derived Antimicrobial Peptides for “Active” Packaging. International Journal of Molecular Sciences, 20(3), 601. https://doi.org/10.3390/ijms20030601