Diuretic Effects of Sodium Glucose Cotransporter 2 Inhibitors and Their Influence on the Renin-Angiotensin System
Abstract
:1. Introduction
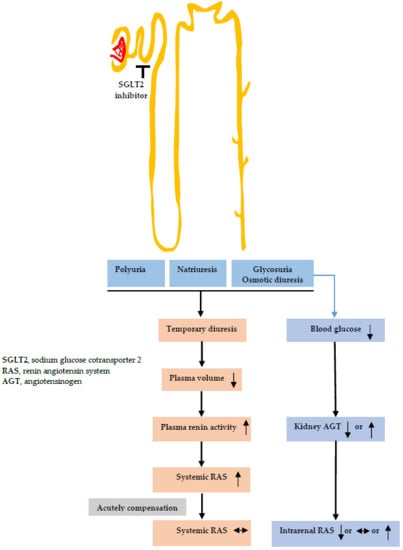
2. Diuretic Effects of SGLT2 Inhibitors
2.1. Changes in Urine Volume and Urinary Sodium Excretion
2.2. Changes in Tubular Functions
3. Effects of SGLT2 Inhibitors on RAS Activity
3.1. Systemic RAS
3.2. Intrarenal RAS
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aguillón, A.R.; Mascarello, A.; Segretti, N.D.; de Azevedo, H.F.Z.; Guimaraes, C.R.W.; Miranda, L.S.M.; de Souza, R.O.M.A. Synthetic Strategies toward SGLT2 Inhibitors. Org. Process Res. Dev. 2018, 22, 467–488. [Google Scholar] [CrossRef]
- Hsia, D.S.; Grove, O.; Cefalu, W.T. An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Tahara, A.; Takasu, T.; Yokono, M.; Imamura, M.; Kurosaki, E. Characterization and comparison of sodium-glucose cotransporter 2 inhibitors: Part 2. Antidiabetic effects in type 2 diabetic mice. J. Pharmacol. Sci. 2016, 131, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Solini, A. SGLT2 inhibition in diabetes mellitus: Rationale and clinical prospects. Nat. Rev. Endocrinol. 2012, 8, 495–502. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Davidson, J.A.; Del Prato, S. The role of the kidneys in glucose homeostasis: A new path towards normalizing glycaemia. Diabetes Obes. Metab. 2012, 14, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Lee, T.; DeFronzo, R.A. Why Do SGLT2 inhibitors inhibit only 30–50% of renal glucose reabsorption in humans? Diabetes 2012, 61, 2199–2204. [Google Scholar] [CrossRef] [PubMed]
- Rahmoune, H.; Thompson, P.W.; Ward, J.M.; Smith, C.D.; Hong, G.; Brown, J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 2005, 54, 3427–3434. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; DeFronzo, R.A.; Norton, L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30–50% of filtered glucose load in humans. Diabetes 2013, 62, 3324–3328. [Google Scholar] [CrossRef]
- Vallon, V.; Platt, K.A.; Cunard, R.; Schroth, J.; Whaley, J.; Thomson, S.C.; Koepsell, H.; Rieg, T. SGLT2 mediates glucose reabsorption in the early proximal tubule. J. Am. Soc. Nephrol. 2011, 22, 104–112. [Google Scholar] [CrossRef]
- Rieg, T.; Masuda, T.; Gerasimova, M.; Mayoux, E.; Platt, K.; Powell, D.R.; Thomson, S.C.; Koepsell, H.; Vallon, V. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am. J. Physiol. Ren. Physiol. 2014, 306, F188–F193. [Google Scholar] [CrossRef]
- Heerspink, H.J.; Perkins, B.A.; Fitchett, D.H.; Husain, M.; Cherney, D.Z. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation 2016, 134, 752–772. [Google Scholar] [CrossRef] [PubMed]
- Lytvyn, Y.; Skrtic, M.; Yang, G.K.; Yip, P.M.; Perkins, B.A.; Cherney, D.Z. Glycosuria-mediated urinary uric acid excretion in patients with uncomplicated type 1 diabetes mellitus. Am. J. Physiol. Ren. Physiol. 2015, 308, F77–F83. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, D.; Hitomi, H.; Nishiyama, A. Hypertension with diabetes mellitus complications. Hypertens. Res. 2018, 41, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Radholm, K.; Figtree, G.; Perkovic, V.; Solomon, S.D.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Barrett, T.D.; Shaw, W.; Desai, M.; et al. Canagliflozin and Heart Failure in Type 2 Diabetes Mellitus: Results From the CANVAS Program (Canagliflozin Cardiovascular Assessment Study). Circulation 2018. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Takano, K.; Iijima, H.; Kubo, H.; Maruyama, N.; Hashimoto, T.; Arakawa, K.; Togo, M.; Inagaki, N.; Kaku, K. Factors Affecting Canagliflozin-Induced Transient Urine Volume Increase in Patients with Type 2 Diabetes Mellitus. Adv. Ther. 2017, 34, 436–451. [Google Scholar] [CrossRef] [PubMed]
- Rajasekeran, H.; Lytvyn, Y.; Cherney, D.Z. Sodium-glucose cotransporter 2 inhibition and cardiovascular risk reduction in patients with type 2 diabetes: The emerging role of natriuresis. Kidney Int. 2016, 89, 524–526. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.; Polidori, D.; Heise, T.; Natarajan, J.; Farrell, K.; Wang, S.S.; Sica, D.; Rothenberg, P.; Plum-Morschel, L. Effect of the sodium glucose co-transporter 2 inhibitor canagliflozin on plasma volume in patients with type 2 diabetes mellitus. Diabetes Obes. Metab. 2014, 16, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Iijima, H.; Kifuji, T.; Maruyama, N.; Inagaki, N. Pharmacokinetics, Pharmacodynamics, and Safety of Canagliflozin in Japanese Patients with Type 2 Diabetes Mellitus. Adv. Ther. 2015, 32, 768–782. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Kittikulsuth, W.; Fujisawa, Y.; Sufiun, A.; Rafiq, K.; Hitomi, H.; Nakano, D.; Sohara, E.; Uchida, S.; Nishiyama, A. Effects of diuretics on sodium-dependent glucose cotransporter 2 inhibitor-induced changes in blood pressure in obese rats suffering from the metabolic syndrome. J. Hypertens. 2016, 34, 893–906. [Google Scholar] [CrossRef]
- Ansary, T.M.; Fujisawa, Y.; Rahman, A.; Nakano, D.; Hitomi, H.; Kobara, H.; Masaki, T.; Titze, J.M.; Kitada, K.; Nishiyama, A. Responses of renal hemodynamics and tubular functions to acute sodium-glucose cotransporter 2 inhibitor administration in non-diabetic anesthetized rats. Sci. Rep. 2017, 7, 9555. [Google Scholar] [CrossRef]
- Thomson, S.C.; Rieg, T.; Miracle, C.; Mansoury, H.; Whaley, J.; Vallon, V.; Singh, P. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R75–R83. [Google Scholar] [CrossRef]
- Takeshige, Y.; Fujisawa, Y.; Rahman, A.; Kittikulsuth, W.; Nakano, D.; Mori, H.; Masaki, T.; Ohmori, K.; Kohno, M.; Ogata, H.; et al. A sodium-glucose co-transporter 2 inhibitor empagliflozin prevents abnormality of circadian rhythm of blood pressure in salt-treated obese rats. Hypertens. Res. 2016, 39, 415–422. [Google Scholar] [CrossRef]
- Ferrannini, E.; Baldi, S.; Frascerra, S.; Astiarraga, B.; Barsotti, E.; Clerico, A.; Muscelli, E. Renal Handling of Ketones in Response to Sodium-Glucose Cotransporter 2 Inhibition in Patients With Type 2 Diabetes. Diabetes Care 2017, 40, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Lambers Heerspink, H.J.; de Zeeuw, D.; Wie, L.; Leslie, B.; List, J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes. Metab. 2013, 15, 853–862. [Google Scholar] [CrossRef]
- Reed, J.W. Impact of sodium-glucose cotransporter 2 inhibitors on blood pressure. Vasc. Health Risk Manag. 2016, 12, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; McMurray, J.J.V. SGLT2 inhibitors and mechanisms of cardiovascular benefit: A state-of-the-art review. Diabetologia 2018, 61, 2108–2117. [Google Scholar] [CrossRef]
- Lytvyn, Y.; Bjornstad, P.; Udell, J.A.; Lovshin, J.A.; Cherney, D.Z.I. Sodium Glucose Cotransporter-2 Inhibition in Heart Failure: Potential Mechanisms, Clinical Applications, and Summary of Clinical Trials. Circulation 2017, 136, 1643–1658. [Google Scholar] [CrossRef]
- Titze, J. A different view on sodium balance. Curr. Opin. Nephrol. Hypertens. 2015, 24, 14–20. [Google Scholar] [CrossRef]
- Schneider, M.P.; Raff, U.; Kopp, C.; Scheppach, J.B.; Toncar, S.; Wanner, C.; Schlieper, G.; Saritas, T.; Floege, J.; Schmid, M.; et al. Skin Sodium Concentration Correlates with Left Ventricular Hypertrophy in CKD. J. Am. Soc. Nephrol. 2017, 28, 1867–1876. [Google Scholar] [CrossRef]
- Karg, M.V.; Bosch, A.; Kannenkeril, D.; Striepe, K.; Ott, C.; Schneider, M.P.; Boemke-Zelch, F.; Linz, P.; Nagel, A.M.; Titze, J.; et al. SGLT-2-inhibition with dapagliflozin reduces tissue sodium content: A randomised controlled trial. Cardiovasc. Diabetol. 2018, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Heise, T.; Seewaldt-Becker, E.; Macha, S.; Hantel, S.; Pinnetti, S.; Seman, L.; Woerle, H.J. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks’ treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes. Metab. 2013, 15, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Weir, M.R.; Januszewicz, A.; Gilbert, R.E.; Vijapurkar, U.; Kline, I.; Fung, A.; Meininger, G. Effect of canagliflozin on blood pressure and adverse events related to osmotic diuresis and reduced intravascular volume in patients with type 2 diabetes mellitus. J. Clin. Hypertens. (Greenwich) 2014, 16, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Bode, B.; Stenlof, K.; Sullivan, D.; Fung, A.; Usiskin, K. Efficacy and safety of canagliflozin treatment in older subjects with type 2 diabetes mellitus: A randomized trial. Hosp. Pract. (1995) 2013, 41, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Yale, J.F.; Bakris, G.; Cariou, B.; Yue, D.; David-Neto, E.; Xi, L.; Figueroa, K.; Wajs, E.; Usiskin, K.; Meininger, G. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes. Metab. 2013, 15, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.; Blonde, L.; Leiter, L.A.; Cerdas, S.; Tong, C.; Yee, J.; Meininger, G. Efficacy and safety of canagliflozin by baseline HbA1c and known duration of type 2 diabetes mellitus. J. Diabetes Complicat. 2015, 29, 438–444. [Google Scholar] [CrossRef]
- Yasui, A.; Lee, G.; Hirase, T.; Kaneko, T.; Kaspers, S.; von Eynatten, M.; Okamura, T. Empagliflozin Induces Transient Diuresis Without Changing Long-Term Overall Fluid Balance in Japanese Patients with Type 2 Diabetes. Diabetes Ther. 2018, 9, 863–871. [Google Scholar] [CrossRef]
- List, J.F.; Woo, V.; Morales, E.; Tang, W.; Fiedorek, F.T. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 2009, 32, 650–657. [Google Scholar] [CrossRef]
- Han, S.; Hagan, D.L.; Taylor, J.R.; Xin, L.; Meng, W.; Biller, S.A.; Wetterau, J.R.; Washburn, W.N.; Whaley, J.M. Dapagliflozin, a selective SGLT2 inhibitor, improves glucose homeostasis in normal and diabetic rats. Diabetes 2008, 57, 1723–1729. [Google Scholar] [CrossRef]
- Zhang, W.; Welihinda, A.; Mechanic, J.; Ding, H.; Zhu, L.; Lu, Y.; Deng, Z.; Sheng, Z.; Lv, B.; Chen, Y.; et al. EGT1442, a potent and selective SGLT2 inhibitor, attenuates blood glucose and HbA(1c) levels in db/db mice and prolongs the survival of stroke-prone rats. Pharmacol. Res. 2011, 63, 284–293. [Google Scholar] [CrossRef]
- Yamamoto, K.; Uchida, S.; Kitano, K.; Fukuhara, N.; Okumura-Kitajima, L.; Gunji, E.; Kozakai, A.; Tomoike, H.; Kojima, N.; Asami, J.; et al. TS-071 is a novel, potent and selective renal sodium-glucose cotransporter 2 (SGLT2) inhibitor with anti-hyperglycaemic activity. Br. J. Pharmacol. 2011, 164, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Nagata, T.; Fukuzawa, T.; Takeda, M.; Fukazawa, M.; Mori, T.; Nihei, T.; Honda, K.; Suzuki, Y.; Kawabe, Y. Tofogliflozin, a novel sodium-glucose co-transporter 2 inhibitor, improves renal and pancreatic function in db/db mice. Br. J. Pharmacol. 2013, 170, 519–531. [Google Scholar] [CrossRef]
- Tahara, A.; Kurosaki, E.; Yokono, M.; Yamajuku, D.; Kihara, R.; Hayashizaki, Y.; Takasu, T.; Imamura, M.; Li, Q.; Tomiyama, H.; et al. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur. J. Pharmacol. 2013, 715, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Koibuchi, N.; Hasegawa, Y.; Sueta, D.; Toyama, K.; Uekawa, K.; Ma, M.; Nakagawa, T.; Kusaka, H.; Kim-Mitsuyama, S. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc. Diabetol. 2014, 13, 148. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.; Fasching, A.; Pihl, L.; Patinha, D.; Franzen, S.; Palm, F. Acute SGLT inhibition normalizes O2 tension in the renal cortex but causes hypoxia in the renal medulla in anaesthetized control and diabetic rats. Am. J. Physiol. Ren. Physiol. 2015, 309, F227–F234. [Google Scholar] [CrossRef] [PubMed]
- Gallo, L.A.; Ward, M.S.; Fotheringham, A.K.; Zhuang, A.; Borg, D.J.; Flemming, N.B.; Harvie, B.M.; Kinneally, T.L.; Yeh, S.M.; McCarthy, D.A.; et al. Once daily administration of the SGLT2 inhibitor, empagliflozin, attenuates markers of renal fibrosis without improving albuminuria in diabetic db/db mice. Sci. Rep. 2016, 6, 26428. [Google Scholar] [CrossRef] [PubMed]
- Salim, H.M.; Fukuda, D.; Yagi, S.; Soeki, T.; Shimabukuro, M.; Sata, M. Glycemic Control with Ipragliflozin, a Novel Selective SGLT2 Inhibitor, Ameliorated Endothelial Dysfunction in Streptozotocin-Induced Diabetic Mouse. Front. Cardiovasc. Med. 2016, 3, 43. [Google Scholar] [CrossRef]
- Xu, L.; Nagata, N.; Nagashimada, M.; Zhuge, F.; Ni, Y.; Chen, G.; Mayoux, E.; Kaneko, S.; Ota, T. SGLT2 Inhibition by Empagliflozin Promotes Fat Utilization and Browning and Attenuates Inflammation and Insulin Resistance by Polarizing M2 Macrophages in Diet-induced Obese Mice. EBioMedicine 2017, 20, 137–149. [Google Scholar] [CrossRef]
- Wilding, J.P.; Norwood, P.; T’Joen, C.; Bastien, A.; List, J.F.; Fiedorek, F.T. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: Applicability of a novel insulin-independent treatment. Diabetes Care 2009, 32, 1656–1662. [Google Scholar] [CrossRef]
- Cherney, D.Z.; Perkins, B.A.; Soleymanlou, N.; Maione, M.; Lai, V.; Lee, A.; Fagan, N.M.; Woerle, H.J.; Johansen, O.E.; Broedl, U.C.; et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014, 129, 587–597. [Google Scholar] [CrossRef]
- Sha, S.; Devineni, D.; Ghosh, A.; Polidori, D.; Hompesch, M.; Arnolds, S.; Morrow, L.; Spitzer, H.; Demarest, K.; Rothenberg, P. Pharmacodynamic effects of canagliflozin, a sodium glucose co-transporter 2 inhibitor, from a randomized study in patients with type 2 diabetes. PLoS ONE 2014, 9, e110069. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Seino, Y.; Fukatsu, A.; Ubukata, M.; Sakai, S.; Samukawa, Y. Pharmacokinetics, Pharmacodynamics, and Safety of Luseogliflozin in Japanese Patients with Type 2 Diabetes Mellitus: A Randomized, Single-blind, Placebo-controlled Trial. Adv. Ther. 2015, 32, 319–340. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Dohi, K.; Omori, T.; Moriwaki, K.; Sato, Y.; Nakamori, S.; Fujimoto, N.; Fujii, E.; Yamada, N.; Ito, M. Diuretic effects of sodium-glucose cotransporter 2 inhibitor in patients with type 2 diabetes mellitus and heart failure. Int. J. Cardiol. 2015, 201, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kawasoe, S.; Maruguchi, Y.; Kajiya, S.; Uenomachi, H.; Miyata, M.; Kawasoe, M.; Kubozono, T.; Ohishi, M. Mechanism of the blood pressure-lowering effect of sodium-glucose cotransporter 2 inhibitors in obese patients with type 2 diabetes. BMC Pharmacol. Toxicol. 2017, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.D.; Rash, A.; Sands, J.M.; Ecelbarger, C.M.; Tiwari, S. Candesartan Differentially Regulates Epithelial Sodium Channel in Cortex Versus Medulla of Streptozotocin-Induced Diabetic Rats. J. Epithel. Biol. Pharmacol. 2009, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Knepper, M.A.; Verbalis, J.G.; Ecelbarger, C.A. Increased renal ENaC subunit and sodium transporter abundances in streptozotocin-induced type 1 diabetes. Am. J. Physiol. Ren. Physiol. 2003, 285, F1125–F1137. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Sands, J.M.; Klein, J.D. Changes in renal medullary transport proteins during uncontrolled diabetes mellitus in rats. Am. J. Physiol. Ren. Physiol. 2003, 285, F303–F309. [Google Scholar] [CrossRef]
- Harris, R.C.; Brenner, B.M.; Seifter, J.L. Sodium-hydrogen exchange and glucose transport in renal microvillus membrane vesicles from rats with diabetes mellitus. J. Clin. Investig. 1986, 77, 724–733. [Google Scholar] [CrossRef]
- Madala Halagappa, V.K.; Tiwari, S.; Riazi, S.; Hu, X.; Ecelbarger, C.M. Chronic candesartan alters expression and activity of NKCC2, NCC, and ENaC in the obese Zucker rat. Am. J. Physiol. Ren. Physiol. 2008, 294, F1222–F1231. [Google Scholar] [CrossRef]
- Chavez-Canales, M.; Arroyo, J.P.; Ko, B.; Vazquez, N.; Bautista, R.; Castaneda-Bueno, M.; Bobadilla, N.A.; Hoover, R.S.; Gamba, G. Insulin increases the functional activity of the renal NaCl cotransporter. J. Hypertens. 2013, 31, 303–311. [Google Scholar] [CrossRef]
- Layton, A.T.; Vallon, V.; Edwards, A. Predicted consequences of diabetes and SGLT inhibition on transport and oxygen consumption along a rat nephron. Am. J. Physiol. Ren. Physiol. 2016, 310, F1269–F1283. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, T.D.; Campos, L.C.; Carraro-Lacroix, L.; Girardi, A.C.; Malnic, G. Functional role of glucose metabolism, osmotic stress, and sodium-glucose cotransporter isoform-mediated transport on Na+/H+ exchanger isoform 3 activity in the renal proximal tubule. J. Am. Soc. Nephrol. 2014, 25, 2028–2039. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Gerasimova, M.; Mayoux, E.; Masuda, T.; Vallon, V. Acute and Chronic Cmplications. Diabetes 2014, 63, A103–A170. [Google Scholar] [CrossRef]
- Novikov, A.; Vallon, V. Sodium glucose cotransporter 2 inhibition in the diabetic kidney: An update. Curr. Opin. Nephrol. Hypertens. 2016, 25, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Cassis, P.; Locatelli, M.; Cerullo, D.; Corna, D.; Buelli, S.; Zanchi, C.; Villa, S.; Morigi, M.; Remuzzi, G.; Benigni, A.; et al. SGLT2 inhibitor dapagliflozin limits podocyte damage in proteinuric nondiabetic nephropathy. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Takiyama, Y.; Sera, T.; Nakamura, M.; Ishizeki, K.; Saijo, Y.; Yanagimachi, T.; Maeda, M.; Bessho, R.; Takiyama, T.; Kitsunai, H.; et al. Impacts of Diabetes and an SGLT2 Inhibitor on the Glomerular Number and Volume in db/db Mice, as Estimated by Synchrotron Radiation Micro-CT at SPring-8. EBioMedicine 2018, 36, 329–346. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Gerasimova, M.; Rose, M.A.; Masuda, T.; Satriano, J.; Mayoux, E.; Koepsell, H.; Thomson, S.C.; Rieg, T. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am. J. Physiol. Ren. Physiol. 2014, 306, F194–F204. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, A.; Kobori, H. Independent regulation of renin-angiotensin-aldosterone system in the kidney. Clin. Exp. Nephrol. 2018, 22, 1231–1239. [Google Scholar] [CrossRef]
- Li, L.; Konishi, Y.; Morikawa, T.; Zhang, Y.; Kitabayashi, C.; Kobara, H.; Masaki, T.; Nakano, D.; Hitomi, H.; Kobori, H.; et al. Effect of a SGLT2 inhibitor on the systemic and intrarenal renin-angiotensin system in subtotally nephrectomized rats. J. Pharmacol. Sci. 2018, 137, 220–223. [Google Scholar] [CrossRef]
- Kobori, H.; Navar, L.G. Urinary Angiotensinogen as a Novel Biomarker of Intrarenal Renin-Angiotensin System in Chronic Kidney Disease. Int. Rev. Thromb. 2011, 6, 108–116. [Google Scholar]
- Nijst, P.; Verbrugge, F.H.; Martens, P.; Bertrand, P.B.; Dupont, M.; Francis, G.S.; Tang, W.W.; Mullens, W. Plasma renin activity in patients with heart failure and reduced ejection fraction on optimal medical therapy. J. Renin Angiotensin Aldosterone Syst. 2017, 18, 1470320317729919. [Google Scholar] [CrossRef] [PubMed]
- Kobori, H.; Harrison-Bernard, L.M.; Navar, L.G. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002, 61, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Satirapoj, B.; Siritaweesuk, N.; Supasyndh, O. Urinary angiotensinogen as a potential biomarker of diabetic nephropathy. Clin. Kidney J. 2014, 7, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.J.; Chung, S.; Kim, S.J.; Lee, E.M.; Yoo, Y.H.; Kim, J.W.; Ahn, Y.B.; Kim, E.S.; Moon, S.D.; Kim, M.J.; et al. Effect of Sodium-Glucose Co-Transporter 2 Inhibitor, Dapagliflozin, on Renal Renin-Angiotensin System in an Animal Model of Type 2 Diabetes. PLoS ONE 2016, 11, e0165703. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, T.; Furuki, T.; Kobori, H.; Miyakawa, M.; Imachi, H.; Murao, K.; Nishiyama, A. Effects of sodium-glucose cotransporter 2 inhibitors on urinary excretion of intact and total angiotensinogen in patients with type 2 diabetes. J. Investig. Med. 2017, 65, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- MORI, I.; ISHIZUKA, T. Effects of SGLT2 Inhibitors on Renin-Aldosterone System for One Month and Six Months in Type 2 Diabetes. Diabetes 2018, 67. [Google Scholar] [CrossRef]
- Nomiyama, T.; Shimono, D.; Horikawa, T.; Fujimura, Y.; Ohsako, T.; Terawaki, Y.; Fukuda, T.; Motonaga, R.; Tanabe, M.; Yanase, T.; et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitor ipragliflozin on glycemic control and cardiovascular parameters in Japanese patients with type 2 diabetes mellitus; Fukuoka Study of Ipragliflozin (FUSION). Endocr. J. 2018, 65, 859–867. [Google Scholar] [CrossRef]
- Lijnen, P.; Fagard, R.; Staessen, J.; Amery, A. Effect of chronic diuretic treatment on the plasma renin-angiotensin-aldosterone system in essential hypertension. Br. J. Clin. Pharmacol. 1981, 12, 387–392. [Google Scholar] [CrossRef]
- Vaughan, E.D., Jr.; Carey, R.M.; Peach, M.J.; Ackerly, J.A.; Ayers, C.R. The renin response to diuretic therapyl A limitation of antihypertensive potential. Circ. Res. 1978, 42, 376–381. [Google Scholar] [CrossRef]
- Komoroski, B.; Vachharajani, N.; Feng, Y.; Li, L.; Kornhauser, D.; Pfister, M. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin. Pharmacol. Ther. 2009, 85, 513–519. [Google Scholar] [CrossRef]
- Hollenberg, N.K.; Stevanovic, R.; Agarwal, A.; Lansang, M.C.; Price, D.A.; Laffel, L.M.; Williams, G.H.; Fisher, N.D. Plasma aldosterone concentration in the patient with diabetes mellitus. Kidney Int. 2004, 65, 1435–1439. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J.; Wilding, J.P.H. Effects of canagliflozin on cardiovascular risk factors in patients with type 2 diabetes mellitus. Int. J. Clin. Pract. 2017, 71. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, M.; Townsend, R.R.; Davies, M.J.; Vijapurkar, U.; Ren, J. Effects of canagliflozin, a sodium glucose co-transporter 2 inhibitor, on blood pressure and markers of arterial stiffness in patients with type 2 diabetes mellitus: A post hoc analysis. Cardiovasc. Diabetol. 2017, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Fujisawa, Y.; Nakano, D.; Hitomi, H.; Nishiyama, A. Effect of a selective SGLT2 inhibitor, luseogliflozin, on circadian rhythm of sympathetic nervous function and locomotor activities in metabolic syndrome rats. Clin. Exp. Pharmacol. Physiol. 2017, 44, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Wan, N.; Rahman, A.; Hitomi, H.; Nishiyama, A. The Effects of Sodium-Glucose Cotransporter 2 Inhibitors on Sympathetic Nervous Activity. Front. Endocrinol. (Lausanne) 2018, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- Matsusaka, T.; Niimura, F.; Shimizu, A.; Pastan, I.; Saito, A.; Kobori, H.; Nishiyama, A.; Ichikawa, I. Liver angiotensinogen is the primary source of renal angiotensin II. J. Am. Soc. Nephrol. 2012, 23, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Kobori, H.; Nishiyama, A.; Harrison-Bernard, L.M.; Navar, L.G. Urinary angiotensinogen as an indicator of intrarenal Angiotensin status in hypertension. Hypertension 2003, 41, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shibayama, Y.; Kobori, H.; Liu, Y.; Kobara, H.; Masaki, T.; Wang, Z.; Nishiyama, A. High glucose augments angiotensinogen in human renal proximal tubular cells through hepatocyte nuclear factor-5. PLoS ONE 2017, 12, e0185600. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.Y.; Kobori, H.; Nakano, D.; Hitomi, H.; Mori, H.; Masaki, T.; Sun, Y.X.; Zhi, N.; Zhang, L.; Huang, W.; et al. Aberrant activation of the intrarenal renin-angiotensin system in the developing kidneys of type 2 diabetic rats. Horm. Metab. Res. 2013, 45, 338–343. [Google Scholar] [CrossRef]
- Saito, T.; Urushihara, M.; Kotani, Y.; Kagami, S.; Kobori, H. Increased urinary angiotensinogen is precedent to increased urinary albumin in patients with type 1 diabetes. Am. J. Med. Sci. 2009, 338, 478–480. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, S.S.; Kim, I.J.; Song, S.H.; Kim, E.H.; Seo, J.Y.; Kim, J.H.; Kim, S.; Jeon, Y.K.; Kim, B.H.; et al. Changes in Urinary Angiotensinogen Associated with Deterioration of Kidney Function in Patients with Type 2 Diabetes Mellitus. J. Korean Med. Sci. 2017, 32, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Nishiyama, A. A4490 Effects of esaxerenone (CS-3150), a non-steroidal selective of mineralocorticoid receptor antagonist, on blood pressure and renal injury in Dahl salt-sensitive rats. J. Hypertens. 2018, 36, e18. [Google Scholar] [CrossRef]

| Subjects | Observation Period | Food Restriction, etc. | Urinary Sodium Excretion | Urine Volume | SGLT2 Inhibitor | Reference | |
|---|---|---|---|---|---|---|---|
| Animal Experiments | ZDF rats | 24 h | No | N/A | Increased by ~1.82 fold | dapagliflozin | [39] |
| SD rats | Increased by ~5.0 fold | ||||||
| Dogs | 24 h | No | Increased by ~1.50 fold | Increased by ~3.7 fold | bexagliflozin or EGT1442 | [40] | |
| ZDF rats | 24 h | No | Increased by ~1.80 fold | Increased by ~1.82 fold | luseogliflozin | [41] | |
| db/db mice | 4 weeks | No | N/A | Increased by ~1.27 fold | tofogliflozin | [42] | |
| 8 weeks | No change | ||||||
| Streptozotocin-nicotinamide induced type 2 diabetic mice | 24 h | High fat diet | N/A | Increased by ~4.6 fold | ipragliflozin | [43] | |
| db/db mice | Day 1 | No | Increased by ~1.40 fold | Increased by ~1.67 fold | empagliflozin | [44] | |
| 7 days | No change | Increased by ~1.33 fold | |||||
| Streptozotocin induced type 1 diabetic rats | 70 min | No | Increased by ~4.0 fold | Increased by ~2.28 fold | phlorizin | [45] | |
| SHRcp rats | 5 weeks | No | Increased by ~1.30 fold | Increased by ~4.0 fold | luseogliflozin | [20] | |
| OLETF rats | 12 h | 0.5% NaCl diet | Increased by ~1.30 fold | N/A | empagliflozin | [23] | |
| 5 weeks | N/A | ||||||
| db/db mice | 10 weeks | No | N/A | Increased by ~1.06 fold | empagliflozin | [46] | |
| Streptozotocin induced type 1 diabetic mice | 3 weeks | No | N/A | Increased by ~1.17 fold | ipragliflozin | [47] | |
| C57BL/6J mice | 16 weeks | High fat diet | N/A | Increased by ~4.0 fold | empagliflozin | [48] | |
| SD rats | 120 min | No | Increased by ~7.0 fold | Increased by ~1.90 fold | luseogliflozin | [21] | |
| Clinical Studies | Type 2 diabetes | 12 weeks | Standard diet | No change | Increased by ~.26 fold | dapagliflozin | [38] |
| Type 2 diabetes | 12 weeks | Standard diet | N/A | Increased by ~1.25 fold | dapagliflozin | [49] | |
| Type 1 diabetes with hyperfiltration | 8 weeks | High sodium (>140 mmol/d) and moderate protein (<1.5 g/kg/d) diet for 14 days | Increased by ~1.10 fold | Increased by ~1.56 fold | empagliflozin | [50] | |
| Type 2 diabetes | 12 weeks | Sodium restricted diet (∼200 mmol/day) | Increased by ~1.22 fold | Increased by ~1.49 fold | canagliflozin | [18] | |
| Type 2 diabetes | Day 1 | Isocaloric diet | N/A | Increased by ~1.14 fold | canagliflozin | [51] | |
| 2 weeks | No change | ||||||
| Type 2 diabetes | 7 days | Standardized meal of approximately 600 kcal | N/A | Increased by ~1.19 fold | luseogliflozin | [52] | |
| Type 2 diabetes | 4 days | No | Increased by ~1.28 fold | Increased by ~1.6 fold | ipragliflozin | [53] | |
| Type 2 diabetes | 24 h | Standard diet | No change | Increased by ~1.27 fold | canagliflozin | [19] | |
| 18 days | No change | ||||||
| Type 2 diabetes | Day 1 | No | Increased by ~1.33 fold | Increased by ~3.71 fold | canagliflozin | [16] | |
| Day 5 | No change | Increased by ~1.03 fold | |||||
| Type 2 diabetes | 6 months | Standard diet | Increased by ~1.40 fold | Increased by ~1.86 fold | ipragliflozin, dapagliflozin, tofogliflozin, luseogliflozin | [54] |
| Subjects | Observation Period | RAS Parameters | Urinary Sodium Excretion | Urine Volume | SGLT2 Inhibitor | Reference | |
|---|---|---|---|---|---|---|---|
| Animal Experiments | db/db mice | 10 weeks | PRA increased by ~1.5 fold | N/A | Increased by ~1.06 fold | empagliflozin | [46] |
| 5/6 Nx SD rats | 10 weeks | No change | N/A | N/A | luseogliflozin | [69] | |
| OLETF rats | 12 weeks | PRA no change | N/A | N/A | dapagliflozin | [74] | |
| Urinary Ang II decreased by ~30 fold | |||||||
| Urinary AGT decreased by ~5 fold | |||||||
| Plasma aldosterone no change | |||||||
| Clinical Studies | Type 2 diabetes | Day 1 | PRA no change | Increased by ~1.33 fold | Increased by ~3.71 fold | canagliflozin | [16] |
| Plasma aldosterone no change | |||||||
| Day 5 | PRA increased by ~2 fold | No change | Increased by ~1.03 fold | ||||
| Plasma aldosterone no change | |||||||
| Type 1 diabetes with hyperfiltration | 8 weeks | PRA increased by ~1.11 fold, but this change was not significant | Increased by ~1.10 fold | Increased by ~1.56 fold | empagliflozin | [50] | |
| Plasma Ang II increased by ~1.56 fold | |||||||
| Plasma aldosterone increased by ~1.72 fold | |||||||
| Type 2 diabetes | 4 days | PRA increased by ~1.0 fold, but this change was not significant | Increased by ~1.28 fold | Increased by ~1.6 fold | ipragliflozin | [53] | |
| Plasma Ang II no change | |||||||
| Plasma aldosterone no change | |||||||
| Type 2 diabetes | 1 month | total urinary AGT/creatinine ratio no changed | N/A | N/A | canagliflozin, ipragliflozin, dapagliflozin, tofogliflozin, luseogliflozin | [75] | |
| Type 2 diabetes | 1 month | PRA increased by ~3.0 fold | N/A | N/A | tofogliflozin, empagliflozin, canagliflozin | [76] | |
| Plasma aldosterone no change | |||||||
| 6 months | PRA no change | ||||||
| Plasma aldosterone no change | |||||||
| Type 2 diabetes | 24 weeks | PRA increased by ~1.59 fold, but this change was not significant | N/A | N/A | Ipragliflozin | [77] | |
| Plasma aldosterone increased by ~1.27 fold |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansary, T.M.; Nakano, D.; Nishiyama, A. Diuretic Effects of Sodium Glucose Cotransporter 2 Inhibitors and Their Influence on the Renin-Angiotensin System. Int. J. Mol. Sci. 2019, 20, 629. https://doi.org/10.3390/ijms20030629
Ansary TM, Nakano D, Nishiyama A. Diuretic Effects of Sodium Glucose Cotransporter 2 Inhibitors and Their Influence on the Renin-Angiotensin System. International Journal of Molecular Sciences. 2019; 20(3):629. https://doi.org/10.3390/ijms20030629
Chicago/Turabian StyleAnsary, Tuba M., Daisuke Nakano, and Akira Nishiyama. 2019. "Diuretic Effects of Sodium Glucose Cotransporter 2 Inhibitors and Their Influence on the Renin-Angiotensin System" International Journal of Molecular Sciences 20, no. 3: 629. https://doi.org/10.3390/ijms20030629
APA StyleAnsary, T. M., Nakano, D., & Nishiyama, A. (2019). Diuretic Effects of Sodium Glucose Cotransporter 2 Inhibitors and Their Influence on the Renin-Angiotensin System. International Journal of Molecular Sciences, 20(3), 629. https://doi.org/10.3390/ijms20030629