Biomaterials: Foreign Bodies or Tuners for the Immune Response?
Abstract
:1. Introduction
2. Immune System—Biomaterial Interplay
3. Immunological Profile of Biomaterials
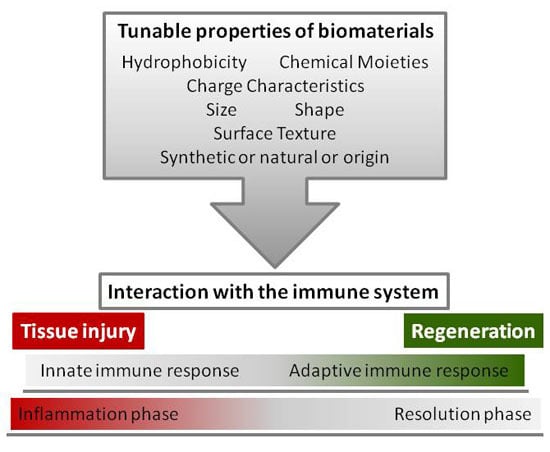
4. Tunable Properties of Biomaterials
4.1. Surface Chemistry: Hydrophobicity, Chemical Moieties, and Charge Characteristics
4.2. Topography: Size, Shape, and Surface Texture
5. Immune-Interactive Strategies
5.1. Immune Modulation by Decellularized ECM
5.2. Immunomodulation by Pro-Inflammatory Molecules
5.3. Immunomodulation by Anti-Inflammatory Molecules
5.4. Immunomodulation by Integrins, Pro-Resolving Mediators, Cells, and Regulatory Pathways
6. Concluding Remarks and Open Questions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| aAPCs | artificial antigen presenting cells |
| Arg | arginase |
| Arg1 | Arginase 1 |
| CCL | C chemokine ligand |
| CD | cluster of differentiation |
| CH3 | methyl |
| CO2 | carbon dioxide |
| COOH | carboxyl |
| CR | complement receptor |
| CXCL | CX chemokine ligand |
| DAMPs | damage-associated molecular patterns |
| DCs | dendritic cells |
| ECM | extra-cellular matrix |
| EDC | 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide |
| FBGC | foreign body giant cell |
| FBR | foreign body reaction |
| GAG | glycosaminoglycans |
| IFN | interferon |
| Ig | immunoglobulin |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| lPS | lipopolysaccharide |
| MBVs | microvesicles |
| MHC | Major histocompatibility complex |
| miRNA | micro RiboNucleic Acid |
| NETs | neutrophil extracellular traps |
| NH2 | amino |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| OH | hydroxyl |
| PAMPs | pathogen-associated molecular patterns |
| PCL | poly(caprolactone) |
| PDGF | platelet-derived growth factor |
| PEEK | poly(ether ether ketone) |
| PEG | poly(ethylene glycol) |
| PEO | poly(ethylene oxide) |
| PGA | poly(glycolic acid) |
| PLA | poly(lactic acid) |
| PLGA | poly(lactic-co-glycolic acid) |
| PPF | poly(propylene fumarate) |
| PRRs | pattern recognition receptors |
| ROS | reactive oxygen species |
| SF | silk fibroin |
| SIS | small intestine submucosa |
| SLA | sand-blasted, acid etched |
| TGF | transforming growth factor |
| Th | T helper |
| Ti | titanium |
| TLR | Toll-like receptors |
| TNF | tumor necrosis factor |
| VEGF | vascular endothelial growth factor |
References
- Vishwakarma, A.; Bhise, N.S.; Evangelista, M.B.; Rouwkema, J.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Vrana, N.E.; Khademhosseini, A. Engineering Immunomodulatory Biomaterials to Tune the Inflammatory Response. Trends Biotechnol. 2016, 34, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Wiles, K.; Fishman, J.M.; De Coppi, P.; Birchall, M.A. The Host Immune Response to Tissue-Engineered Organs: Current Problems and Future Directions. Tissue Eng. Part B Rev. 2016, 22, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Santoro, M.; Perale, G. Polymeric scaffolds as stem cell carriers in bone repair. J. Tissue Eng. Regen. Med. 2015, 9, 1093–1119. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of biomaterial-cell interactions by adsorbed proteins: A review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Keselowsky, B.G.; Collard, D.M.; Garcia, A.J. Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Biomaterials 2004, 25, 5947–5954. [Google Scholar] [CrossRef]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar] [CrossRef]
- Thevenot, P.; Hu, W.; Tang, L. Surface chemistry influences implant biocompatibility. Curr. Top. Med. Chem. 2008, 8, 270–280. [Google Scholar]
- Matlaga, B.F.; Yasenchak, L.P.; Salthouse, T.N. Tissue response to implanted polymers: The significance of sample shape. J. Biomed. Mater. Res. 1976, 10, 391–397. [Google Scholar] [CrossRef]
- Delves, P.J.; Martin, S.J.; Burton, D.R.; Roitt, I.M. Roitt’s Essential Immunology; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.; Gibson, M.; Singh, A.; Taube, J.; Furlong, C.; Murcia, M.; Elisseeff, J. PEG hydrogel degradation and the role of the surrounding tissue environment. J. Tissue Eng. Regen. Med. 2015, 9, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M. Inflammatory response to implants. ASAIO Trans. 1988, 34, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Boccafoschi, F.; Mosca, C.; Cannas, M. Cardiovascular biomaterials: When the inflammatory response helps to efficiently restore tissue functionality? J. Tissue Eng. Regen. Med. 2014, 8, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Badylak, S.F. Expanded applications, shifting paradigms and an improved understanding of host-biomaterial interactions. Acta Biomater. 2013, 9, 4948–4955. [Google Scholar] [CrossRef] [PubMed]
- Christo, S.N.; Diener, K.R.; Bachhuka, A.; Vasilev, K.; Hayball, J.D. Innate Immunity and Biomaterials at the Nexus: Friends or Foes. Biomed. Res. Int. 2015, 2015, 342304. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.; Maestas, D.R., Jr.; Housseau, F.; Elisseeff, J.H. Key players in the immune response to biomaterial scaffolds for regenerative medicine. Adv. Drug Deliv. Rev. 2017, 114, 184–192. [Google Scholar] [CrossRef]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune responses to implants—A review of the implications for the design of immunomodulatory biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef]
- Ward, W.K. A review of the foreign-body response to subcutaneously-implanted devices: The role of Macrophages and cytokines in biofouling and fibrosis. J. Diabetes Sci. Technol. 2008, 2, 768–777. [Google Scholar] [CrossRef]
- Tang, L.; Eaton, J.W. Fibrin(ogen) mediates acute inflammatory responses to biomaterials. J. Exp. Med. 1993, 178, 2147–2156. [Google Scholar] [CrossRef]
- Milleret, V.; Buzzi, S.; Gehrig, P.; Ziogas, A.; Grossmann, J.; Schilcher, K.; Zinkernagel, A.S.; Zucker, A.; Ehrbar, M. Protein adsorption steers blood contact activation on engineered cobalt chromium alloy oxide layers. Acta Biomater. 2015, 24, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Vitte, J.; Benoliel, A.M.; Pierres, A.; Bongrand, P. Is there a predictable relationship between surface physical-chemical properties and cell behaviour at the interface? Eur. Cells Mater. 2004, 7, 52–63; discussion 63. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Z.; Bai, T.; Carr, L.; Ella-Menye, J.R.; Irvin, C.; Ratner, B.D.; Jiang, S. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol. 2013, 31, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Que, R.; Wang, S.W.; Liu, W.F. Modification of biomaterials with a self-protein inhibits the macrophage response. Adv. Healthc. Mater. 2014, 3, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Ekdahl, K.N.; Lambris, J.D.; Elwing, H.; Ricklin, D.; Nilsson, P.H.; Teramura, Y.; Nicholls, I.A.; Nilsson, B. Innate immunity activation on biomaterial surfaces: A mechanistic model and coping strategies. Adv. Drug Deliv. Rev. 2011, 63, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Gorbet, M.B.; Sefton, M.V. Biomaterial-associated thrombosis: Roles of coagulation factors, complement, platelets and leukocytes. In The Biomaterials: Silver Jubilee Compendium; Elsevier Ltd.: Oxford, UK, 2006. [Google Scholar]
- Chiumiento, A.; Lamponi, S.; Barbucci, R. Role of fibrinogen conformation in platelet activation. Biomacromolecules 2007, 8, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Simonovsky, F.I.; Ratner, B.D.; Horbett, T.A. The role of adsorbed fibrinogen in platelet adhesion to polyurethane surfaces: A comparison of surface hydrophobicity, protein adsorption, monoclonal antibody binding, and platelet adhesion. J. Biomed. Mater. Res. A 2005, 74, 722–738. [Google Scholar] [CrossRef]
- Engelmann, B.; Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013, 13, 34–45. [Google Scholar] [CrossRef]
- Andersson, J.; Ekdahl, K.N.; Lambris, J.D.; Nilsson, B. Binding of C3 fragments on top of adsorbed plasma proteins during complement activation on a model biomaterial surface. Biomaterials 2005, 26, 1477–1485. [Google Scholar] [CrossRef]
- Hed, J.; Johansson, M.; Lindroth, M. Complement activation according to the alternate pathway by glass and plastic surfaces and its role in neutrophil adhesion. Immunol. Lett. 1984, 8, 295–299. [Google Scholar] [CrossRef]
- Nilsson, B.; Ekdahl, K.N.; Mollnes, T.E.; Lambris, J.D. The role of complement in biomaterial-induced inflammation. Mol. Immunol. 2007, 44, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Flick, M.J.; Du, X.; Witte, D.P.; Jirouskova, M.; Soloviev, D.A.; Busuttil, S.J.; Plow, E.F.; Degen, J.L. Leukocyte engagement of fibrin(ogen) via the integrin receptor alphaMbeta2/Mac-1 is critical for host inflammatory response in vivo. J. Clin. Investig. 2004, 113, 1596–1606. [Google Scholar] [CrossRef] [PubMed]
- Szaba, F.M.; Smiley, S.T. Roles for thrombin and fibrin(ogen) in cytokine/chemokine production and macrophage adhesion in vivo. Blood 2002, 99, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Esche, C.; Stellato, C.; Beck, L.A. Chemokines: Key players in innate and adaptive immunity. J. Investig. Dermatol. 2005, 125, 615–628. [Google Scholar] [CrossRef] [PubMed]
- McFarland, C.D.; Thomas, C.H.; DeFilippis, C.; Steele, J.G.; Healy, K.E. Protein adsorption and cell attachment to patterned surfaces. J. Biomed. Mater. Res. 2000, 49, 200–210. [Google Scholar] [CrossRef]
- Groth, T.; Zlatanov, I.; Altankov, G. Adhesion of Human Peripheral Lymphocytes on Biomaterials Preadsorbed with Fibronectin and Vitronectin. J. Biomater. Sci. Polym. Ed. 1995. [Google Scholar] [CrossRef]
- Jenney, C.R.; Anderson, J.M. Adsorbed serum proteins responsible for surface dependent human macrophage behavior. J. Biomed. Mater. Res. 2000, 49, 435–447. [Google Scholar] [CrossRef]
- McNally, A.K.; Jones, J.A.; Macewan, S.R.; Colton, E.; Anderson, J.M. Vitronectin is a critical protein adhesion substrate for IL-4-induced foreign body giant cell formation. J. Biomed. Mater. Res. A 2008, 86, 535–543. [Google Scholar] [CrossRef]
- Keselowsky, B.G.; Bridges, A.W.; Burns, K.L.; Tate, C.C.; Babensee, J.E.; LaPlaca, M.C.; Garcia, A.J. Role of plasma fibronectin in the foreign body response to biomaterials. Biomaterials 2007, 28, 3626–3631. [Google Scholar] [CrossRef]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef]
- Shen, M.; Garcia, I.; Maier, R.V.; Horbett, T.A. Effects of adsorbed proteins and surface chemistry on foreign body giant cell formation, tumor necrosis factor alpha release and procoagulant activity of monocytes. J. Biomed. Mater. Res. A 2004, 70, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Keselowsky, B.G.; Collard, D.M.; Garcia, A.J. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proc. Natl. Acad. Sci. USA 2005, 102, 5953–5957. [Google Scholar] [CrossRef] [PubMed]
- McNally, A.K.; Macewan, S.R.; Anderson, J.M. alpha subunit partners to beta1 and beta2 integrins during IL-4-induced foreign body giant cell formation. J. Biomed. Mater. Res. A 2007, 82, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Babensee, J.E. Interaction of dendritic cells with biomaterials. Semin. Immunol. 2008, 20, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.E. DAMPs, PAMPs and alarmins: All we need to know about danger. J. Leukoc. Biol. 2007, 81, 1–5. [Google Scholar] [CrossRef]
- Grandjean-Laquerriere, A.; Tabary, O.; Jacquot, J.; Richard, D.; Frayssinet, P.; Guenounou, M.; Laurent-Maquin, D.; Laquerriere, P.; Gangloff, S. Involvement of toll-like receptor 4 in the inflammatory reaction induced by hydroxyapatite particles. Biomaterials 2007, 28, 400–404. [Google Scholar] [CrossRef]
- Labow, R.S.; Meek, E.; Santerre, J.P. Neutrophil-mediated biodegradation of medical implant materials. J. Cell. Physiol. 2001, 186, 95–103. [Google Scholar] [CrossRef]
- Nimeri, G.; Majeed, M.; Elwing, H.; Ohman, L.; Wettero, J.; Bengtsson, T. Oxygen radical production in neutrophils interacting with platelets and surface-immobilized plasma proteins: Role of tyrosine phosphorylation. J. Biomed. Mater. Res. A 2003, 67, 439–447. [Google Scholar] [CrossRef]
- Nimeri, G.; Ohman, L.; Elwing, H.; Wettero, J.; Bengtsson, T. The influence of plasma proteins and platelets on oxygen radical production and F-actin distribution in neutrophils adhering to polymer surfaces. Biomaterials 2002, 23, 1785–1795. [Google Scholar] [CrossRef]
- Wettero, J.; Bengtsson, T.; Tengvall, P. Complement activation on immunoglobulin G-coated hydrophobic surfaces enhances the release of oxygen radicals from neutrophils through an actin-dependent mechanism. J. Biomed. Mater. Res. 2000, 51, 742–751. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.; Schauer, C.; Czegley, C.; Kling, L.; Petru, L.; Schmid, B.; Weidner, D.; Reinwald, C.; Biermann, M.H.C.; Blunder, S.; et al. Aggregated neutrophil extracellular traps resolve inflammation by proteolysis of cytokines and chemokines and protection from antiproteases. FASEB J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Schauer, C.; Janko, C.; Munoz, L.E.; Zhao, Y.; Kienhofer, D.; Frey, B.; Lell, M.; Manger, B.; Rech, J.; Naschberger, E.; et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 2014, 20, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Selders, G.S.; Fetz, A.E.; Radic, M.Z.; Bowlin, G.L. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen. Biomater. 2017, 4, 55–68. [Google Scholar] [CrossRef]
- Branzk, N.; Lubojemska, A.; Hardison, S.E.; Wang, Q.; Gutierrez, M.G.; Brown, G.D.; Papayannopoulos, V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 2014, 15, 1017–1025. [Google Scholar] [CrossRef]
- McNally, A.K.; Anderson, J.M. Macrophage fusion and multinucleated giant cells of inflammation. Adv. Exp. Med. Biol. 2011, 713, 97–111. [Google Scholar] [CrossRef]
- Scapini, P.; Lapinet-Vera, J.A.; Gasperini, S.; Calzetti, F.; Bazzoni, F.; Cassatella, M.A. The neutrophil as a cellular source of chemokines. Immunol. Rev. 2000, 177, 195–203. [Google Scholar] [CrossRef]
- Yamashiro, S.; Kamohara, H.; Wang, J.M.; Yang, D.; Gong, W.H.; Yoshimura, T. Phenotypic and functional change of cytokine-activated neutrophils: Inflammatory neutrophils are heterogeneous and enhance adaptive immune responses. J. Leukoc. Biol. 2001, 69, 698–704. [Google Scholar] [PubMed]
- Altieri, D.C.; Mannucci, P.M.; Capitanio, A.M. Binding of fibrinogen to human monocytes. J. Clin. Investig. 1986, 78, 968–976. [Google Scholar] [CrossRef]
- Trezzini, C.; Jungi, T.W.; Kuhnert, P.; Peterhans, E. Fibrinogen association with human monocytes: Evidence for constitutive expression of fibrinogen receptors and for involvement of Mac-1 (CD18, CR3) in the binding. Biochem. Biophys. Res. Commun. 1988, 156, 477–484. [Google Scholar] [CrossRef]
- Mesure, L.; De Visscher, G.; Vranken, I.; Lebacq, A.; Flameng, W. Gene expression study of monocytes/macrophages during early foreign body reaction and identification of potential precursors of myofibroblasts. PLoS ONE 2010, 5, e12949. [Google Scholar] [CrossRef]
- Badylak, S.F.; Gilbert, T.W. Immune response to biologic scaffold materials. Semin. Immunol. 2008, 20, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, R.; Cameron, A.R.; Kelly, D.J.; Kearney, C.J.; O’Brien, F.J. Biomaterial Based Modulation of macrophage polarization: A review and suggested design principles. Mater. Today 2015, 18, 313–325. [Google Scholar] [CrossRef]
- Jones, J.A.; Chang, D.T.; Meyerson, H.; Colton, E.; Kwon, I.K.; Matsuda, T.; Anderson, J.M. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. J. Biomed. Mater. Res. A 2007, 83, 585–596. [Google Scholar] [CrossRef]
- Lynn, A.D.; Kyriakides, T.R.; Bryant, S.J. Characterization of the in vitro macrophage response and in vivo host response to poly(ethylene glycol)-based hydrogels. J. Biomed. Mater. Res. A 2010, 93, 941–953. [Google Scholar] [CrossRef]
- Zhao, Q.; Topham, N.; Anderson, J.M.; Hiltner, A.; Lodoen, G.; Payet, C.R. Foreign-body giant cells and polyurethane biostability: In vivo correlation of cell adhesion and surface cracking. J. Biomed. Mater. Res. 1991, 25, 177–183. [Google Scholar] [CrossRef]
- Labrousse, A.M.; Meunier, E.; Record, J.; Labernadie, A.; Beduer, A.; Vieu, C.; Ben Safta, T.; Maridonneau-Parini, I. Frustrated phagocytosis on micro-patterned immune complexes to characterize lysosome movements in live macrophages. Front. Immunol. 2011, 2, 51. [Google Scholar] [CrossRef]
- Underhill, D.M.; Goodridge, H.S. Information processing during phagocytosis. Nat. Rev. Immunol. 2012, 12, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Pullen, N.A.; Oskeritzian, C.A.; Ryan, J.J.; Bowlin, G.L. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials 2013, 34, 4439–4451. [Google Scholar] [CrossRef] [PubMed]
- Broughton, G., 2nd; Janis, J.E.; Attinger, C.E. The basic science of wound healing. Plast. Reconstr. Surg. 2006, 117, 12S–34S. [Google Scholar] [CrossRef] [PubMed]
- Kyriakides, T.R.; Foster, M.J.; Keeney, G.E.; Tsai, A.; Giachelli, C.M.; Clark-Lewis, I.; Rollins, B.J.; Bornstein, P. The CC chemokine ligand, CCL2/MCP1, participates in macrophage fusion and foreign body giant cell formation. Am. J. Pathol. 2004, 165, 2157–2166. [Google Scholar] [CrossRef]
- Brodbeck, W.G.; Macewan, M.; Colton, E.; Meyerson, H.; Anderson, J.M. Lymphocytes and the foreign body response: Lymphocyte enhancement of macrophage adhesion and fusion. J. Biomed. Mater. Res. A 2005, 74, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Burd, P.R.; Thompson, W.C.; Max, E.E.; Mills, F.C. Activated mast cells produce interleukin 13. J. Exp. Med. 1995, 181, 1373–1380. [Google Scholar] [CrossRef]
- Venkayya, R.; Lam, M.; Willkom, M.; Grunig, G.; Corry, D.B.; Erle, D.J. The Th2 lymphocyte products IL-4 and IL-13 rapidly induce airway hyperresponsiveness through direct effects on resident airway cells. Am. J. Respir. Cell Mol. Biol. 2002, 26, 202–208. [Google Scholar] [CrossRef]
- Rezzani, R.; Rodella, L.; Tartaglia, G.M.; Paganelli, C.; Sapelli, P.; Bianchi, R. Mast cells and the inflammatory response to different implanted biomaterials. Arch. Histol. Cytol. 2004, 67, 211–217. [Google Scholar] [CrossRef]
- Tang, L.; Jennings, T.A.; Eaton, J.W. Mast cells mediate acute inflammatory responses to implanted biomaterials. Proc. Natl. Acad. Sci. USA 1998, 95, 8841–8846. [Google Scholar] [CrossRef]
- Christenson, L.; Wahlberg, L.; Aebischer, P. Mast cells and tissue reaction to intraperitoneally implanted polymer capsules. J. Biomed. Mater. Res. 1991, 25, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Zdolsek, J.; Eaton, J.W.; Tang, L. Histamine release and fibrinogen adsorption mediate acute inflammatory responses to biomaterial implants in humans. J. Transl. Med. 2007, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Kanbe, N.; Kurosawa, M.; Nagata, H.; Saitoh, H.; Miyachi, Y. Cord blood-derived human cultured mast cells produce transforming growth factor beta1. Clin. Exp. Allergy 1999, 29, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Crivellato, E. Mast cells, angiogenesis, and tumour growth. Biochim. Biophys. Acta 2012, 1822, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Tacchini-Cottier, F.; Zweifel, C.; Belkaid, Y.; Mukankundiye, C.; Vasei, M.; Launois, P.; Milon, G.; Louis, J.A. An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major. J. Immunol. 2000, 165, 2628–2636. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yabluchanskiy, A.; Iyer, R.P.; Cannon, P.L.; Flynn, E.R.; Jung, M.; Henry, J.; Cates, C.A.; Deleon-Pennell, K.Y.; Lindsey, M.L. Temporal neutrophil polarization following myocardial infarction. Cardiovasc. Res. 2016, 110, 51–61. [Google Scholar] [CrossRef]
- Chen, F.; Wu, W.; Millman, A.; Craft, J.F.; Chen, E.; Patel, N.; Boucher, J.L.; Urban, J.F., Jr.; Kim, C.C.; Gause, W.C. Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat. Immunol. 2014, 15, 938–946. [Google Scholar] [CrossRef]
- Yokoyama, M.; Nakahashi, T.; Nishimura, T.; Maeda, M.; Inoue, S.; Kataoka, K.; Sakurai, Y. Adhesion behavior of rat lymphocytes to poly(ether)-poly(amino acid) block and graft copolymers. J. Biomed. Mater. Res. 1986, 20, 867–878. [Google Scholar] [CrossRef]
- Curtsinger, J.M.; Mescher, M.F. Inflammatory cytokines as a third signal for T cell activation. Curr. Opin. Immunol. 2010, 22, 333–340. [Google Scholar] [CrossRef]
- Curtsinger, J.M.; Schmidt, C.S.; Mondino, A.; Lins, D.C.; Kedl, R.M.; Jenkins, M.K.; Mescher, M.F. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J. Immunol. 1999, 162, 3256–3262. [Google Scholar]
- Rodriguez, A.; Voskerician, G.; Meyerson, H.; MacEwan, S.R.; Anderson, J.M. T cell subset distributions following primary and secondary implantation at subcutaneous biomaterial implant sites. J. Biomed. Mater. Res. A 2008, 85, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.T.; Colton, E.; Anderson, J.M. Paracrine and juxtacrine lymphocyte enhancement of adherent macrophage and foreign body giant cell activation. J. Biomed. Mater. Res. A 2009, 89, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M. In Vitro and In Vivo Monocyte, Macrophage, Foreign Body Giant Cell, and Lymphocyte Interactions with Biomaterials. In Biological Interactions on Materials Surfaces; Springer: New York, NY, USA, 2009. [Google Scholar]
- Shen, E.C.; Chou, T.C.; Gau, C.H.; Tu, H.P.; Chen, Y.T.; Fu, E. Releasing growth factors from activated human platelets after chitosan stimulation: A possible bio-material for platelet-rich plasma preparation. Clin. Oral Implants Res. 2006, 17, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.S.; Ehrlich, H.P.; Brem, H. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 2009, 153, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jones, J.A.; Xu, Y.; Low, H.Y.; Anderson, J.M.; Leong, K.W. Characterization of topographical effects on macrophage behavior in a foreign body response model. Biomaterials 2010, 31, 3479–3491. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Sell, S.A.; Madurantakam, P.; Bowlin, G.L. Angiogenic potential of human macrophages on electrospun bioresorbable vascular grafts. Biomed. Mater. 2009, 4, 031001. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, K.; Rubinstein, J.; Lopez, B.S.; Arvidson, K. Production of transforming growth factor beta1 and prostaglandin E2 by osteoblast-like cells cultured on titanium surfaces blasted with TiO2 particles. Clin. Oral Implants Res. 2003, 14, 50–56. [Google Scholar] [CrossRef]
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef]
- Castro, P.R.; Marques, S.M.; Campos, P.P.; Cardoso, C.C.; Sampaio, F.P.; Ferreira, M.A.; Andrade, S.P. Kinetics of implant-induced inflammatory angiogenesis in abdominal muscle wall in mice. Microvasc. Res. 2012, 84, 9–15. [Google Scholar] [CrossRef]
- Oviedo-Socarras, T.; Vasconcelos, A.C.; Barbosa, I.X.; Pereira, N.B.; Campos, P.P.; Andrade, S.P. Diabetes alters inflammation, angiogenesis, and fibrogenesis in intraperitoneal implants in rats. Microvasc. Res. 2014, 93, 23–29. [Google Scholar] [CrossRef]
- Ratner, B.D. Reducing capsular thickness and enhancing angiogenesis around implant drug release systems. J. Control. Release 2002, 78, 211–218. [Google Scholar] [CrossRef]
- Ratner, B.D.; Bryant, S.J. Biomaterials: Where we have been and where we are going. Annu. Rev. Biomed. Eng. 2004, 6, 41–75. [Google Scholar] [CrossRef] [PubMed]
- Rockey, D.C.; Bell, P.D.; Hill, J.A. Fibrosis—A common pathway to organ injury and failure. N. Engl. J. Med. 2015, 372, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, D.M.; van Loosdregt, J.; Gorlani, A.; Bekker, C.P.; Grone, A.; Sibilia, M.; van Bergen en Henegouwen, P.M.; Roovers, R.C.; Coffer, P.J.; Sijts, A.J. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity 2013, 38, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Green, J.A.; Moltedo, B.; Arvey, A.; Hemmers, S.; Yuan, S.; Treuting, P.M.; Rudensky, A.Y. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell 2015, 162, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, D.M.W.; Gause, W.C.; Osborne, L.C.; Artis, D. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity 2015, 42, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Burzyn, D.; Kuswanto, W.; Kolodin, D.; Shadrach, J.L.; Cerletti, M.; Jang, Y.; Sefik, E.; Tan, T.G.; Wagers, A.J.; Benoist, C.; et al. A special population of regulatory T cells potentiates muscle repair. Cell 2013, 155, 1282–1295. [Google Scholar] [CrossRef] [PubMed]
- Weirather, J.; Hofmann, U.D.; Beyersdorf, N.; Ramos, G.C.; Vogel, B.; Frey, A.; Ertl, G.; Kerkau, T.; Frantz, S. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ. Res. 2014, 115, 55–67. [Google Scholar] [CrossRef]
- Nosbaum, A.; Prevel, N.; Truong, H.A.; Mehta, P.; Ettinger, M.; Scharschmidt, T.C.; Ali, N.H.; Pauli, M.L.; Abbas, A.K.; Rosenblum, M.D. Cutting Edge: Regulatory T Cells Facilitate Cutaneous Wound Healing. J. Immunol. 2016, 196, 2010–2014. [Google Scholar] [CrossRef]
- Vinish, M.; Cui, W.; Stafford, E.; Bae, L.; Hawkins, H.; Cox, R.; Toliver-Kinsky, T. Dendritic cells modulate burn wound healing by enhancing early proliferation. Wound Repair Regen. 2016, 24, 6–13. [Google Scholar] [CrossRef]
- Anzai, A.; Anzai, T.; Nagai, S.; Maekawa, Y.; Naito, K.; Kaneko, H.; Sugano, Y.; Takahashi, T.; Abe, H.; Mochizuki, S.; et al. Regulatory role of dendritic cells in postinfarction healing and left ventricular remodeling. Circulation 2012, 125, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Rutella, S.; Danese, S.; Leone, G. Tolerogenic dendritic cells: Cytokine modulation comes of age. Blood 2006, 108, 1435–1440. [Google Scholar] [CrossRef]
- Lutz, M.B.; Schuler, G. Immature, semi-mature and fully mature dendritic cells: Which signals induce tolerance or immunity? Trends Immunol. 2002, 23, 445–449. [Google Scholar] [CrossRef]
- Babensee, J.E.; Stein, M.M.; Moore, L. Interconnections between inflammatory and immune responses in tissue engineering. Ann. N. Y. Acad. Sci. 2002, 961, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Babensee, J.E. Poly(lactic-co-glycolic acid) enhances maturation of human monocyte-derived dendritic cells. J. Biomed. Mater. Res. A 2004, 71, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Keselowsky, B.G.; Lewis, J.S. Dendritic cells in the host response to implanted materials. Semin. Immunol. 2017, 29, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef]
- Wu, L.; Ong, S.; Talor, M.V.; Barin, J.G.; Baldeviano, G.C.; Kass, D.A.; Bedja, D.; Zhang, H.; Sheikh, A.; Margolick, J.B.; et al. Cardiac fibroblasts mediate IL-17A-driven inflammatory dilated cardiomyopathy. J. Exp. Med. 2014, 211, 1449–1464. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Hirota, K.; Cua, D.J.; Stockinger, B.; Veldhoen, M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity 2009, 31, 321–330. [Google Scholar] [CrossRef]
- Housseau, F.; Wu, S.; Wick, E.C.; Fan, H.; Wu, X.; Llosa, N.J.; Smith, K.N.; Tam, A.; Ganguly, S.; Wanyiri, J.W.; et al. Redundant Innate and Adaptive Sources of IL17 Production Drive Colon Tumorigenesis. Cancer Res. 2016, 76, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, K.; Witherden, D.A.; Havran, W.L. All hands on DE(T)C: Epithelial-resident gammadelta T cells respond to tissue injury. Cell. Immunol. 2015, 296, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.; Ugarte, K.; Chen, N.; Yachi, P.; Fuchs, E.; Boismenu, R.; Havran, W.L. A role for skin gammadelta T cells in wound repair. Science 2002, 296, 747–749. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Okamoto, K.; Nakashima, T.; Nitta, T.; Hori, S.; Iwakura, Y.; Takayanagi, H. IL-17-producing gammadelta T cells enhance bone regeneration. Nat. Commun. 2016, 7, 10928. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Burns, A.R.; Miller, S.B.; Smith, C.W. CCL20, gammadelta T cells, and IL-22 in corneal epithelial healing. FASEB J. 2011, 25, 2659–2668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Magadi, S.; Li, Z.; Smith, C.W.; Burns, A.R. IL-20 promotes epithelial healing of the injured mouse cornea. Exp. Eye Res. 2017, 154, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.A.; Corless, D.J.; Aspinall, R.; Wastell, C. Effect of CD4(+) and CD8(+) cell depletion on wound healing. Br. J. Surg. 2001, 88, 298–304. [Google Scholar] [CrossRef]
- Reinke, S.; Geissler, S.; Taylor, W.R.; Schmidt-Bleek, K.; Juelke, K.; Schwachmeyer, V.; Dahne, M.; Hartwig, T.; Akyuz, L.; Meisel, C.; et al. Terminally differentiated CD8(+) T cells negatively affect bone regeneration in humans. Sci. Transl. Med. 2013, 5, 177ra136. [Google Scholar] [CrossRef]
- Konnecke, I.; Serra, A.; El Khassawna, T.; Schlundt, C.; Schell, H.; Hauser, A.; Ellinghaus, A.; Volk, H.D.; Radbruch, A.; Duda, G.N.; et al. T and B cells participate in bone repair by infiltrating the fracture callus in a two-wave fashion. Bone 2014, 64, 155–165. [Google Scholar] [CrossRef]
- Klose, C.S.; Artis, D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat. Immunol. 2016, 17, 765–774. [Google Scholar] [CrossRef]
- Halim, T.Y.; Steer, C.A.; Matha, L.; Gold, M.J.; Martinez-Gonzalez, I.; McNagny, K.M.; McKenzie, A.N.; Takei, F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 2014, 40, 425–435. [Google Scholar] [CrossRef]
- Mirchandani, A.S.; Besnard, A.G.; Yip, E.; Scott, C.; Bain, C.C.; Cerovic, V.; Salmond, R.J.; Liew, F.Y. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J. Immunol. 2014, 192, 2442–2448. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Paul, W.E. Inflammatory group 2 innate lymphoid cells. Int. Immunol. 2016, 28, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Sadtler, K.; Estrellas, K.; Allen, B.W.; Wolf, M.T.; Fan, H.; Tam, A.J.; Patel, C.H.; Luber, B.S.; Wang, H.; Wagner, K.R.; et al. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science 2016, 352, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; McNally, A.K. Biocompatibility of implants: Lymphocyte/macrophage interactions. Semin. Immunopathol. 2011, 33, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Matassi, F.; Nistri, L.; Chicon Paez, D.; Innocenti, M. New biomaterials for bone regeneration. Clin. Cases Miner. Bone Metab. 2011, 8, 21–24. [Google Scholar] [PubMed]
- Gao, C.; Deng, Y.; Feng, P.; Mao, Z.; Li, P.; Yang, B.; Deng, J.; Cao, Y.; Shuai, C.; Peng, S. Current progress in bioactive ceramic scaffolds for bone repair and regeneration. Int. J. Mol. Sci. 2014, 15, 4714–4732. [Google Scholar] [CrossRef] [PubMed]
- Vroman, I.; Tighzert, L. Biodegradable polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Paknejad, Z.; Rad, M.R.; Motamedian, S.R.; Eghbal, M.J.; Nadjmi, N.; Khojasteh, A. Polymeric scaffolds in tissue engineering: A literature review. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 431–459. [Google Scholar] [CrossRef] [PubMed]
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive polymeric scaffolds for tissue engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Janouskova, O. Synthetic polymer scaffolds for soft tissue engineering. Physiol. Res. 2018, 67, S335–S348. [Google Scholar] [PubMed]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Tang, L. Influence of scaffold design on host immune and stem cell responses. Semin. Immunol. 2017, 29, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Rnjak-Kovacina, J.; Tang, F.; Whitelock, J.M.; Lord, M.S. Glycosaminoglycan and Proteoglycan-Based Biomaterials: Current Trends and Future Perspectives. Adv. Healthc. Mater. 2018, 7, e1701042. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Wlodarczyk-Biegun, M.K.; Del Campo, A. 3D bioprinting of structural proteins. Biomaterials 2017, 134, 180–201. [Google Scholar] [CrossRef] [PubMed]
- Sproul, E.; Nandi, S.; Brown, A. Fibrin biomaterials for tissue regeneration and repair. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Barbosa, M.A., Martins, M.C.L., Eds.; Woodhead Publishing: Sawston, Cambridge, UK, 2018; pp. 151–173. [Google Scholar]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wang, H.; Wei, K.; Yang, Y.; Zheng, R.Y.; Kim, I.S.; Zhang, K.Q. A Review of Structure Construction of Silk Fibroin Biomaterials from Single Structures to Multi-Level Structures. Int. J. Mol. Sci. 2017, 18, 237. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Gerber, M.H.; Babensee, J.E. Phenotype and polarization of autologous T cells by biomaterial-treated dendritic cells. J. Biomed. Mater. Res. A 2015, 103, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.H.; Stamer, D.K.; Kyriakides, T.R. The host response to naturally-derived extracellular matrix biomaterials. Semin. Immunol. 2017, 29, 72–91. [Google Scholar] [CrossRef] [PubMed]
- Hotaling, N.A.; Tang, L.; Irvine, D.J.; Babensee, J.E. Biomaterial Strategies for Immunomodulation. Annu. Rev. Biomed. Eng. 2015, 17, 317–349. [Google Scholar] [CrossRef]
- Mora-Solano, C.; Collier, J.H. Engaging adaptive immunity with biomaterials. J. Mater. Chem. B 2014, 2, 2409–2421. [Google Scholar] [CrossRef] [PubMed]
- Panilaitis, B.; Altman, G.H.; Chen, J.; Jin, H.J.; Karageorgiou, V.; Kaplan, D.L. Macrophage responses to silk. Biomaterials 2003, 24, 3079–3085. [Google Scholar] [CrossRef]
- Reeves, A.R.; Spiller, K.L.; Freytes, D.O.; Vunjak-Novakovic, G.; Kaplan, D.L. Controlled release of cytokines using silk-biomaterials for macrophage polarization. Biomaterials 2015, 73, 272–283. [Google Scholar] [CrossRef]
- Bhattacharjee, M.; Schultz-Thater, E.; Trella, E.; Miot, S.; Das, S.; Loparic, M.; Ray, A.R.; Martin, I.; Spagnoli, G.C.; Ghosh, S. The role of 3D structure and protein conformation on the innate and adaptive immune responses to silk-based biomaterials. Biomaterials 2013, 34, 8161–8171. [Google Scholar] [CrossRef]
- Nakamura, K.; Yokohama, S.; Yoneda, M.; Okamoto, S.; Tamaki, Y.; Ito, T.; Okada, M.; Aso, K.; Makino, I. High, but not low, molecular weight hyaluronan prevents T-cell-mediated liver injury by reducing proinflammatory cytokines in mice. J. Gastroenterol. 2004, 39, 346–354. [Google Scholar] [CrossRef]
- Je, J.Y.; Kim, S.K. Reactive oxygen species scavenging activity of aminoderivatized chitosan with different degree of deacetylation. Bioorg. Med. Chem. 2006, 14, 5989–5994. [Google Scholar] [CrossRef] [PubMed]
- Orenstein, S.B.; Qiao, Y.; Klueh, U.; Kreutzer, D.L.; Novitsky, Y.W. Activation of human mononuclear cells by porcine biologic meshes in vitro. Hernia 2010, 14, 401–407. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Maitra, R.; Clement, C.C.; Scharf, B.; Crisi, G.M.; Chitta, S.; Paget, D.; Purdue, P.E.; Cobelli, N.; Santambrogio, L. Endosomal damage and TLR2 mediated inflammasome activation by alkane particles in the generation of aseptic osteolysis. Mol. Immunol. 2009, 47, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Seong, S.Y.; Matzinger, P. Hydrophobicity: An ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 2004, 4, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Moyano, D.F.; Goldsmith, M.; Solfiell, D.J.; Landesman-Milo, D.; Miranda, O.R.; Peer, D.; Rotello, V.M. Nanoparticle hydrophobicity dictates immune response. J. Am. Chem. Soc. 2012, 134, 3965–3967. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ravensbergen, K.; Alabanza, A.; Soldin, D.; Hahm, J.I. Distinct adsorption configurations and self-assembly characteristics of fibrinogen on chemically uniform and alternating surfaces including block copolymer nanodomains. ACS Nano 2014, 8, 5257–5269. [Google Scholar] [CrossRef]
- Kumar, N.; Parajuli, O.; Gupta, A.; Hahm, J.I. Elucidation of protein adsorption behavior on polymeric surfaces: Toward high-density, high-payload protein templates. Langmuir 2008, 24, 2688–2694. [Google Scholar] [CrossRef]
- Absolom, D.R.; Zingg, W.; Neumann, A.W. Protein adsorption to polymer particles: Role of surface properties. J. Biomed. Mater. Res. 1987, 21, 161–171. [Google Scholar] [CrossRef]
- Ouberai, M.M.; Xu, K.; Welland, M.E. Effect of the interplay between protein and surface on the properties of adsorbed protein layers. Biomaterials 2014, 35, 6157–6163. [Google Scholar] [CrossRef]
- Kakizawa, Y.; Lee, J.S.; Bell, B.; Fahmy, T.M. Precise manipulation of biophysical particle parameters enables control of proinflammatory cytokine production in presence of TLR 3 and 4 ligands. Acta Biomater. 2017, 57, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yang, P.; Guo, X.; Huang, N.; Shen, R. An in vitro evaluation of inflammation response of titanium functionalized with heparin/fibronectin complex. Cytokine 2011, 56, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Alfarsi, M.A.; Hamlet, S.M.; Ivanovski, S. Titanium surface hydrophilicity modulates the human macrophage inflammatory cytokine response. J. Biomed. Mater. Res. A 2014, 102, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.T.; Colton, E.; Matsuda, T.; Anderson, J.M. Lymphocyte adhesion and interactions with biomaterial adherent macrophages and foreign body giant cells. J. Biomed. Mater. Res. A 2009, 91, 1210–1220. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Peppas, N.A.; Kavimandan, N.J. Nanoscale analysis of protein and peptide absorption: Insulin absorption using complexation and pH-sensitive hydrogels as delivery vehicles. Eur. J. Pharm. Sci. 2006, 29, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Tiller, J.C.; Bonner, G.; Pan, L.C.; Klibanov, A.M. Improving biomaterial properties of collagen films by chemical modification. Biotechnol Bioeng 2001, 73, 246–252. [Google Scholar] [CrossRef]
- Blaszykowski, C.; Sheikh, S.; Thompson, M. Biocompatibility and antifouling: Is there really a link? Trends Biotechnol. 2014, 32, 61–62. [Google Scholar] [CrossRef]
- Maitra, R.; Clement, C.C.; Crisi, G.M.; Cobelli, N.; Santambrogio, L. Immunogenecity of modified alkane polymers is mediated through TLR1/2 activation. PloS ONE 2008, 3, e2438. [Google Scholar] [CrossRef]
- Andorko, J.I.; Jewell, C.M. Designing biomaterials with immunomodulatory properties for tissue engineering and regenerative medicine. Bioeng. Transl. Med. 2017, 2, 139–155. [Google Scholar] [CrossRef]
- Barbosa, J.N.; Barbosa, M.A.; Aguas, A.P. Inflammatory responses and cell adhesion to self-assembled monolayers of alkanethiolates on gold. Biomaterials 2004, 25, 2557–2563. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.N.; Madureira, P.; Barbosa, M.A.; Aguas, A.P. The influence of functional groups of self-assembled monolayers on fibrous capsule formation and cell recruitment. J. Biomed. Mater. Res. A 2006, 76, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Bartneck, M.; Keul, H.A.; Singh, S.; Czaja, K.; Bornemann, J.; Bockstaller, M.; Moeller, M.; Zwadlo-Klarwasser, G.; Groll, J. Rapid uptake of gold nanorods by primary human blood phagocytes and immunomodulatory effects of surface chemistry. ACS Nano 2010, 4, 3073–3086. [Google Scholar] [CrossRef] [PubMed]
- Christo, S.N.; Bachhuka, A.; Diener, K.R.; Mierczynska, A.; Hayball, J.D.; Vasilev, K. The Role of Surface Nanotopography and Chemistry on Primary Neutrophil and Macrophage Cellular Responses. Adv. Healthc. Mater. 2016, 5, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Getts, D.R.; Terry, R.L.; Getts, M.T.; Deffrasnes, C.; Muller, M.; van Vreden, C.; Ashhurst, T.M.; Chami, B.; McCarthy, D.; Wu, H.; et al. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci. Transl. Med. 2014, 6, 219ra217. [Google Scholar] [CrossRef] [PubMed]
- Kamath, S.; Bhattacharyya, D.; Padukudru, C.; Timmons, R.B.; Tang, L. Surface chemistry influences implant-mediated host tissue responses. J. Biomed. Mater. Res. A 2008, 86, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Keselowsky, B.G.; Collard, D.M.; Garcia, A.J. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J. Biomed. Mater. Res. A 2003, 66, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.A.; Gersbach, C.A.; Michael, K.E.; Keselowsky, B.G.; Garcia, A.J. Myoblast proliferation and differentiation on fibronectin-coated self assembled monolayers presenting different surface chemistries. Biomaterials 2005, 26, 4523–4531. [Google Scholar] [CrossRef]
- Lee, M.H.; Ducheyne, P.; Lynch, L.; Boettiger, D.; Composto, R.J. Effect of biomaterial surface properties on fibronectin-alpha5beta1 integrin interaction and cellular attachment. Biomaterials 2006, 27, 1907–1916. [Google Scholar] [CrossRef]
- Sperling, C.; Schweiss, R.B.; Streller, U.; Werner, C. In vitro hemocompatibility of self-assembled monolayers displaying various functional groups. Biomaterials 2005, 26, 6547–6557. [Google Scholar] [CrossRef]
- Tang, L.; Wu, Y.; Timmons, R.B. Fibrinogen adsorption and host tissue responses to plasma functionalized surfaces. J. Biomed. Mater. Res. 1998, 42, 156–163. [Google Scholar] [CrossRef]
- Nair, A.; Zou, L.; Bhattacharyya, D.; Timmons, R.B.; Tang, L. Species and density of implant surface chemistry affect the extent of foreign body reactions. Langmuir 2008, 24, 2015–2024. [Google Scholar] [CrossRef]
- Sastry, S.K.; Burridge, K. Focal adhesions: A nexus for intracellular signaling and cytoskeletal dynamics. Exp. Cell Res. 2000, 261, 25–36. [Google Scholar] [CrossRef]
- Kalltorp, M.; Oblogina, S.; Jacobsson, S.; Karlsson, A.; Tengvall, P.; Thomsen, P. In vivo cell recruitment, cytokine release and chemiluminescence response at gold, and thiol functionalized surfaces. Biomaterials 1999, 20, 2123–2137. [Google Scholar] [CrossRef]
- Benesch, J.; Svedhem, S.; Svensson, S.C.; Valiokas, R.; Liedberg, B.; Tengvall, P. Protein adsorption to oligo(ethylene glycol) self-assembled monolayers: Experiments with fibrinogen, heparinized plasma, and serum. J. Biomater. Sci. Polym. Ed. 2001, 12, 581–597. [Google Scholar] [CrossRef]
- Hirata, I.; Hioki, Y.; Toda, M.; Kitazawa, T.; Murakami, Y.; Kitano, E.; Kitamura, H.; Ikada, Y.; Iwata, H. Deposition of complement protein C3b on mixed self-assembled monolayers carrying surface hydroxyl and methyl groups studied by surface plasmon resonance. J. Biomed. Mater. Res. A 2003, 66, 669–676. [Google Scholar] [CrossRef]
- Kim, J.; Somorjai, G.A. Molecular packing of lysozyme, fibrinogen, and bovine serum albumin on hydrophilic and hydrophobic surfaces studied by infrared-visible sum frequency generation and fluorescence microscopy. J. Am. Chem. Soc. 2003, 125, 3150–3158. [Google Scholar] [CrossRef]
- Szott, L.M.; Horbett, T.A. Protein interactions with surfaces: Cellular responses, complement activation, and newer methods. Curr. Opin. Chem. Biol. 2011, 15, 677–682. [Google Scholar] [CrossRef]
- Sivaraman, B.; Fears, K.P.; Latour, R.A. Investigation of the effects of surface chemistry and solution concentration on the conformation of adsorbed proteins using an improved circular dichroism method. Langmuir 2009, 25, 3050–3056. [Google Scholar] [CrossRef]
- Vieira, E.P.; Rocha, S.; Carmo Pereira, M.; Mohwald, H.; Coelho, M.A. Adsorption and diffusion of plasma proteins on hydrophilic and hydrophobic surfaces: Effect of trifluoroethanol on protein structure. Langmuir 2009, 25, 9879–9886. [Google Scholar] [CrossRef]
- Neumann, S.; Burkert, K.; Kemp, R.; Rades, T.; Rod Dunbar, P.; Hook, S. Activation of the NLRP3 inflammasome is not a feature of all particulate vaccine adjuvants. Immunol. Cell Biol. 2014, 92, 535–542. [Google Scholar] [CrossRef]
- Wen, Y.; Waltman, A.; Han, H.; Collier, J.H. Switching the Immunogenicity of Peptide Assemblies Using Surface Properties. ACS Nano 2016. [Google Scholar] [CrossRef]
- Gallorini, S.; Berti, F.; Parente, P.; Baronio, R.; Aprea, S.; D’Oro, U.; Pizza, M.; Telford, J.L.; Wack, A. Introduction of zwitterionic motifs into bacterial polysaccharides generates TLR2 agonists able to activate APCs. J. Immunol. 2007, 179, 8208–8215. [Google Scholar] [CrossRef]
- Betancourt, T.; Brannon-Peppas, L. Micro- and nanofabrication methods in nanotechnological medical and pharmaceutical devices. Int. J. Nanomed. 2006, 1, 483–495. [Google Scholar] [CrossRef]
- Refai, A.K.; Textor, M.; Brunette, D.M.; Waterfield, J.D. Effect of titanium surface topography on macrophage activation and secretion of proinflammatory cytokines and chemokines. J. Biomed. Mater. Res. A 2004, 70, 194–205. [Google Scholar] [CrossRef]
- Soskolne, W.A.; Cohen, S.; Sennerby, L.; Wennerberg, A.; Shapira, L. The effect of titanium surface roughness on the adhesion of monocytes and their secretion of TNF-alpha and PGE2. Clin. Oral Implants Res. 2002, 13, 86–93. [Google Scholar] [CrossRef]
- Bota, P.C.; Collie, A.M.; Puolakkainen, P.; Vernon, R.B.; Sage, E.H.; Ratner, B.D.; Stayton, P.S. Biomaterial topography alters healing in vivo and monocyte/macrophage activation in vitro. J. Biomed. Mater. Res. A 2010, 95, 649–657. [Google Scholar] [CrossRef]
- Collie, A.M.; Bota, P.C.; Johns, R.E.; Maier, R.V.; Stayton, P.S. Differential monocyte/macrophage interleukin-1beta production due to biomaterial topography requires the beta2 integrin signaling pathway. J. Biomed. Mater. Res. A 2011, 96, 162–169. [Google Scholar] [CrossRef]
- Mohiuddin, M.; Pan, H.A.; Hung, Y.C.; Huang, G.S. Control of growth and inflammatory response of macrophages and foam cells with nanotopography. Nanoscale Res. Lett. 2012, 7, 394. [Google Scholar] [CrossRef]
- Fink, J.; Fuhrmann, R.; Scharnweber, T.; Franke, R.P. Stimulation of monocytes and macrophages: Possible influence of surface roughness. Clin. Hemorheol. Microcirc. 2008, 39, 205–212. [Google Scholar]
- Gamboa, J.R.; Mohandes, S.; Tran, P.L.; Slepian, M.J.; Yoon, J.Y. Linear fibroblast alignment on sinusoidal wave micropatterns. Colloids Surf. B Biointerfaces 2013, 104, 318–325. [Google Scholar] [CrossRef]
- Hulander, M.; Lundgren, A.; Faxalv, L.; Lindahl, T.L.; Palmquist, A.; Berglin, M.; Elwing, H. Gradients in surface nanotopography used to study platelet adhesion and activation. Colloids Surf. B Biointerfaces 2013, 110, 261–269. [Google Scholar] [CrossRef]
- Gilchrist, C.L.; Ruch, D.S.; Little, D.; Guilak, F. Micro-scale and meso-scale architectural cues cooperate and compete to direct aligned tissue formation. Biomaterials 2014, 35, 10015–10024. [Google Scholar] [CrossRef]
- Lopacinska, J.M.; Gradinaru, C.; Wierzbicki, R.; Kobler, C.; Schmidt, M.S.; Madsen, M.T.; Skolimowski, M.; Dufva, M.; Flyvbjerg, H.; Molhave, K. Cell motility, morphology, viability and proliferation in response to nanotopography on silicon black. Nanoscale 2012, 4, 3739–3745. [Google Scholar] [CrossRef]
- Vogel, V.; Sheetz, M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006, 7, 265–275. [Google Scholar] [CrossRef]
- Yim, E.K.; Reano, R.M.; Pang, S.W.; Yee, A.F.; Chen, C.S.; Leong, K.W. Nanopattern-induced changes in morphology and motility of smooth muscle cells. Biomaterials 2005, 26, 5405–5413. [Google Scholar] [CrossRef]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef]
- Ma, Q.L.; Zhao, L.Z.; Liu, R.R.; Jin, B.Q.; Song, W.; Wang, Y.; Zhang, Y.S.; Chen, L.H.; Zhang, Y.M. Improved implant osseointegration of a nanostructured titanium surface via mediation of macrophage polarization. Biomaterials 2014, 35, 9853–9867. [Google Scholar] [CrossRef]
- Tan, K.S.; Qian, L.; Rosado, R.; Flood, P.M.; Cooper, L.F. The role of titanium surface topography on J774A.1 macrophage inflammatory cytokines and nitric oxide production. Biomaterials 2006, 27, 5170–5177. [Google Scholar] [CrossRef]
- Hulander, M.; Lundgren, A.; Berglin, M.; Ohrlander, M.; Lausmaa, J.; Elwing, H. Immune complement activation is attenuated by surface nanotopography. Int. J. Nanomed. 2011, 6, 2653–2666. [Google Scholar] [CrossRef]
- Roach, P.; Eglin, D.; Rohde, K.; Perry, C.C. Modern biomaterials: A review—bulk properties and implications of surface modifications. J. Mater. Sci. Mater. Med. 2007, 18, 1263–1277. [Google Scholar] [CrossRef] [PubMed]
- Scopelliti, P.E.; Borgonovo, A.; Indrieri, M.; Giorgetti, L.; Bongiorno, G.; Carbone, R.; Podesta, A.; Milani, P. The effect of surface nanometre-scale morphology on protein adsorption. PLoS ONE 2010, 5, e11862. [Google Scholar] [CrossRef] [PubMed]
- Rechendorff, K.; Hovgaard, M.B.; Foss, M.; Zhdanov, V.P.; Besenbacher, F. Enhancement of protein adsorption induced by surface roughness. Langmuir 2006, 22, 10885–10888. [Google Scholar] [CrossRef] [PubMed]
- Hovgaard, M.B.; Rechendorff, K.; Chevallier, J.; Foss, M.; Besenbacher, F. Fibronectin adsorption on tantalum: The influence of nanoroughness. J. Phys. Chem. B 2008, 112, 8241–8249. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U.; Buchter, A.; Wiesmann, H.P.; Joos, U.; Jones, D.B. Basic reactions of osteoblasts on structured material surfaces. Eur. Cells Mater. 2005, 9, 39–49. [Google Scholar] [CrossRef]
- Mrksich, M.; Whitesides, G.M. Using self-assembled monolayers to understand the interactions of man-made surfaces with proteins and cells. Annu. Rev. Biophys. Biomol. Struct. 1996, 25, 55–78. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.A.; Chalouni, C.; Williams, A.; Hartl, D.; Lee, C.G.; Elias, J.A. Chitin is a size-dependent regulator of macrophage TNF and IL-10 production. J. Immunol. 2009, 182, 3573–3582. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, T.D.; Pearson, J.R.; Leal, M.P.; Torres, M.J.; Blanca, M.; Mayorga, C.; Le Guevel, X. Intracellular accumulation and immunological properties of fluorescent gold nanoclusters in human dendritic cells. Biomaterials 2015, 43, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choi, J.; Shin, S.; Im, Y.M.; Song, J.; Kang, S.S.; Nam, T.H.; Webster, T.J.; Kim, S.H.; Khang, D. Analysis on migration and activation of live macrophages on transparent flat and nanostructured titanium. Acta Biomater. 2011, 7, 2337–2344. [Google Scholar] [CrossRef]
- Lu, J.; Webster, T.J. Reduced immune cell responses on nano and submicron rough titanium. Acta Biomater. 2015, 16, 223–231. [Google Scholar] [CrossRef]
- McWhorter, F.Y.; Wang, T.; Nguyen, P.; Chung, T.; Liu, W.F. Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. USA 2013, 110, 17253–17258. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Doloff, J.C.; Ma, M.; Vegas, A.J.; Tam, H.H.; Bader, A.R.; Li, J.; Langan, E.; Wyckoff, J.; Loo, W.S.; et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater. 2015, 14, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.W.; Liu, X.; Weng, H.; Luo, C.; Tang, L. Fibroblast/fibrocyte: Surface interaction dictates tissue reactions to micropillar implants. Biomacromolecules 2011, 12, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Jahed, Z.; Molladavoodi, S.; Seo, B.B.; Gorbet, M.; Tsui, T.Y.; Mofrad, M.R. Cell responses to metallic nanostructure arrays with complex geometries. Biomaterials 2014, 35, 9363–9371. [Google Scholar] [CrossRef] [PubMed]
- Hornung, V.; Bauernfeind, F.; Halle, A.; Samstad, E.O.; Kono, H.; Rock, K.L.; Fitzgerald, K.A.; Latz, E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008, 9, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Niikura, K.; Matsunaga, T.; Suzuki, T.; Kobayashi, S.; Yamaguchi, H.; Orba, Y.; Kawaguchi, A.; Hasegawa, H.; Kajino, K.; Ninomiya, T.; et al. Gold nanoparticles as a vaccine platform: Influence of size and shape on immunological responses in vitro and in vivo. ACS Nano 2013, 7, 3926–3938. [Google Scholar] [CrossRef] [PubMed]
- Padmore, T.; Stark, C.; Turkevich, L.A.; Champion, J.A. Quantitative analysis of the role of fiber length on phagocytosis and inflammatory response by alveolar macrophages. Biochim. Biophys. Acta Gen Subj 2017, 1861, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Schanen, B.C.; Karakoti, A.S.; Seal, S.; Drake, D.R., 3rd; Warren, W.L.; Self, W.T. Exposure to titanium dioxide nanomaterials provokes inflammation of an in vitro human immune construct. ACS Nano 2009, 3, 2523–2532. [Google Scholar] [CrossRef]
- Sunshine, J.C.; Perica, K.; Schneck, J.P.; Green, J.J. Particle shape dependence of CD8+ T cell activation by artificial antigen presenting cells. Biomaterials 2014, 35, 269–277. [Google Scholar] [CrossRef]
- Vaine, C.A.; Patel, M.K.; Zhu, J.; Lee, E.; Finberg, R.W.; Hayward, R.C.; Kurt-Jones, E.A. Tuning innate immune activation by surface texturing of polymer microparticles: The role of shape in inflammasome activation. J. Immunol. 2013, 190, 3525–3532. [Google Scholar] [CrossRef]
- Kosmides, A.K.; Meyer, R.A.; Hickey, J.W.; Aje, K.; Cheung, K.N.; Green, J.J.; Schneck, J.P. Biomimetic biodegradable artificial antigen presenting cells synergize with PD-1 blockade to treat melanoma. Biomaterials 2017, 118, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.A.; Sunshine, J.C.; Perica, K.; Kosmides, A.K.; Aje, K.; Schneck, J.P.; Green, J.J. Biodegradable nanoellipsoidal artificial antigen presenting cells for antigen specific T-cell activation. Small 2015, 11, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Neacsu, P.; Mazare, A.; Cimpean, A.; Park, J.; Costache, M.; Schmuki, P.; Demetrescu, I. Reduced inflammatory activity of RAW 264.7 macrophages on titania nanotube modified Ti surface. Int. J. Biochem. Cell Biol. 2014, 55, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Rajyalakshmi, A.; Ercan, B.; Balasubramanian, K.; Webster, T.J. Reduced adhesion of macrophages on anodized titanium with select nanotube surface features. Int. J. Nanomed. 2011, 6, 1765–1771. [Google Scholar]
- Moura, C.C.; Zanetta-Barbosa, D.; Dechichi, P.; Carvalho, V.F.; Soares, P.B. Effects of titanium surfaces on the developmental profile of monocytes/macrophages. Braz. Dent. J. 2014, 25, 96–103. [Google Scholar] [CrossRef]
- Mahyudin, F.; Widhiyanto, L.; Hermawan, H. Biomaterials in orthopaedics. In Advanced Structured Materials; Springer Publishing: New York, NY, USA, 2016. [Google Scholar]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Matena, J.; Petersen, S.; Gieseke, M.; Kampmann, A.; Teske, M.; Beyerbach, M.; Murua Escobar, H.; Haferkamp, H.; Gellrich, N.C.; Nolte, I. SLM produced porous titanium implant improvements for enhanced vascularization and osteoblast seeding. Int. J. Mol. Sci. 2015, 16, 7478–7492. [Google Scholar] [CrossRef]
- Kuboki, Y.; Jin, Q.; Takita, H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. J. Bone Joint Surg. Am. 2001, 83 (Suppl. 1), S105–S115. [Google Scholar] [CrossRef]
- Artel, A.; Mehdizadeh, H.; Chiu, Y.C.; Brey, E.M.; Cinar, A. An agent-based model for the investigation of neovascularization within porous scaffolds. Tissue Eng. Part A 2011, 17, 2133–2141. [Google Scholar] [CrossRef]
- Jiang, J.; Li, Z.; Wang, H.; Wang, Y.; Carlson, M.A.; Teusink, M.J.; MacEwan, M.R.; Gu, L.; Xie, J. Expanded 3D Nanofiber Scaffolds: Cell Penetration, Neovascularization, and Host Response. Adv. Healthc. Mater. 2016, 5, 2993–3003. [Google Scholar] [CrossRef]
- Muller, D.; Chim, H.; Bader, A.; Whiteman, M.; Schantz, J.T. Vascular guidance: Microstructural scaffold patterning for inductive neovascularization. Stem Cells Int. 2010, 2011, 547247. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, U.J.; Vunjak-Novakovic, G.; Min, B.H.; Kaplan, D.L. Influence of macroporous protein scaffolds on bone tissue engineering from bone marrow stem cells. Biomaterials 2005, 26, 4442–4452. [Google Scholar] [CrossRef] [PubMed]
- Madden, L.R.; Mortisen, D.J.; Sussman, E.M.; Dupras, S.K.; Fugate, J.A.; Cuy, J.L.; Hauch, K.D.; Laflamme, M.A.; Murry, C.E.; Ratner, B.D. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc. Natl. Acad. Sci. USA 2010, 107, 15211–15216. [Google Scholar] [CrossRef] [PubMed]
- Bridges, A.W.; Singh, N.; Burns, K.L.; Babensee, J.E.; Andrew Lyon, L.; Garcia, A.J. Reduced acute inflammatory responses to microgel conformal coatings. Biomaterials 2008, 29, 4605–4615. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Hammerschmidt, M.; Krieg, T.; Roers, A. Interrelation of immunity and tissue repair or regeneration. Semin. Cell Dev. Biol. 2009, 20, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F. TISSUE REGENERATION. A scaffold immune microenvironment. Science 2016, 352, 298. [Google Scholar] [CrossRef]
- Mescher, A.L.; Neff, A.W.; King, M.W. Inflammation and immunity in organ regeneration. Dev. Comp. Immunol. 2017, 66, 98–110. [Google Scholar] [CrossRef]
- Julier, Z.; Park, A.J.; Briquez, P.S.; Martino, M.M. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017, 53, 13–28. [Google Scholar] [CrossRef]
- Gower, R.M.; Boehler, R.M.; Azarin, S.M.; Ricci, C.F.; Leonard, J.N.; Shea, L.D. Modulation of leukocyte infiltration and phenotype in microporous tissue engineering scaffolds via vector induced IL-10 expression. Biomaterials 2014, 35, 2024–2031. [Google Scholar] [CrossRef]
- Hubbell, J.A.; Thomas, S.N.; Swartz, M.A. Materials engineering for immunomodulation. Nature 2009, 462, 449–460. [Google Scholar] [CrossRef]
- Boontheekul, T.; Mooney, D.J. Protein-based signaling systems in tissue engineering. Curr. Opin. Biotechnol. 2003, 14, 559–565. [Google Scholar] [CrossRef]
- Wolf, M.T.; Dearth, C.L.; Sonnenberg, S.B.; Loboa, E.G.; Badylak, S.F. Naturally derived and synthetic scaffolds for skeletal muscle reconstruction. Adv. Drug Deliv. Rev. 2015, 84, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F.; Dziki, J.L.; Sicari, B.M.; Ambrosio, F.; Boninger, M.L. Mechanisms by which acellular biologic scaffolds promote functional skeletal muscle restoration. Biomaterials 2016, 103, 128–136. [Google Scholar] [CrossRef]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.J.; Badylak, S.F. The Use of Biologic Scaffolds in the Treatment of Chronic Nonhealing Wounds. Adv. Wound Care 2015, 4, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Ratner, B.D.; Goodman, S.B.; Amar, S.; Badylak, S.F. Macrophage polarization: An opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 2012, 33, 3792–3802. [Google Scholar] [CrossRef] [PubMed]
- Gowen, B.B.; Borg, T.K.; Ghaffar, A.; Mayer, E.P. Selective adhesion of macrophages to denatured forms of type I collagen is mediated by scavenger receptors. Matrix Biol. 2000, 19, 61–71. [Google Scholar] [CrossRef]
- Veres, S.P.; Brennan-Pierce, E.P.; Lee, J.M. Macrophage-like U937 cells recognize collagen fibrils with strain-induced discrete plasticity damage. J. Biomed. Mater. Res. A 2015, 103, 397–408. [Google Scholar] [CrossRef]
- Huleihel, L.; Hussey, G.S.; Naranjo, J.D.; Zhang, L.; Dziki, J.L.; Turner, N.J.; Stolz, D.B.; Badylak, S.F. Matrix-bound nanovesicles within ECM bioscaffolds. Sci. Adv. 2016, 2, e1600502. [Google Scholar] [CrossRef]
- Dziki, J.L.; Wang, D.S.; Pineda, C.; Sicari, B.M.; Rausch, T.; Badylak, S.F. Solubilized extracellular matrix bioscaffolds derived from diverse source tissues differentially influence macrophage phenotype. J. Biomed. Mater. Res. A 2017, 105, 138–147. [Google Scholar] [CrossRef]
- Keane, T.J.; Dziki, J.; Sobieski, E.; Smoulder, A.; Castleton, A.; Turner, N.; White, L.J.; Badylak, S.F. Restoring Mucosal Barrier Function and Modifying Macrophage Phenotype with an Extracellular Matrix Hydrogel: Potential Therapy for Ulcerative Colitis. J. Crohns Colitis 2017, 11, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Valentin, J.E.; Stewart-Akers, A.M.; McCabe, G.P.; Badylak, S.F. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials 2009, 30, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J.M.; Lowdell, M.W.; Urbani, L.; Ansari, T.; Burns, A.J.; Turmaine, M.; North, J.; Sibbons, P.; Seifalian, A.M.; Wood, K.J.; et al. Immunomodulatory effect of a decellularized skeletal muscle scaffold in a discordant xenotransplantation model. Proc. Natl. Acad. Sci. USA 2013, 110, 14360–14365. [Google Scholar] [CrossRef] [PubMed]
- Keane, T.J.; Dziki, J.; Castelton, A.; Faulk, D.M.; Messerschmidt, V.; Londono, R.; Reing, J.E.; Velankar, S.S.; Badylak, S.F. Preparation and characterization of a biologic scaffold and hydrogel derived from colonic mucosa. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Nassiri, S.; Witherel, C.E.; Anfang, R.R.; Ng, J.; Nakazawa, K.R.; Yu, T.; Vunjak-Novakovic, G. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 2015, 37, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Deeken, C.R.; Eliason, B.J.; Pichert, M.D.; Grant, S.A.; Frisella, M.M.; Matthews, B.D. Differentiation of biologic scaffold materials through physicomechanical, thermal, and enzymatic degradation techniques. Ann. Surg. 2012, 255, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Delgado, L.M.; Bayon, Y.; Pandit, A.; Zeugolis, D.I. To cross-link or not to cross-link? Cross-linking associated foreign body response of collagen-based devices. Tissue Eng. Part B Rev. 2015, 21, 298–313. [Google Scholar] [CrossRef] [PubMed]
- McDade, J.K.; Brennan-Pierce, E.P.; Ariganello, M.B.; Labow, R.S.; Michael Lee, J. Interactions of U937 macrophage-like cells with decellularized pericardial matrix materials: Influence of crosslinking treatment. Acta Biomater. 2013, 9, 7191–7199. [Google Scholar] [CrossRef]
- Keane, T.J.; Londono, R.; Turner, N.J.; Badylak, S.F. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials 2012, 33, 1771–1781. [Google Scholar] [CrossRef]
- Beachley, V.Z.; Wolf, M.T.; Sadtler, K.; Manda, S.S.; Jacobs, H.; Blatchley, M.R.; Bader, J.S.; Pandey, A.; Pardoll, D.; Elisseeff, J.H. Tissue matrix arrays for high-throughput screening and systems analysis of cell function. Nat. Methods 2015, 12, 1197–1204. [Google Scholar] [CrossRef]
- Asea, A.; Rehli, M.; Kabingu, E.; Boch, J.A.; Bare, O.; Auron, P.E.; Stevenson, M.A.; Calderwood, S.K. Novel signal transduction pathway utilized by extracellular HSP70: Role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 2002, 277, 15028–15034. [Google Scholar] [CrossRef] [PubMed]
- Kovalchin, J.T.; Wang, R.; Wagh, M.S.; Azoulay, J.; Sanders, M.; Chandawarkar, R.Y. In vivo delivery of heat shock protein 70 accelerates wound healing by up-regulating macrophage-mediated phagocytosis. Wound Repair Regen. 2006, 14, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Laschke, M.W.; Augustin, V.; Kleer, S.; Tschernig, T.; Menger, M.D. Locally applied macrophage-activating lipopeptide-2 (MALP-2) promotes early vascularization of implanted porous polyethylene (Medpor(R)). Acta Biomater. 2014, 10, 4661–4669. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Sato, T.; Beren, J.; Verthelyi, D.; Klinman, D.M. The acceleration of wound healing in primates by the local administration of immunostimulatory CpG oligonucleotides. Biomaterials 2011, 32, 4238–4242. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, K.; Ishikawa, I. The roles of cyclooxygenase-2 and prostaglandin E2 in periodontal disease. Periodontology 2000 2007, 43, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Kalinski, P.; Hilkens, C.M.; Snijders, A.; Snijdewint, F.G.; Kapsenberg, M.L. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J. Immunol. 1997, 159, 28–35. [Google Scholar] [PubMed]
- Katamura, K.; Shintaku, N.; Yamauchi, Y.; Fukui, T.; Ohshima, Y.; Mayumi, M.; Furusho, K. Prostaglandin E2 at priming of naive CD4+ T cells inhibits acquisition of ability to produce IFN-gamma and IL-2, but not IL-4 and IL-5. J. Immunol. 1995, 155, 4604–4612. [Google Scholar] [PubMed]
- Paralkar, V.M.; Borovecki, F.; Ke, H.Z.; Cameron, K.O.; Lefker, B.; Grasser, W.A.; Owen, T.A.; Li, M.; DaSilva-Jardine, P.; Zhou, M.; et al. An EP2 receptor-selective prostaglandin E2 agonist induces bone healing. Proc. Natl. Acad. Sci. USA 2003, 100, 6736–6740. [Google Scholar] [CrossRef] [PubMed]
- Kamolratanakul, P.; Hayata, T.; Ezura, Y.; Kawamata, A.; Hayashi, C.; Yamamoto, Y.; Hemmi, H.; Nagao, M.; Hanyu, R.; Notomi, T.; et al. Nanogel-based scaffold delivery of prostaglandin E(2) receptor-specific agonist in combination with a low dose of growth factor heals critical-size bone defects in mice. Arthritis Rheumatol. 2011, 63, 1021–1033. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Hasegawa, U.; Morimoto, N.; Saita, Y.; Nakashima, K.; Ezura, Y.; Kurosawa, H.; Akiyoshi, K.; Noda, M. Nanogel-based delivery system enhances PGE2 effects on bone formation. J. Cell Biochem. 2007, 101, 1063–1070. [Google Scholar] [CrossRef]
- Toyoda, H.; Terai, H.; Sasaoka, R.; Oda, K.; Takaoka, K. Augmentation of bone morphogenetic protein-induced bone mass by local delivery of a prostaglandin E EP4 receptor agonist. Bone 2005, 37, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Kucia, M.; Jankowski, K.; Reca, R.; Wysoczynski, M.; Bandura, L.; Allendorf, D.J.; Zhang, J.; Ratajczak, J.; Ratajczak, M.Z. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J. Mol. Histol. 2004, 35, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Lau, T.T.; Wang, D.A. Stromal cell-derived factor-1 (SDF-1): Homing factor for engineered regenerative medicine. Expert Opin. Biol. Ther. 2011, 11, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Son, B.R.; Marquez-Curtis, L.A.; Kucia, M.; Wysoczynski, M.; Turner, A.R.; Ratajczak, J.; Ratajczak, M.Z.; Janowska-Wieczorek, A. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells 2006, 24, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Tabata, Y. Recruitment of mesenchymal stem cells and macrophages by dual release of stromal cell-derived factor-1 and a macrophage recruitment agent enhances wound closure. J. Biomed. Mater. Res. A 2016, 104, 942–956. [Google Scholar] [CrossRef]
- Shen, W.; Chen, X.; Chen, J.; Yin, Z.; Heng, B.C.; Chen, W.; Ouyang, H.W. The effect of incorporation of exogenous stromal cell-derived factor-1 alpha within a knitted silk-collagen sponge scaffold on tendon regeneration. Biomaterials 2010, 31, 7239–7249. [Google Scholar] [CrossRef]
- Projahn, D.; Simsekyilmaz, S.; Singh, S.; Kanzler, I.; Kramp, B.K.; Langer, M.; Burlacu, A.; Bernhagen, J.; Klee, D.; Zernecke, A.; et al. Controlled intramyocardial release of engineered chemokines by biodegradable hydrogels as a treatment approach of myocardial infarction. J. Cell. Mol. Med. 2014, 18, 790–800. [Google Scholar] [CrossRef]
- Kimura, Y.; Tabata, Y. Controlled release of stromal-cell-derived factor-1 from gelatin hydrogels enhances angiogenesis. J. Biomater. Sci. Polym. Ed. 2010, 21, 37–51. [Google Scholar] [CrossRef]
- Boehler, R.M.; Graham, J.G.; Shea, L.D. Tissue engineering tools for modulation of the immune response. Biotechniques 2011, 51, 239–240, 242, 244 passim. [Google Scholar] [CrossRef]
- Hume, P.S.; He, J.; Haskins, K.; Anseth, K.S. Strategies to reduce dendritic cell activation through functional biomaterial design. Biomaterials 2012, 33, 3615–3625. [Google Scholar] [CrossRef]
- Chen, J.; Li, M.; Yang, C.; Yin, X.; Duan, K.; Wang, J.; Feng, B. Macrophage phenotype switch by sequential action of immunomodulatory cytokines from hydrogel layers on titania nanotubes. Colloids Surf. B Biointerfaces 2018, 163, 336–345. [Google Scholar] [CrossRef]
- Lin, C.C.; Boyer, P.D.; Aimetti, A.A.; Anseth, K.S. Regulating MCP-1 diffusion in affinity hydrogels for enhancing immuno-isolation. J. Control. Release 2010, 142, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Metters, A.T.; Anseth, K.S. Functional PEG-peptide hydrogels to modulate local inflammation induced by the pro-inflammatory cytokine TNFalpha. Biomaterials 2009, 30, 4907–4914. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Boehler, R.M.; Kuo, R.; Shin, S.; Goodman, A.G.; Pilecki, M.A.; Gower, R.M.; Leonard, J.N.; Shea, L.D. Lentivirus delivery of IL-10 to promote and sustain macrophage polarization towards an anti-inflammatory phenotype. Biotechnol. Bioeng. 2014, 111, 1210–1221. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.; Glaser, J.; Liu, M.T.; Lane, T.E.; Keirstead, H.S. Reducing inflammation decreases secondary degeneration and functional deficit after spinal cord injury. Exp. Neurol. 2003, 184, 456–463. [Google Scholar] [CrossRef]
- Mokarram, N.; Merchant, A.; Mukhatyar, V.; Patel, G.; Bellamkonda, R.V. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials 2012, 33, 8793–8801. [Google Scholar] [CrossRef] [PubMed]
- Hachim, D.; LoPresti, S.T.; Yates, C.C.; Brown, B.N. Shifts in macrophage phenotype at the biomaterial interface via IL-4 eluting coatings are associated with improved implant integration. Biomaterials 2017, 112, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Mori, R.; Shaw, T.J.; Martin, P. Molecular mechanisms linking wound inflammation and fibrosis: Knockdown of osteopontin leads to rapid repair and reduced scarring. J. Exp. Med. 2008, 205, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Holladay, C.; Power, K.; Sefton, M.; O’Brien, T.; Gallagher, W.M.; Pandit, A. Functionalized scaffold-mediated interleukin 10 gene delivery significantly improves survival rates of stem cells in vivo. Mol. Ther. 2011, 19, 969–978. [Google Scholar] [CrossRef]
- Rao, A.J.; Nich, C.; Dhulipala, L.S.; Gibon, E.; Valladares, R.; Zwingenberger, S.; Smith, R.L.; Goodman, S.B. Local effect of IL-4 delivery on polyethylene particle induced osteolysis in the murine calvarium. J. Biomed. Mater. Res. A 2013, 101, 1926–1934. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.P.; Magolbo, N.; De Aquino, R.; Weller, C. Oral aspirin for treating venous leg ulcers. Cochrane Database Syst Rev. 2016, 2, CD009432. [Google Scholar] [CrossRef] [PubMed]
- Canton, I.; McKean, R.; Charnley, M.; Blackwood, K.A.; Fiorica, C.; Ryan, A.J.; MacNeil, S. Development of an Ibuprofen-releasing biodegradable PLA/PGA electrospun scaffold for tissue regeneration. Biotechnol. Bioeng. 2010, 105, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Varatharajan, L.; Thapar, A.; Lane, T.; Munster, A.B.; Davies, A.H. Pharmacological adjuncts for chronic venous ulcer healing: A systematic review. Phlebology 2016, 31, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, E.E.; Sun, L.T.; Natesan, S.; Zamora, D.O.; Christy, R.J.; Washburn, N.R. Effects of hyaluronic acid conjugation on anti-TNF-alpha inhibition of inflammation in burns. J. Biomed. Mater. Res. A 2014, 102, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Park, H.J.; Hong, M.H.; Kang, P.M.; Morgan, J.P.; Jeong, M.H.; Cho, J.G.; Park, J.C.; Ahn, Y. TNF-alpha enhances engraftment of mesenchymal stem cells into infarcted myocardium. Front. Biosci. 2009, 14, 2845–2856. [Google Scholar] [CrossRef]
- Bocker, W.; Docheva, D.; Prall, W.C.; Egea, V.; Pappou, E.; Rossmann, O.; Popov, C.; Mutschler, W.; Ries, C.; Schieker, M. IKK-2 is required for TNF-alpha-induced invasion and proliferation of human mesenchymal stem cells. J. Mol. Med. 2008, 86, 1183–1192. [Google Scholar] [CrossRef]
- Penn, J.W.; Grobbelaar, A.O.; Rolfe, K.J. The role of the TGF-beta family in wound healing, burns and scarring: A review. Int. J. Burns Trauma 2012, 2, 18–28. [Google Scholar]
- Johnston, C.J.; Smyth, D.J.; Dresser, D.W.; Maizels, R.M. TGF-beta in tolerance, development and regulation of immunity. Cell. Immunol. 2016, 299, 14–22. [Google Scholar] [CrossRef]
- Ferguson, M.W.; Duncan, J.; Bond, J.; Bush, J.; Durani, P.; So, K.; Taylor, L.; Chantrey, J.; Mason, T.; James, G.; et al. Prophylactic administration of avotermin for improvement of skin scarring: Three double-blind, placebo-controlled, phase I/II studies. Lancet 2009, 373, 1264–1274. [Google Scholar] [CrossRef]
- Yang, D.; Wei, F.; Tewary, P.; Howard, O.M.; Oppenheim, J.J. Alarmin-induced cell migration. Eur J. Immunol. 2013, 43, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Rankin, A.L.; Mumm, J.B.; Murphy, E.; Turner, S.; Yu, N.; McClanahan, T.K.; Bourne, P.A.; Pierce, R.H.; Kastelein, R.; Pflanz, S. IL-33 induces IL-13-dependent cutaneous fibrosis. J. Immunol. 2010, 184, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Lopetuso, L.R.; Scaldaferri, F.; Pizarro, T.T. Emerging role of the interleukin (IL)-33/ST2 axis in gut mucosal wound healing and fibrosis. Fibrogenes. Tissue Repair. 2012, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Guabiraba, R.; Besnard, A.G.; Komai-Koma, M.; Jabir, M.S.; Zhang, L.; Graham, G.J.; Kurowska-Stolarska, M.; Liew, F.Y.; McSharry, C.; et al. IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J. Allergy Clin. Immunol. 2014, 134, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Smithgall, M.D.; Comeau, M.R.; Yoon, B.R.; Kaufman, D.; Armitage, R.; Smith, D.E. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int. Immunol. 2008, 20, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Li, Y.; Li, M. The potential role of IL-33/ST2 signaling in fibrotic diseases. J. Leukoc. Biol. 2015, 98, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Luzina, I.G.; Kopach, P.; Lockatell, V.; Kang, P.H.; Nagarsekar, A.; Burke, A.P.; Hasday, J.D.; Todd, N.W.; Atamas, S.P. Interleukin-33 potentiates bleomycin-induced lung injury. Am. J. Respir. Cell Mol. Biol. 2013, 49, 999–1008. [Google Scholar] [CrossRef]
- McHedlidze, T.; Waldner, M.; Zopf, S.; Walker, J.; Rankin, A.L.; Schuchmann, M.; Voehringer, D.; McKenzie, A.N.; Neurath, M.F.; Pflanz, S.; et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity 2013, 39, 357–371. [Google Scholar] [CrossRef]
- Franchimont, D.; Kino, T.; Galon, J.; Meduri, G.U.; Chrousos, G. Glucocorticoids and inflammation revisited: The state of the art. NIH clinical staff conference. Neuroimmunomodulation 2002, 10, 247–260. [Google Scholar] [CrossRef]
- Crossley, G.H.; Brinker, J.A.; Reynolds, D.; Spencer, W.; Johnson, W.B.; Hurd, H.; Tonder, L.; Zmijewski, M. Steroid elution improves the stimulation threshold in an active-fixation atrial permanent pacing lead. A randomized, controlled study. Model 4068 Investigators. Circulation 1995, 92, 2935–2939. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Bellamkonda, R.V. Dexamethasone-coated neural probes elicit attenuated inflammatory response and neuronal loss compared to uncoated neural probes. Brain Res. 2007, 1148, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Morais, J.M.; Papadimitrakopoulos, F.; Burgess, D.J. Biomaterials/tissue interactions: Possible solutions to overcome foreign body response. AAPS J. 2010, 12, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.D.; Papadimitrakopoulos, F.; Burgess, D.J. Dexamethasone-loaded poly(lactic-co-glycolic) acid microspheres/poly(vinyl alcohol) hydrogel composite coatings for inflammation control. Diabetes Technol. Ther. 2004, 6, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Minardi, S.; Corradetti, B.; Taraballi, F.; Byun, J.H.; Cabrera, F.; Liu, X.; Ferrari, M.; Weiner, B.K.; Tasciotti, E. IL-4 Release from a Biomimetic Scaffold for the Temporally Controlled Modulation of Macrophage Response. Ann. Biomed. Eng. 2016, 44, 2008–2019. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Martin, D.C. Sustained release of dexamethasone from hydrophilic matrices using PLGA nanoparticles for neural drug delivery. Biomaterials 2006, 27, 3031–3037. [Google Scholar] [CrossRef] [PubMed]
- Tsianakas, A.; Varga, G.; Barczyk, K.; Bode, G.; Nippe, N.; Kran, N.; Roth, J.; Luger, T.A.; Ehrchen, J.; Sunderkoetter, C. Induction of an anti-inflammatory human monocyte subtype is a unique property of glucocorticoids, but can be modified by IL-6 and IL-10. Immunobiology 2012, 217, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.C.; Shin, V.Y.; Liu, E.S.; Ye, Y.N.; Wu, W.K.; So, W.H.; Chang, F.Y.; Cho, C.H. Dexamethasone delays ulcer healing by inhibition of angiogenesis in rat stomachs. Eur. J. Pharmacol. 2004, 485, 275–281. [Google Scholar] [CrossRef]
- Koedam, J.A.; Smink, J.J.; van Buul-Offers, S.C. Glucocorticoids inhibit vascular endothelial growth factor expression in growth plate chondrocytes. Mol. Cell. Endocrinol. 2002, 197, 35–44. [Google Scholar] [CrossRef]
- Patil, S.D.; Papadmitrakopoulos, F.; Burgess, D.J. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. J. Control. Release 2007, 117, 68–79. [Google Scholar] [CrossRef]
- Norton, L.W.; Koschwanez, H.E.; Wisniewski, N.A.; Klitzman, B.; Reichert, W.M. Vascular endothelial growth factor and dexamethasone release from nonfouling sensor coatings affect the foreign body response. J. Biomed. Mater. Res. A 2007, 81, 858–869. [Google Scholar] [CrossRef] [PubMed]
- Kastellorizios, M.; Papadimitrakopoulos, F.; Burgess, D.J. Multiple tissue response modifiers to promote angiogenesis and prevent the foreign body reaction around subcutaneous implants. J. Control. Release 2015, 214, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Doloff, J.C.; Veiseh, O.; Vegas, A.J.; Tam, H.H.; Farah, S.; Ma, M.; Li, J.; Bader, A.; Chiu, A.; Sadraei, A.; et al. Colony stimulating factor-1 receptor is a central component of the foreign body response to biomaterial implants in rodents and non-human primates. Nat. Mater. 2017, 16, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Udipi, K.; Ornberg, R.L.; Thurmond, K.B., 2nd; Settle, S.L.; Forster, D.; Riley, D. Modification of inflammatory response to implanted biomedical materials in vivo by surface bound superoxide dismutase mimics. J. Biomed. Mater. Res. 2000, 51, 549–560. [Google Scholar] [CrossRef]
- Hetrick, E.M.; Prichard, H.L.; Klitzman, B.; Schoenfisch, M.H. Reduced foreign body response at nitric oxide-releasing subcutaneous implants. Biomaterials 2007, 28, 4571–4580. [Google Scholar] [CrossRef]
- Schwentker, A.; Vodovotz, Y.; Weller, R.; Billiar, T.R. Nitric oxide and wound repair: Role of cytokines? Nitric Oxide 2002, 7, 1–10. [Google Scholar] [CrossRef]
- Bogdan, C. Regulation of lymphocytes by nitric oxide. Methods Mol. Biol. 2011, 677, 375–393. [Google Scholar] [CrossRef] [PubMed]
- Cha, K.H.; Wang, X.; Meyerhoff, M.E. Nitric Oxide Release for Improving Performance of Implantable Chemical Sensors—A Review. Appl. Mater. Today 2017, 9, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Kao, W.J.; Lee, D. In vivo modulation of host response and macrophage behavior by polymer networks grafted with fibronectin-derived biomimetic oligopeptides: The role of RGD and PHSRN domains. Biomaterials 2001, 22, 2901–2909. [Google Scholar] [CrossRef]
- VandeVondele, S.; Voros, J.; Hubbell, J.A. RGD-grafted poly-l-lysine-graft-(polyethylene glycol) copolymers block non-specific protein adsorption while promoting cell adhesion. Biotechnol. Bioeng. 2003, 82, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Tang, C.; Kottke-Marchant, K.; Marchant, R.E. Design and synthesis of biomimetic hydrogel scaffolds with controlled organization of cyclic RGD peptides. Bioconjug. Chem. 2009, 20, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Karimi, F.; O’Connor, A.J.; Qiao, G.G.; Heath, D.E. Integrin Clustering Matters: A Review of Biomaterials Functionalized with Multivalent Integrin-Binding Ligands to Improve Cell Adhesion, Migration, Differentiation, Angiogenesis, and Biomedical Device Integration. Adv. Healthc. Mater. 2018, 7, e1701324. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Dalli, J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin. Immunol. 2015, 27, 200–215. [Google Scholar] [CrossRef]
- Schwab, J.M.; Chiang, N.; Arita, M.; Serhan, C.N. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 2007, 447, 869–874. [Google Scholar] [CrossRef]
- Vasconcelos, D.P.; Costa, M.; Amaral, I.F.; Barbosa, M.A.; Aguas, A.P.; Barbosa, J.N. Development of an immunomodulatory biomaterial: Using resolvin D1 to modulate inflammation. Biomaterials 2015, 53, 566–573. [Google Scholar] [CrossRef]
- Vasconcelos, D.P.; Costa, M.; Amaral, I.F.; Barbosa, M.A.; Aguas, A.P.; Barbosa, J.N. Modulation of the inflammatory response to chitosan through M2 macrophage polarization using pro-resolution mediators. Biomaterials 2015, 37, 116–123. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, M.J.; Hellmann, J.; Kosuri, M.; Bhatnagar, A.; Spite, M. Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes 2013, 62, 618–627. [Google Scholar] [CrossRef]
- Hong, S.; Tian, H.; Lu, Y.; Laborde, J.M.; Muhale, F.A.; Wang, Q.; Alapure, B.V.; Serhan, C.N.; Bazan, N.G. Neuroprotectin/protectin D1: Endogenous biosynthesis and actions on diabetic macrophages in promoting wound healing and innervation impaired by diabetes. Am. J. Physiol. Cell Physiol. 2014, 307, C1058–C1067. [Google Scholar] [CrossRef] [PubMed]
- Dohle, E.; Bischoff, I.; Bose, T.; Marsano, A.; Banfi, A.; Unger, R.E.; Kirkpatrick, C.J. Macrophage-mediated angiogenic activation of outgrowth endothelial cells in co-culture with primary osteoblasts. Eur. Cells Mater. 2014, 27, 149–164; discussion 164-145. [Google Scholar] [CrossRef]
- Swartzlander, M.D.; Blakney, A.K.; Amer, L.D.; Hankenson, K.D.; Kyriakides, T.R.; Bryant, S.J. Immunomodulation by mesenchymal stem cells combats the foreign body response to cell-laden synthetic hydrogels. Biomaterials 2015, 41, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, C.; Gu, W.; Klein, T.; Crawford, R.; Xiao, Y. Osteogenic differentiation of bone marrow MSCs by beta-tricalcium phosphate stimulating macrophages via BMP2 signalling pathway. Biomaterials 2014, 35, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, S.; Merhi, Y.; Winnik, F.M.; Tabrizian, M. Investigation of layer-by-layer assembly of polyelectrolytes on fully functional human red blood cells in suspension for attenuated immune response. Biomacromolecules 2011, 12, 585–592. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, H.; Park, T.; Park, J.; Kim, M.H.; Cho, H.; Youn, W.; Kang, S.M.; Choi, I.S. Artificial Spores: Cytocompatible Coating of Living Cells with Plant-Derived Pyrogallol. Chem. Asian J. 2016, 11, 3183–3187. [Google Scholar] [CrossRef]
- Hasturk, O.; Kaplan, D.L. Cell armor for protection against environmental stress: Advances, challenges and applications in micro- and nanoencapsulation of mammalian cells. Acta Biomater. 2018. [Google Scholar] [CrossRef]
- Weiden, J.; Voerman, D.; Dolen, Y.; Das, R.K.; van Duffelen, A.; Hammink, R.; Eggermont, L.J.; Rowan, A.E.; Tel, J.; Figdor, C.G. Injectable Biomimetic Hydrogels as Tools for Efficient T Cell Expansion and Delivery. Front. Immunol. 2018, 9, 2798. [Google Scholar] [CrossRef]
- Kumar, M.; Coburn, J.; Kaplan, D.L.; Mandal, B.B. Immuno-Informed 3D Silk Biomaterials for Tailoring Biological Responses. ACS Appl. Mater. Interfaces 2016, 8, 29310–29322. [Google Scholar] [CrossRef]
- Wang, H.; Morales, R.T.; Cui, X.; Huang, J.; Qian, W.; Tong, J.; Chen, W. A Photoresponsive Hyaluronan Hydrogel Nanocomposite for Dynamic Macrophage Immunomodulation. Adv. Healthc. Mater. 2018, e1801234. [Google Scholar] [CrossRef]
- He, X.T.; Wu, R.X.; Xu, X.Y.; Wang, J.; Yin, Y.; Chen, F.M. Macrophage involvement affects matrix stiffness-related influences on cell osteogenesis under three-dimensional culture conditions. Acta Biomater. 2018, 71, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, L.; Han, J.; Chu, J.; Wang, H.; Chen, X.; Wang, Y.; Tun, N.; Lu, L.; Bai, X.F.; et al. Conformal Nanoencapsulation of Allogeneic T Cells Mitigates Graft-versus-Host Disease and Retains Graft-versus-Leukemia Activity. ACS Nano 2016, 10, 6189–6200. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Kondo, T.; Kimura, A.; Matsushima, K.; Mukaida, N. Absence of IL-1 receptor antagonist impaired wound healing along with aberrant NF-kappaB activation and a reciprocal suppression of TGF-beta signal pathway. J. Immunol. 2006, 176, 5598–5606. [Google Scholar] [CrossRef]
- Chang, J.; Wang, Z.; Tang, E.; Fan, Z.; McCauley, L.; Franceschi, R.; Guan, K.; Krebsbach, P.H.; Wang, C.Y. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat. Med. 2009, 15, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Liu, F.; Lee, M.; Wu, B.; Ting, K.; Zara, J.N.; Soo, C.; Al Hezaimi, K.; Zou, W.; Chen, X.; et al. NF-kappaB inhibits osteogenic differentiation of mesenchymal stem cells by promoting beta-catenin degradation. Proc. Natl. Acad. Sci. USA 2013, 110, 9469–9474. [Google Scholar] [CrossRef] [PubMed]
- Mountziaris, P.M.; Tzouanas, S.N.; Sing, D.C.; Kramer, P.R.; Kasper, F.K.; Mikos, A.G. Intra-articular controlled release of anti-inflammatory siRNA with biodegradable polymer microparticles ameliorates temporomandibular joint inflammation. Acta Biomater. 2012, 8, 3552–3560. [Google Scholar] [CrossRef] [PubMed]
- Leuschner, F.; Dutta, P.; Gorbatov, R.; Novobrantseva, T.I.; Donahoe, J.S.; Courties, G.; Lee, K.M.; Kim, J.I.; Markmann, J.F.; Marinelli, B.; et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat. Biotechnol. 2011, 29, 1005–1010. [Google Scholar] [CrossRef]
- Sager, H.B.; Dutta, P.; Dahlman, J.E.; Hulsmans, M.; Courties, G.; Sun, Y.; Heidt, T.; Vinegoni, C.; Borodovsky, A.; Fitzgerald, K.; et al. RNAi targeting multiple cell adhesion molecules reduces immune cell recruitment and vascular inflammation after myocardial infarction. Sci. Transl. Med. 2016, 8, 342ra380. [Google Scholar] [CrossRef]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef]
- Mehta, A.; Baltimore, D. MicroRNAs as regulatory elements in immune system logic. Nat. Rev. Immunol. 2016, 16, 279–294. [Google Scholar] [CrossRef]
- Li, D.; Wang, A.; Liu, X.; Meisgen, F.; Grunler, J.; Botusan, I.R.; Narayanan, S.; Erikci, E.; Li, X.; Blomqvist, L.; et al. MicroRNA-132 enhances transition from inflammation to proliferation during wound healing. J. Clin. Investig. 2015, 125, 3008–3026. [Google Scholar] [CrossRef] [PubMed]
- Baumjohann, D.; Ansel, K.M. MicroRNA-mediated regulation of T helper cell differentiation and plasticity. Nat. Rev. Immunol. 2013, 13, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Frith, J.E.; Porrello, E.R.; Cooper-White, J.J. Concise review: New frontiers in microRNA-based tissue regeneration. Stem Cells Transl. Med. 2014, 3, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Gori, M.; Trombetta, M.; Santini, D.; Rainer, A. Tissue engineering and microRNAs: Future perspectives in regenerative medicine. Expert Opin. Biol. Ther. 2015, 15, 1601–1622. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kooger, R.; He, M.; Xiao, X.; Zheng, L.; Zhang, Y. A supramolecular hydrogel as a carrier to deliver microRNA into the encapsulated cells. Chem. Commun. (Camb.) 2014, 50, 3722–3724. [Google Scholar] [CrossRef] [PubMed]
- Morishita, Y.; Imai, T.; Yoshizawa, H.; Watanabe, M.; Ishibashi, K.; Muto, S.; Nagata, D. Delivery of microRNA-146a with polyethylenimine nanoparticles inhibits renal fibrosis in vivo. Int. J. Nanomed. 2015, 10, 3475–3488. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Synthetic | Natural |
|---|---|---|
| Polymer Types | Poly(anhydride), Poly(propylene fumarate) (PPF), Poly(caprolactone) (PCL), Poly(phosphazene), Poly(lactic acid) (PLA), Poly(ether ether ketone) (PEEK) poly(glycolic acid) (PGA) poly(lactic-co-glycolic acid) (PLGA) | agarose alginate collagen fibrin, glycosaminoglycans hyaluronic acid, chitosan silk |
| Advantages | inert, high reproducibility, availability on demand, reduced costs, constant quality supporting industrial scale production, possibility to design or tune, mechanical properties, composition adaptable to needs, possibility to fabricate complex shapes, controlled degradation rate, long shelf life, cell attachment improvement, potential to deliver soluble molecules | readily available, mass producible, large quantities constantly available, cost, low immunogenicity, bioactive properties, binding sites for cells and adhesion molecules |
| Drawbacks | immune response, lower ability to interact with cells, strong inflammasome reaction | sterilization cost, in vivo source natural variability, lot-to-lot variability, limited mechanical properties, degradation rate difficult to control, unwanted immune reactions due to impurities |
| Host Innate Immune response | high | low |
| Host Adaptive Immune response | not applicable | low |
| Based on data from [2,3,4,5,8,19,141,142] | ||
| Groups | -NH2 (Amino) | -OH (Hydroxyl) | -COOH (Carboxyl) | -CH3 (Methyl) |
|---|---|---|---|---|
| Surfaces | hydrophilic | hydrophilic | hydrophilic | hydrophobic |
| Charges | positive | neutral | negative | neutral |
| Focal adhesions | medium | high | medium | low |
| Ability to access fibronectin domains, integrin binding, cell adhesion | medium | high | medium | low |
| Inflammatory cell infiltration | high (in vivo) | high (in vivo) | low | high (in vitro) |
| Macrophage response | anti inflammatory | low inflammatory | inflammatory | |
| low inflammatory | ||||
| low inflammatory promoting regulatory T cell phenotypes (mouse model) | ||||
| Thickness of fibrotic capsules around the implant | high (in vivo) | high (in vivo) | low | high (in vitro) |
| Cell differentiation pathways | medium (osteoblasts) | high (osteoblasts) | medium (osteoblasts) | low (osteoblasts and myoblasts) |
| Size | Cell Types | Findings |
|---|---|---|
| Nano scale | Platelets |
|
| Macrophages |
| |
| ||
| Dendritic cells |
| |
| Nano- submicron scale | Macrophages |
|
| Micron scale | Macrophages |
|
| ||
| Meso scale | Macrophages |
|
| Cell Types | Findings |
|---|---|
| Macrophages |
|
| |
| |
| |
| Neutrophils |
|
| Dendritic cells |
|
| |
| T lymphocytes |
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. https://doi.org/10.3390/ijms20030636
Mariani E, Lisignoli G, Borzì RM, Pulsatelli L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? International Journal of Molecular Sciences. 2019; 20(3):636. https://doi.org/10.3390/ijms20030636
Chicago/Turabian StyleMariani, Erminia, Gina Lisignoli, Rosa Maria Borzì, and Lia Pulsatelli. 2019. "Biomaterials: Foreign Bodies or Tuners for the Immune Response?" International Journal of Molecular Sciences 20, no. 3: 636. https://doi.org/10.3390/ijms20030636
APA StyleMariani, E., Lisignoli, G., Borzì, R. M., & Pulsatelli, L. (2019). Biomaterials: Foreign Bodies or Tuners for the Immune Response? International Journal of Molecular Sciences, 20(3), 636. https://doi.org/10.3390/ijms20030636