Postnatal Expression Profile of microRNAs Associated with Cardiovascular and Cerebrovascular Diseases in Children at the Age of 3 to 11 Years in Relation to Previous Occurrence of Pregnancy-Related Complications
Abstract
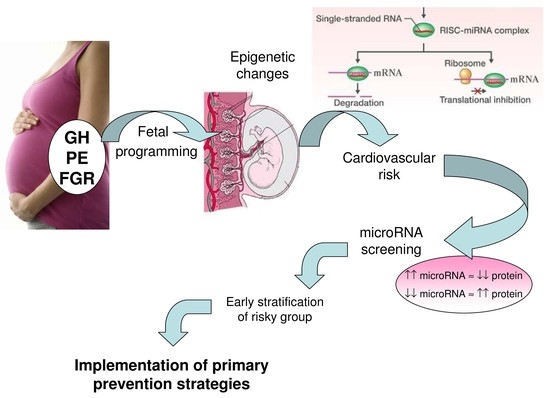
:1. Introduction
2. Results
2.1. Distribution of Children Descending from Normal Pregnancies into Groups Based on Clinical Examination and Consequent Findings
2.2. Up-Regulation of miR-21-5p in Children Descending from Normal Pregnancies that are Overweight/Obese, Prehypertensive/Hypertensive and/or have Abnormal Echocardiogram Findings
2.3. Dysregulation of Cardiovascular/Cerebrovascular Disease Associated microRNAs in Children Descending from Complicated Pregnancies
2.4. Multiple microRNAs are Up-Regulated in Children Descending from GH Pregnancies
2.5. Up-Regulation of miR-21-5p, miR-23a-3p, miR-26a-5p, miR-103a-3p, miR-125b-5p, miR-195-5p, and miR-342-3p in Children with Normal Postnatal Clinical Findings Descending from GH Pregnancies
2.6. Up-Regulation of miR-20a-5p in Children with Abnormal Postnatal Clinical Findings Descending from GH Pregnancies
2.7. Up-Regulation of miR-1-3p, miR-17-5p, miR-29a-3p, miR-126-3p, miR-133a-3p, miR-146a-5p, and miR-181a-5p in Children Descending from GH Pregnancies Irrespective of Postnatal Clinical Findings
2.8. Cardiovascular/Cerebrovascular Disease Associated microRNAs are Dysregulated in Children Descending from PE Pregnancies
2.9. Increased Expression of miR-133a-3p in Children Descending from PE Pregnancies
2.10. Increased Expression of miR-1-3p, miR-20a-5p, miR-103a-3p, and miR-342-3p in Children with Abnormal Clinical Findings Descending from PE Pregnancies
2.11. Up-Regulation of miR-20b-5p in Children with Normal Clinical Findings Descending from Mild PE Pregnancies
2.12. Dysregulation of Cardiovascular/Cerebrovascular Disease Associated microRNAs in Children Descending from FGR Pregnancies
2.13. Increased Expression of miR-17-5p, miR-126-3p and miR-133a-3p in Children with Abnormal Clinical Findings Descending from FGR Pregnancies
2.14. The Association between Postnatal Expression of miR-210-3p and the Severity of PE and/or FGR with regard to Doppler Ultrasonography Parameters
2.15. The Effect of Children Age on Particular microRNA Expression in Children Descending from Normal and Complicated Pregnancies
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. BP Measurements
4.3. BMI Assessment
4.4. Echocardiography Measurements
4.5. Processing of Samples
4.6. Reverse Transcriptase Reaction
4.7. Relative Quantification of microRNAs by Real-Time PCR
4.8. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PE | Preeclampsia |
| FGR | Fetal growth restriction |
| GH | Gestational hypertension |
| DV | Ductus venosus |
| CPR | Cerebro-placental ratio |
| PI | Pulsatility index |
| BMI | Body mass index |
References
- Davis, E.F.; Lazdam, M.; Lewandowski, A.J.; Worton, S.A.; Kelly, B.; Kenworthy, Y.; Adwani, S.; Wilkinson, A.R.; McCormick, K.; Sargent, I.; et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: A systematic review. Pediatrics 2012, 129, e1552–e1561. [Google Scholar] [CrossRef]
- Alsnes, I.V.; Vatten, L.J.; Fraser, A.; Bjørngaard, J.H.; Rich-Edwards, J.; Romundstad, P.R.; Åsvold, B.O. Hypertension in Pregnancy and Offspring Cardiovascular Risk in Young Adulthood: Prospective and Sibling Studies in the HUNT Study (Nord-Trøndelag Health Study) in Norway. Hypertension 2017, 69, 591–598. [Google Scholar] [PubMed]
- Tenhola, S.; Rahiala, E.; Martikainen, A.; Halonen, P.; Voutilainen, R. Blood pressure, serum lipids, fasting insulin, and adrenal hormones in 12-year-old children born with maternal preeclampsia. J. Clin. Endocrinol. Metab. 2003, 88, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Øglaend, B.; Forman, M.R.; Romundstad, P.R.; Nilsen, S.T.; Vatten, L.J. Blood pressure in early adolescence in the offspring of preeclamptic and normotensive pregnancies. J. Hypertens. 2009, 27, 2051–2054. [Google Scholar] [CrossRef]
- Fraser, A.; Nelson, S.M.; Macdonald-Wallis, C.; Sattar, N.; Lawlor, D.A. Hypertensive disorders of pregnancy and cardiometabolic health in adolescent offspring. Hypertension 2013, 62, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Staley, J.R.; Bradley, J.; Silverwood, R.J.; Howe, L.D.; Tilling, K.; Lawlor, D.A.; Macdonald-Wallis, C. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: Findings from a prospective study. J. Am. Heart Assoc. 2015, 4, e001422. [Google Scholar] [CrossRef]
- Lazdam, M.; de la Horra, A.; Diesch, J.; Kenworthy, Y.; Davis, E.; Lewandowski, A.J.; Szmigielski, C.; Shore, A.; Mackillop, L.; Kharbanda, R.; et al. Unique blood pressure characteristics in mother and offspring after early onset preeclampsia. Hypertension 2012, 60, 1338–1345. [Google Scholar] [CrossRef]
- Lim, W.Y.; Lee, Y.S.; Yap, F.K.; Aris, I.M.; Lek, N.; Meaney, M.; Gluckman, P.D.; Godfrey, K.M.; Kwek, K.; Chong, Y.S.; et al. Maternal Blood Pressure During Pregnancy and Early Childhood Blood Pressures in the Offspring: The GUSTO Birth Cohort Study. Medicine (Baltimore) 2015, 94, e1981. [Google Scholar] [CrossRef]
- Jayet, P.Y.; Rimoldi, S.F.; Stuber, T.; Salmòn, C.S.; Hutter, D.; Rexhaj, E.; Thalmann, S.; Schwab, M.; Turini, P.; Sartori-Cucchia, C.; et al. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation 2010, 122, 488–494. [Google Scholar] [CrossRef]
- Sarvari, S.I.; Rodriguez-Lopez, M.; Nuñez-Garcia, M.; Sitges, M.; Sepulveda-Martinez, A.; Camara, O.; Butakoff, C.; Gratacos, E.; Bijnens, B.; Crispi, F. Persistence of Cardiac Remodeling in Preadolescents with Fetal Growth Restriction. Circ. Cardiovasc. Imaging 2017, 10, e005270. [Google Scholar] [CrossRef]
- Yiallourou, S.R.; Wallace, E.M.; Whatley, C.; Odoi, A.; Hollis, S.; Weichard, A.J.; Muthusamy, J.S.; Varma, S.; Cameron, J.; Narayan, O.; et al. Sleep: A Window Into Autonomic Control in Children Born Preterm and Growth Restricted. Sleep 2017, 40. [Google Scholar] [CrossRef] [PubMed]
- Rostand, S.G.; Cliver, S.P.; Goldenberg, R.L. Racial disparities in the association of foetal growth retardation to childhood blood pressure. Nephrol. Dial. Transplant. 2005, 20, 1592–1597. [Google Scholar] [CrossRef] [PubMed]
- Libby, G.; Murphy, D.J.; McEwan, N.F.; Greene, S.A.; Forsyth, J.S.; Chien, P.W.; Morris, A.D.; DARTS/MEMO Collaboration. Pre-eclampsia and the later development of type 2 diabetes in mothers and their children: An intergenerational study from the Walker cohort. Diabetologia 2007, 50, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Tappia, P.S.; Gabriel, C.A. Role of nutrition in the development of the fetal cardiovascular system. Expert Rev. Cardiovasc. Ther. 2006, 4, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef]
- Elmén, J.; Lindow, M.; Silahtaroglu, A.; Bak, M.; Christensen, M.; Lind-Thomsen, A.; Hedtjärn, M.; Hansen, J.B.; Hansen, H.F.; Straarup, E.M.; et al. Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Res. 2008, 36, 1153–1162. [Google Scholar] [CrossRef]
- Yang, K.; He, Y.S.; Wang, X.Q.; Lu, L.; Chen, Q.J.; Liu, J.; Sun, Z.; Shen, W.F. MiR-146a inhibits oxidized low-density lipoprotein-induced lipid accumulation and inflammatory response via targeting toll-like receptor 4. FEBS Lett. 2011, 585, 854–860. [Google Scholar] [CrossRef]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008, 456, 980–984. [Google Scholar] [CrossRef]
- Xin, M.; Small, E.M.; Sutherland, L.B.; Qi, X.; McAnally, J.; Plato, C.F.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009, 23, 2166–2178. [Google Scholar] [CrossRef]
- Li, S.; Zhu, J.; Zhang, W.; Chen, Y.; Zhang, K.; Popescu, L.M.; Ma, X.; Lau, W.B.; Rong, R.; Yu, X.; et al. Signature microRNA expression profile of essential hypertension and its novel link to human cytomegalovirus infection. Circulation 2011, 124, 175–184. [Google Scholar] [CrossRef]
- Harris, T.A.; Yamakuchi, M.; Ferlito, M.; Mendell, J.T.; Lowenstein, C.J. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc. Natl. Acad. Sci. USA 2008, 105, 1516–1521. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Wang, H.Y.; Liao, Y.C.; Tsai, P.C.; Chen, K.C.; Cheng, H.Y.; Lin, R.T.; Juo, S.H. MicroRNA-195 regulates vascular smooth muscle cell phenotype and prevents neointimal formation. Cardiovasc. Res. 2012, 95, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhu, J.; Han, W.; Jiang, X.; Xu, M.; Zhao, Y.; Dong, Q.; Pang, Z.; Guan, Q.; Gao, L.; et al. Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: A clinical study. Acta Diabetol. 2011, 48, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Cheng, Y.; Yue, J.; Yang, J.; Liu, X.; Chen, H.; Dean, D.B.; Zhang, C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ. Res. 2007, 100, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Raitoharju, E.; Lyytikäinen, L.P.; Levula, M.; Oksala, N.; Mennander, A.; Tarkka, M.; Klopp, N.; Illig, T.; Kähönen, M.; Karhunen, P.J.; et al. miR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atherosclerosis 2011, 219, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Poliseno, L.; Tuccoli, A.; Mariani, L.; Evangelista, M.; Citti, L.; Woods, K.; Mercatanti, A.; Hammond, S.; Rainaldi, G. MicroRNAs modulate the angiogenic properties of HUVECs. Blood 2006, 108, 3068–3071. [Google Scholar] [CrossRef] [PubMed]
- Doebele, C.; Bonauer, A.; Fischer, A.; Scholz, A.; Reiss, Y.; Urbich, C.; Hofmann, W.K.; Zeiher, A.M.; Dimmeler, S. Members of the microRNA-17-92 cluster exhibit a cell-intrinsic antiangiogenic function in endothelial cells. Blood 2010, 115, 4944–4950. [Google Scholar] [CrossRef] [PubMed]
- Fichtlscherer, S.; De Rosa, S.; Fox, H.; Schwietz, T.; Fischer, A.; Liebetrau, C.; Weber, M.; Hamm, C.W.; Röxe, T.; Müller-Ardogan, M.; et al. Circulating microRNAs in patients with coronary artery disease. Circ. Res. 2010, 107, 677–684. [Google Scholar] [CrossRef]
- van Rooij, E.; Sutherland, L.B.; Liu, N.; Williams, A.H.; McAnally, J.; Gerard, R.D.; Richardson, J.A.; Olson, E.N. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc. Natl. Acad. Sci. USA 2006, 103, 18255–18260. [Google Scholar] [CrossRef]
- Ikeda, S.; Kong, S.W.; Lu, J.; Bisping, E.; Zhang, H.; Allen, P.D.; Golub, T.R.; Pieske, B.; Pu, W.T. Altered microRNA expression in human heart disease. Physiol. Genom. 2007, 31, 367–373. [Google Scholar] [CrossRef]
- D’Alessandra, Y.; Devanna, P.; Limana, F.; Straino, S.; Di Carlo, A.; Brambilla, P.G.; Rubino, M.; Carena, M.C.; Spazzafumo, L.; De Simone, M.; et al. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur. Heart. J. 2010, 31, 2765–2773. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, J.; López, B.; Hermida, N.; Schroen, B.; San José, G.; Heymans, S.; Valencia, F.; Gómez-Doblas, J.J.; De Teresa, E.; Díez, J.; et al. microRNA-122 down-regulation may play a role in severe myocardial fibrosis in human aortic stenosis through TGF-β1 up-regulation. Clin. Sci. (Lond.) 2014, 126, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Maitrias, P.; Metzinger-Le Meuth, V.; Nader, J.; Reix, T.; Caus, T.; Metzinger, L. The Involvement of miRNA in Carotid-Related Stroke. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1608–1617. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, M.; He, H.; Chen, J.; Zeng, H.; Li, J.; Duan, R. MicroRNA/mRNA profiling and regulatory network of intracranial aneurysm. BMC Med. Genom. 2013, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Bienertova-Vasku, J.; Novak, J.; Vasku, A. MicroRNAs in pulmonary arterial hypertension: Pathogenesis, diagnosis and treatment. J. Am. Soc. Hypertens. 2015, 9, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Halkein, J.; Tabruyn, S.P.; Ricke-Hoch, M.; Haghikia, A.; Nguyen, N.Q.; Scherr, M.; Castermans, K.; Malvaux, L.; Lambert, V.; Thiry, M.; et al. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J. Clin. Investig. 2013, 123, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.C. Micro RNAs are complementary to 3′ UTR sequence motifs that mediate negative post-transcriptional regulation. Nat. Genet. 2002, 30, 363–364. [Google Scholar] [CrossRef]
- Rosenfeld, N.; Aharonov, R.; Meiri, E.; Rosenwald, S.; Spector, Y.; Zepeniuk, M.; Benjamin, H.; Shabes, N.; Tabak, S.; Levy, A.; et al. MicroRNAs accurately identify cancer tissue origin. Nat. Biotechnol. 2008, 26, 462–469. [Google Scholar] [CrossRef]
- Pineles, B.L.; Romero, R.; Montenegro, D.; Tarca, A.L.; Han, Y.M.; Kim, Y.M.; Draghici, S.; Espinoza, J.; Kusanovic, J.P.; Mittal, P.; et al. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am. J. Obstet. Gynecol. 2007, 196, 261.e1–261.e6. [Google Scholar] [CrossRef]
- Hu, Y.; Li, P.; Hao, S.; Liu, L.; Zhao, J.; Hou, Y. Differential expression of microRNAs in the placentae of Chinese patients with severe pre-eclampsia. Clin. Chem. Lab. Med. 2009, 47, 923–929. [Google Scholar] [CrossRef]
- Maccani, M.A.; Padbury, J.F.; Marsit, C.J. miR-16 and miR-21 expression in the placenta is associated with fetal growth. PLoS ONE 2011, 6, e21210. [Google Scholar] [CrossRef] [PubMed]
- Higashijima, A.; Miura, K.; Mishima, H.; Kinoshita, A.; Jo, O.; Abe, S.; Hasegawa, Y.; Miura, S.; Yamasaki, K.; Yoshida, A.; et al. Characterization of placenta-specific microRNAs in fetal growth restriction pregnancy. Prenat. Diagn. 2013, 33, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhao, Y.; Liu, M.; Wang, Y.; Wang, H.; Li, Y.X.; Zhu, X.; Yao, Y.; Wang, H.; Qiao, J.; et al. Variations of microRNAs in human placentas and plasma from preeclamptic pregnancy. Hypertension 2014, 63, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Hromadnikova, I.; Kotlabova, K.; Ivankova, K.; Vedmetskaya, Y.; Krofta, L. Profiling of cardiovascular and cerebrovascular disease associated microRNA expression in umbilical cord blood in gestational hypertension, preeclampsia and fetal growth restriction. Int. J. Cardiol. 2017, 249, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Hromadnikova, I.; Kotlabova, K.; Hympanova, L.; Krofta, L. Cardiovascular and Cerebrovascular Disease Associated microRNAs Are Dysregulated in Placental Tissues Affected with Gestational Hypertension, Preeclampsia and Intrauterine Growth Restriction. PLoS ONE 2015, 10, e0138383. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dong, X.; Wang, Z.; Wu, J. MicroRNA-1 in Cardiac Diseases and Cancers. Korean J. Physiol. Pharmacol. 2014, 18, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Zhang, M.F.; Wen, H.Y.; Hu, C.L.; Liu, R.; Wei, H.Y.; Ai, C.M.; Wang, G.; Liao, X.X.; Li, X. Comparing the diagnostic values of circulating microRNAs and cardiac troponin T in patients with acute myocardial infarction. Clinics (Sao Paulo) 2013, 68, 75–80. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Cardiac-specific miRNA in cardiogenesis, heart function, and cardiac pathology (with focus on myocardial infarction). J. Mol. Cell. Cardiol. 2016, 94, 107–121. [Google Scholar] [CrossRef]
- Tang, Y.; Zheng, J.; Sun, Y.; Wu, Z.; Liu, Z.; Huang, G. MicroRNA-1 regulates cardiomyocyte apoptosis by targeting Bcl-2. Int. Heart J. 2009, 50, 377–387. [Google Scholar] [CrossRef]
- Terentyev, D.; Belevych, A.E.; Terentyeva, R.; Martin, M.M.; Malana, G.E.; Kuhn, D.E.; Abdellatif, M.; Feldman, D.S.; Elton, T.S.; Györke, S. miR-1 overexpression enhances Ca(2+) release and promotes cardiac arrhythmogenesis by targeting PP2A regulatory subunit B56alpha and causing CaMKII-dependent hyperphosphorylation of RyR2. Circ. Res. 2009, 104, 514–521. [Google Scholar] [CrossRef]
- Mogilyansky, E.; Rigoutsos, I. The miR-17/92 cluster: A comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ. 2013, 20, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Qi, R.Q.; Liu, M.; Xu, Y.P.; Li, G.; Weiland, M.; Kaplan, D.H.; Mi, Q.S. microRNA miR-17-92 cluster is highly expressed in epidermal Langerhans cells but not required for its development. Genes Immun. 2014, 15, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Bonauer, A.; Carmona, G.; Iwasaki, M.; Mione, M.; Koyanagi, M.; Fischer, A.; Burchfield, J.; Fox, H.; Doebele, C.; Ohtani, K.; et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 2009, 324, 1710–1713. [Google Scholar] [CrossRef] [PubMed]
- Danielson, L.S.; Park, D.S.; Rotllan, N.; Chamorro-Jorganes, A.; Guijarro, M.V.; Fernandez-Hernando, C.; Fishman, G.I.; Phoon, C.K.; Hernando, E. Cardiovascular dysregulation of miR-17-92 causes a lethal hypertrophic cardiomyopathy and arrhythmogenesis. FASEB J. 2013, 27, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Pan, Z.; Chen, X.; Wang, L.; Zhang, Y.; Li, S.; Liang, H.; Xu, C.; Zhang, Y.; Wu, Y.; et al. By targeting Stat3 microRNA-17-5p promotes cardiomyocyte apoptosis in response to ischemia followed by reperfusion. Cell. Physiol. Biochem. 2014, 34, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Fan, T.; Hu, Q.; Xu, W.; Yang, J.; Xu, C.; Zhang, B.; Chen, J.; Jiang, H. Downregulation of microRNA-17-5p improves cardiac function after myocardial infarction via attenuation of apoptosis in endothelial cells. Mol. Genet. Genom. 2018, 293, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Kaucsár, T.; Révész, C.; Godó, M.; Krenács, T.; Albert, M.; Szalay, C.I.; Rosivall, L.; Benyó, Z.; Bátkai, S.; Thum, T.; et al. Activation of the miR-17 family and miR-21 during murine kidney ischemia-reperfusion injury. Nucleic Acid Ther. 2013, 23, 344–354. [Google Scholar] [CrossRef]
- Fang, L.; Ellims, A.H.; Moore, X.L.; White, D.A.; Taylor, A.J.; Chin-Dusting, J.; Dart, A.M. Circulating microRNAs as biomarkers for diffuse myocardial fibrosis in patients with hypertrophic cardiomyopathy. J. Transl. Med. 2015, 13, 314. [Google Scholar] [CrossRef]
- Wu, J.; Du, K.; Lu, X. Elevated expressions of serum miR-15a, miR-16, and miR-17-5p are associated with acute ischemic stroke. Int. J. Clin. Exp. Med. 2015, 8, 21071–21079. [Google Scholar]
- Chen, J.; Xu, L.; Hu, Q.; Yang, S.; Zhang, B.; Jiang, H. MiR-17-5p as circulating biomarkers for the severity of coronary atherosclerosis in coronary artery disease. Int. J. Cardiol. 2015, 197, 123–124. [Google Scholar] [CrossRef]
- Mendell, J.T. miRiad roles for the miR-17-92 cluster in development and disease. Cell 2008, 133, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Brock, M.; Samillan, V.J.; Trenkmann, M.; Schwarzwald, C.; Ulrich, S.; Gay, R.E.; Gassmann, M.; Ostergaard, L.; Gay, S.; Speich, R.; et al. AntagomiR directed against miR-20a restores functional BMPR2 signalling and prevents vascular remodelling in hypoxia-induced pulmonary hypertension. Eur. Heart J. 2014, 35, 3203–3211. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tian, F.; Li, H.; Zhou, Y.; Lu, J.; Ge, Q. Profiling maternal plasma microRNA expression in early pregnancy to predict gestational diabetes mellitus. Int. J. Gynaecol. Obstet. 2015, 130, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, B.A.; Semus, H.M.; Montgomery, R.L.; Stack, C.; Latimer, P.A.; Lewton, S.M.; Lynch, J.M.; Hullinger, T.G.; Seto, A.G.; et al. Plasma microRNAs serve as biomarkers of therapeutic efficacy and disease progression in hypertension-induced heart failure. Eur. J. Heart Fail. 2013, 15, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Rodosthenous, R.S.; Burris, H.H.; Sanders, A.P.; Just, A.C.; Dereix, A.E.; Svensson, K.; Solano, M.; Téllez-Rojo, M.M.; Wright, R.O.; Baccarelli, A.A. Second trimester extracellular microRNAs in maternal blood and fetal growth: An exploratory study. Epigenetics 2017, 12, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Sekar, D.; Venugopal, B.; Sekar, P.; Ramalingam, K. Role of microRNA 21 in diabetes and associated/related diseases. Gene 2016, 582, 14–18. [Google Scholar] [CrossRef]
- Suárez, Y.; Fernández-Hernando, C.; Pober, J.S.; Sessa, W.C. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ. Res. 2007, 100, 1164–1173. [Google Scholar] [CrossRef]
- Dong, S.; Ma, W.; Hao, B.; Hu, F.; Yan, L.; Yan, X.; Wang, Y.; Chen, Z.; Wang, Z. microRNA-21 promotes cardiac fibrosis and development of heart failure with preserved left ventricular ejection fraction by up-regulating Bcl-2. Int. J. Clin. Exp. Pathol. 2014, 7, 565–574. [Google Scholar]
- Liu, Y.; Nie, H.; Zhang, K.; Ma, D.; Yang, G.; Zheng, Z.; Liu, K.; Yu, B.; Zhai, C.; Yang, S. A feedback regulatory loop between HIF-1α and miR-21 in response to hypoxia in cardiomyocytes. FEBS Lett. 2014, 588, 3137–3146. [Google Scholar] [CrossRef]
- Zhang, J.; Xing, Q.; Zhou, X.; Li, J.; Li, Y.; Zhang, L.; Zhou, Q.; Tang, B. Circulating miRNA-21 is a promising biomarker for heart failure. Mol. Med. Rep. 2017, 16, 7766–7774. [Google Scholar] [CrossRef]
- Long, B.; Gan, T.Y.; Zhang, R.C.; Zhang, Y.H. miR-23a Regulates Cardiomyocyte Apoptosis by Targeting Manganese Superoxide Dismutase. Mol. Cells 2017, 40, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, W.; Wang, C. MiR-23a Regulates the Vasculogenesis of Coronary Artery Disease by Targeting Epidermal Growth Factor Receptor. Cardiovasc. Ther. 2016, 34, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Li, Y.; Lu, N.; Dai, Y.; Zhang, H.; Zhao, X.; Liu, Y. Resveratrol attenuates the inflammatory reaction induced by ischemia/reperfusion in the rat heart. Mol. Med. Rep. 2014, 9, 2528–2532. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, Q.G. The role of miR-26 in tumors and normal tissues. Oncol. Lett. 2011, 2, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Lin, S.; Lv, C. MiR-26a-5p regulates cardiac fibroblasts collagen expression by targeting ULK1. Sci. Rep. 2018, 8, 2104. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Hu, Z.; Lin, Y.; Zhang, C.; Perez-Polo, J.R. Downregulation of microRNA-29 by antisense inhibitors and a PPAR-gamma agonist protects against myocardial ischaemia-reperfusion injury. Cardiovasc. Res. 2010, 87, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Moraes, L.N.; Fernandez, G.J.; Vechetti-Júnior, I.J.; Freire, P.P.; Souza, R.W.A.; Villacis, R.A.R.; Rogatto, S.R.; Reis, P.P.; Dal-Pai-Silva, M.; Carvalho, R.F. Integration of miRNA and mRNA expression profiles reveals microRNA-regulated networks during muscle wasting in cardiac cachexia. Sci. Rep. 2017, 7, 6998. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yuan, Y.; Qiu, C. Underexpression of CACNA1C Caused by Overexpression of microRNA-29a Underlies the Pathogenesis of Atrial Fibrillation. Med. Sci. Monit. 2016, 22, 2175–2181. [Google Scholar] [CrossRef] [PubMed]
- Widlansky, M.E.; Jensen, D.M.; Wang, J.; Liu, Y.; Geurts, A.M.; Kriegel, A.J.; Liu, P.; Ying, R.; Zhang, G.; Casati, M.; et al. miR-29 contributes to normal endothelial function and can restore it in cardiometabolic disorders. EMBO Mol. Med. 2018, 10, e8046. [Google Scholar] [CrossRef] [PubMed]
- Moncini, S.; Salvi, A.; Zuccotti, P.; Viero, G.; Quattrone, A.; Barlati, S.; De Petro, G.; Venturin, M.; Riva, P. The role of miR-103 and miR-107 in regulation of CDK5R1 expression and in cellular migration. PLoS ONE 2011, 6, e20038. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, L.; Chen, X.; Zhang, H.; Shi, Z. MiR-103a targeting Piezo1 is involved in acute myocardial infarction through regulating endothelium function. Cardiol. J. 2016, 23, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Zhang, X.J.; Li, Q.; Wang, K.; Wang, Y.; Jiao, J.Q.; Feng, C.; Teng, S.; Zhou, L.Y.; Gong, Y.; et al. MicroRNA-103/107 Regulate Programmed Necrosis and Myocardial Ischemia/Reperfusion Injury Through Targeting FADD. Circ. Res. 2015, 117, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Du, J.; Hu, R.; Wang, A.P.; Wu, W.H.; Hu, C.P.; Li, Y.J.; Li, X.H. MicroRNA-103/107 is involved in hypoxia-induced proliferation of pulmonary arterial smooth muscle cells by targeting HIF-1β. Life Sci. 2016, 147, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Trajkovski, M.; Hausser, J.; Soutschek, J.; Bhat, B.; Akin, A.; Zavolan, M.; Heim, M.H.; Stoffel, M. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature 2011, 474, 649–653. [Google Scholar] [CrossRef]
- Shaham, L.; Binder, V.; Gefen, N.; Borkhardt, A.; Izraeli, S. MiR-125 in normal and malignant hematopoiesis. Leukemia 2012, 26, 2011–2018. [Google Scholar] [CrossRef]
- Tiedt, S.; Prestel, M.; Malik, R.; Schieferdecker, N.; Duering, M.; Kautzky, V.; Stoycheva, I.; Böck, J.; Northoff, B.H.; Klein, M.; et al. RNA-Seq Identifies Circulating miR-125a-5p, miR-125b-5p, and miR-143-3p as Potential Biomarkers for Acute Ischemic Stroke. Circ. Res. 2017, 121, 970–980. [Google Scholar] [CrossRef]
- Jia, K.; Shi, P.; Han, X.; Chen, T.; Tang, H.; Wang, J. Diagnostic value of miR-30d-5p and miR-125b-5p in acute myocardial infarction. Mol. Med. Rep. 2016, 14, 184–194. [Google Scholar] [CrossRef]
- Wei, M.; Gan, L.; Liu, Z.; Kong, L.H.; Chang, J.R.; Chen, L.H.; Su, X.L. MiR125b-5p protects endothelial cells from apoptosis under oxidative stress. Biomed. Pharmacother. 2017, 95, 453–460. [Google Scholar] [CrossRef]
- Bayoumi, A.S.; Park, K.M.; Wang, Y.; Teoh, J.P.; Aonuma, T.; Tang, Y.; Su, H.; Weintraub, N.L.; Kim, I.M. A carvedilol-responsive microRNA, miR-125b-5p protects the heart from acute myocardial infarction by repressing pro-apoptotic bak1 and klf13 in cardiomyocytes. J. Mol. Cell. Cardiol. 2018, 114, 72–82. [Google Scholar] [CrossRef]
- Wu, X.J.; Zhao, Z.F.; Kang, X.J.; Wang, H.J.; Zhao, J.; Pu, X.M. MicroRNA-126-3p suppresses cell proliferation by targeting PIK3R2 in Kaposi’s sarcoma cells. Oncotarget 2016, 7, 36614–36621. [Google Scholar]
- Hsu, A.; Chen, S.J.; Chang, Y.S.; Chen, H.C.; Chu, P.H. Systemic approach to identify serum microRNAs as potential biomarkers for acute myocardial infarction. Biomed. Res. Int. 2014, 2014, 418628. [Google Scholar] [CrossRef]
- Olivieri, F.; Spazzafumo, L.; Bonafè, M.; Recchioni, R.; Prattichizzo, F.; Marcheselli, F.; Micolucci, L.; Mensà, E.; Giuliani, A.; Santini, G.; et al. MiR-21-5p and miR-126a-3p levels in plasma and circulating angiogenic cells: Relationship with type 2 diabetes complications. Oncotarget 2015, 6, 35372–35382. [Google Scholar] [CrossRef]
- Jansen, F.; Stumpf, T.; Proebsting, S.; Franklin, B.S.; Wenzel, D.; Pfeifer, P.; Flender, A.; Schmitz, T.; Yang, X.; Fleischmann, B.K.; et al. Intercellular transfer of miR-126-3p by endothelial microparticles reduces vascular smooth muscle cell proliferation and limits neointima formation by inhibiting LRP6. J. Mol. Cell. Cardiol. 2017, 104, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.W.; Yang, X.; Wen, D.Y.; Gao, L.; Zhang, X.Y.; Ye, Z.H.; Luo, J.; Li, Z.Y.; He, Y.; Pang, Y.Y.; et al. Utility of miR-133a-3p as a diagnostic indicator for hepatocellular carcinoma: An investigation combined with GEO, TCGA, meta-analysis and bioinformatics. Mol. Med. Rep. 2018, 17, 1469–1484. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, R.; Lin, F.; Zhang, S.; Zhang, G.; Hu, S.; Zheng, Z. MicroRNA: Novel regulators involved in the remodeling and reverse remodeling of the heart. Cardiology 2009, 113, 81–88. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, E.; Olson, E.N. MicroRNAs: Powerful new regulators of heart disease and provocative therapeutic targets. J. Clin. Investig. 2007, 117, 2369–2376. [Google Scholar] [CrossRef]
- Kukreja, R.C.; Yin, C.; Salloum, F.N. MicroRNAs: New players in cardiac injury and protection. Mol. Pharmacol. 2011, 80, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Duisters, R.F.; Tijsen, A.J.; Schroen, B.; Leenders, J.J.; Lentink, V.; van der Made, I.; Herias, V.; van Leeuwen, R.E.; Schellings, M.W.; Barenbrug, P.; et al. miR-133 and miR-30 regulate connective tissue growth factor: Implications for a role of microRNAs in myocardial matrix remodeling. Circ. Res. 2009, 104, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ling, S.; Sun, W.; Liu, T.; Li, Y.; Zhong, G.; Zhao, D.; Zhang, P.; Song, J.; Jin, X.; et al. Circulating microRNAs correlated with the level of coronary artery calcification in symptomatic patients. Sci. Rep. 2015, 5, 16099. [Google Scholar] [CrossRef] [PubMed]
- Taganov, K.D.; Boldin, M.P.; Chang, K.J.; Baltimore, D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486. [Google Scholar] [CrossRef]
- Paterson, M.R.; Kriegel, A.J. MiR-146a/b: A family with shared seeds and different roots. Physiol. Genom. 2017, 49, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ye, Z.H.; Liang, H.W.; Ren, F.H.; Li, P.; Dang, Y.W.; Chen, G. Down-regulation of miR-146a-5p and its potential targets in hepatocellular carcinoma validated by a TCGA- and GEO-based study. FEBS Open Bio 2017, 7, 504–521. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Chen, L.; Pang, X.M.; Su, S.Y.; Zhou, X.; Chen, C.Y.; Huang, L.G.; Li, J.P.; Liu, J.L. Decreased miR-146a expression in acute ischemic stroke directly targets the Fbxl10 mRNA and is involved in modulating apoptosis. Neurochem. Int. 2017, 107, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ha, T.; Liu, L.; Zou, J.; Zhang, X.; Kalbfleisch, J.; Gao, X.; Williams, D.; Li, C. Increased expression of microRNA-146a decreases myocardial ischaemia/reperfusion injury. Cardiovasc. Res. 2013, 97, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Ji, Y.; Zhang, C.; Guo, X.; Zhang, Y.; Jia, S.; Ma, W.; Fan, Y.; Wang, C. Circulating MiR-146a May be a Potential Biomarker of Coronary Heart Disease in Patients with Subclinical Hypothyroidism. Cell. Physiol. Biochem. 2018, 45, 226–236. [Google Scholar] [CrossRef]
- Sun, X.; Sit, A.; Feinberg, M.W. Role of miR-181 family in regulating vascular inflammation and immunity. Trends Cardiovasc. Med. 2014, 24, 105–112. [Google Scholar] [CrossRef]
- Hulsmans, M.; Sinnaeve, P.; Van der Schueren, B.; Mathieu, C.; Janssens, S.; Holvoet, P. Decreased miR-181a expression in monocytes of obese patients is associated with the occurrence of metabolic syndrome and coronary artery disease. J. Clin. Endocrinol. Metab. 2012, 97, E1213–E1218. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Bartolomé, J.; Llauradó, G.; Portero-Otin, M.; Altuna-Coy, A.; Rojo-Martínez, G.; Vendrell, J.; Jorba, R.; Rodríguez-Gallego, E.; Chacón, M.R. Altered Expression of miR-181a-5p and miR-23a-3p Is Associated With Obesity and TNFα-Induced Insulin Resistance. J. Clin. Endocrinol. Metab. 2018, 103, 1447–1458. [Google Scholar] [CrossRef]
- Du, X.; Yang, Y.; Xu, C.; Peng, Z.; Zhang, M.; Lei, L.; Gao, W.; Dong, Y.; Shi, Z.; Sun, X.; et al. Upregulation of miR-181a impairs hepatic glucose and lipid homeostasis. Oncotarget 2017, 8, 91362–91378. [Google Scholar] [CrossRef]
- Nabih, E.S.; Andrawes, N.G. The Association Between Circulating Levels of miRNA-181a and Pancreatic Beta Cells Dysfunction via SMAD7 in Type 1 Diabetic Children and Adolescents. J. Clin. Lab. Anal. 2016, 30, 727–731. [Google Scholar] [CrossRef]
- Wu, J.; Fan, C.L.; Ma, L.J.; Liu, T.; Wang, C.; Song, J.X.; Lv, Q.S.; Pan, H.; Zhang, C.N.; Wang, J.J. Distinctive expression signatures of serum microRNAs in ischaemic stroke and transient ischaemic attack patients. Thromb. Haemost. 2017, 117, 992–1001. [Google Scholar] [PubMed]
- Zhu, J.; Yao, K.; Wang, Q.; Guo, J.; Shi, H.; Ma, L.; Liu, H.; Gao, W.; Zou, Y.; Ge, J. Circulating miR-181a as a Potential Novel Biomarker for Diagnosis of Acute Myocardial Infarction. Cell. Physiol. Biochem. 2016, 40, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- He, J.F.; Luo, Y.M.; Wan, X.H.; Jiang, D. Biogenesis of MiRNA-195 and its role in biogenesis, the cell cycle, and apoptosis. J. Biochem. Mol. Toxicol. 2011, 25, 404–408. [Google Scholar] [CrossRef] [PubMed]
- You, X.Y.; Huang, J.H.; Liu, B.; Liu, S.J.; Zhong, Y.; Liu, S.M. HMGA1 is a new target of miR-195 involving isoprenaline-induced cardiomyocyte hypertrophy. Biochemistry 2014, 79, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Zampetaki, A.; Attia, R.; Mayr, U.; Gomes, R.S.; Phinikaridou, A.; Yin, X.; Langley, S.R.; Willeit, P.; Lu, R.; Fanshawe, B.; et al. Role of miR-195 in aortic aneurysmal disease. Circ. Res. 2014, 115, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zheng, R.; Xiao, F.; Zhang, S.; He, K.; Zhang, J.; Shao, Y. Downregulated MicroRNA-195 in the Bicuspid Aortic Valve Promotes Calcification of Valve Interstitial Cells via Targeting SMAD7. Cell. Physiol. Biochem. 2017, 44, 884–896. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, C.; Zhang, L.; Yang, P. MicroRNA-210 induces endothelial cell apoptosis by directly targeting PDK1 in the setting of atherosclerosis. Cell. Mol. Biol. Lett. 2017, 22, 3. [Google Scholar] [CrossRef]
- Zaccagnini, G.; Maimone, B.; Di Stefano, V.; Fasanaro, P.; Greco, S.; Perfetti, A.; Capogrossi, M.C.; Gaetano, C.; Martelli, F. Hypoxia-induced miR-210 modulates tissue response to acute peripheral ischemia. Antioxid. Redox Signal. 2014, 21, 1177–1188. [Google Scholar] [CrossRef]
- Diao, H.; Liu, B.; Shi, Y.; Song, C.; Guo, Z.; Liu, N.; Song, X.; Lu, Y.; Lin, X.; Li, Z. MicroRNA-210 alleviates oxidative stress-associated cardiomyocyte apoptosis by regulating BNIP3. Biosci. Biotechnol. Biochem. 2017, 81, 1712–1720. [Google Scholar] [CrossRef]
- Li, T.; Cao, H.; Zhuang, J.; Wan, J.; Guan, M.; Yu, B.; Li, X.; Zhang, W. Identification of miR-130a, miR-27b and miR-210 as serum biomarkers for atherosclerosis obliterans. Clin. Chim. Acta 2011, 412, 66–70. [Google Scholar] [CrossRef]
- Zhao, D.S.; Chen, Y.; Jiang, H.; Lu, J.P.; Zhang, G.; Geng, J.; Zhang, Q.; Shen, J.H.; Zhou, X.; Zhu, W.; et al. Serum miR-210 and miR-30a expressions tend to revert to fetal levels in Chinese adult patients with chronic heart failure. Cardiovasc. Pathol. 2013, 22, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.L.; Guo, F.; Liu, F.; Gao, F.L.; Zhang, P.Q.; Niu, X.; Guo, S.C.; Yin, J.H.; Wang, Y.; Deng, Z.F. miR-210 activates notch signaling pathway in angiogenesis induced by cerebral ischemia. Mol. Cell. Biochem. 2012, 370, 45–51. [Google Scholar] [CrossRef]
- Wang, L.; Xu, L.; Xu, M.; Liu, G.; Xing, J.; Sun, C.; Ding, H. Obesity-Associated MiR-342-3p Promotes Adipogenesis of Mesenchymal Stem Cells by Suppressing CtBP2 and Releasing C/EBPα from CtBP2 Binding. Cell. Physiol. Biochem. 2015, 35, 2285–2298. [Google Scholar] [CrossRef]
- Collares, C.V.; Evangelista, A.F.; Xavier, D.J.; Rassi, D.M.; Arns, T.; Foss-Freitas, M.C.; Foss, M.C.; Puthier, D.; Sakamoto-Hojo, E.T.; Passos, G.A.; et al. Identifying common and specific microRNAs expressed in peripheral blood mononuclear cell of type 1, type 2, and gestational diabetes mellitus patients. BMC Res. Notes 2013, 6, 491. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Matboli, M.; Bekhet, M.M. Clinical verification of a novel urinary microRNA panal: 133b, -342 and -30 as biomarkers for diabetic nephropathy identified by bioinformatics analysis. Biomed. Pharmacother. 2016, 83, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Cui, Y.; Fan, L.; Mu, X.; Hua, Y. T2DM inhibition of endothelial miR-342-3p facilitates angiogenic dysfunction via repression of FGF11 signaling. Biochem. Biophys. Res. Commun. 2018, 503, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Hezova, R.; Slaby, O.; Faltejskova, P.; Mikulkova, Z.; Buresova, I.; Raja, K.R.; Hodek, J.; Ovesna, J.; Michalek, J. microRNA-342, microRNA-191 and microRNA-510 are differentially expressed in T regulatory cells of type 1 diabetic patients. Cell. Immunol. 2010, 260, 70–74. [Google Scholar] [CrossRef]
- Khalyfa, A.; Kheirandish-Gozal, L.; Bhattacharjee, R.; Khalyfa, A.A.; Gozal, D. Circulating microRNAs as Potential Biomarkers of Endothelial Dysfunction in Obese Children. Chest 2016, 149, 786–800. [Google Scholar] [CrossRef]
- ACOG Practice Bulletin. Diagnosis and management of preeclampsia and eclampsia. Obstet. Gynecol. 2002, 99, 159–167. [Google Scholar]
- Figueras, F.; Gratacos, E. Stage-based approach to the management of fetal growth restriction. Prenat. Diagn. 2014, 34, 655–659. [Google Scholar] [CrossRef]
- Baschat, A.A. Neurodevelopment following fetal growth restriction and its relationship with antepartum parameters of placental dysfunction. Ultrasound. Obstet. Gynecol. 2011, 37, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Nardozza, L.M.; Caetano, A.C.; Zamarian, A.C.; Mazzola, J.B.; Silva, C.P.; Marçal, V.M.; Lobo, T.F.; Peixoto, A.B.; Araujo Júnior, E. Fetal growth restriction: Current knowledge. Arch. Gynecol. Obstet. 2017, 295, 1061–1077. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Nicolaides, K.H.; Bower, S.; Campbell, S. Middle cerebral artery flow velocity waveforms in fetal hypoxaemia. Br. J. Obstet. Gynaecol. 1990, 97, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Cohn, H.E.; Sacks, E.J.; Heymann, M.A.; Rudolph, A.M. Cardiovascular responses to hypoxemia and acidemia in fetal lambs. Am. J. Obstet. Gynecol. 1974, 120, 817–824. [Google Scholar] [CrossRef]
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004, 114, 555–576. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; de Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; de Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]




























| MicroRNA Expression in Children Descending from Pregnancy-Related Complications | |||
|---|---|---|---|
| miRBase ID | Gestational Hypertension (GH) | Preeclampsia (PE) | Fetal Growth Restriction (FGR) |
| hsa-miR-1-3p | ↑ children with both normal and abnormal clinical findings | ↑ late PE, only children with abnormal clinical findings | |
| hsa-miR-17-5p | ↑ children with both normal and abnormal clinical findings | ↑ only children with abnormal clinical findings | |
| hsa-miR-20a-5p | ↑ only children with abnormal clinical findings | ↑ late PE, only children with abnormal clinical findings | |
| hsa-miR-20b-5p | ↑ mild PE, only children with normal clinical findings | ||
| hsa-miR-21-5p | ↑ only children with normal clinical findings | ||
| hsa-miR-23a-3p | ↑ only children with normal clinical findings | ||
| hsa-miR-26a-5p | ↑ only children with normal clinical findings | ||
| hsa-miR-29a-3p | ↑ children with both normal and abnormal clinical findings | ||
| hsa-miR-103a-3p | ↑ severe PE, late PE, only children with abnormal clinical findings | ||
| hsa-miR-125b-5p | ↑ only children with normal clinical findings | ||
| hsa-miR-126-3p | ↑ children with both normal and abnormal clinical findings | ↑ only children with abnormal clinical findings | |
| hsa-miR-133a-3p | ↑ children with both normal and abnormal clinical findings | ↑ PE, severe PE, late PE children with both normal and abnormal clinical findings early PE, only children with normal clinical findings | ↑ only children with abnormal clinical findings |
| hsa-miR-146a-5p | ↑ children with both normal and abnormal clinical findings | ||
| hsa-miR-181a-5p | ↑ children with both normal and abnormal clinical findings | ||
| hsa-miR-195-5p | ↑ only children with normal clinical findings | ||
| hsa-miR-210-3p | ↑ children descending from PE and/or FGR complicated pregnancies with increased PI in the ductus venosus during gestation | ||
| hsa-miR-342-3p | ↓ early PE, only children with abnormal clinical findings | ||
| miRBase ID | Gene Location on Chromosome | Expression | Role in the Pathogenesis of Cardiovascular/Cerebrovascular Diseases | Potential Therapeutic Target in Treatment of Cardiovascular Diseases |
|---|---|---|---|---|
| hsa-miR-1-3p | 20q13.3 18q11.2 [46] | Cardiac and skeletal muscles, myocardium | Acute myocardial infarction, heart ischemia, post-myocardial infarction complications [47] | + [48,49,50] |
| hsa-miR-17-5p | 13q31.3 [51,52] | Endothelial cells, vascular smooth muscle cells [53] | Cardiac development [54], ischemia/reperfusion-induced cardiac injury [55], kidney ischemia-reperfusion injury [57], diffuse myocardial fibrosis in hypertrophic cardiomyopathy [58], acute ischemic stroke [59], coronary artery disease [60] | + [55,56] |
| hsa-miR-20a-5p | 13q31.3 [61] | Pulmonary arteries [62] | Pulmonary hypertension [62], gestational diabetes mellitus [63] | + [62] |
| hsa-miR-20b-5p | Xq26.2 [61] | Hypertension-induced heart failure [64], small for gestational age foetuses [65] | ||
| hsa-miR-21-5p | 17q23.2 [66] | Cardiomyocytes | Homeostasis of the cardiovascular system [67], cardiac fibrosis and heart failure [68,70] | + [68,69] |
| hsa-miR-23a-3p | 19p13.12 | Cardiomyocytes | Heart failure [71], coronary artery disease [72], cerebral ischemia-reperfusion [73] | |
| hsa-miR-26a-5p | 3p22.2 12q14.1 [74] | Cardiac fibroblasts [75] | Heart failure, cardiac hypertrophy [75] | |
| hsa-miR-29a-3p | 7q32.3 | Heart | Ischemia/reperfusion-induced cardiac injury [76], cardiac cachexia, heart failure [77], atrial fibrillation [78], diffuse myocardial fibrosis in hypertrophic cardiomyopathy [58], gestational diabetes mellitus [63], T2DM [23,80] | + [76] |
| hsa-miR-103a-3p | 5q34 20p13 [81] | Heart Pulmonary arterial smooth muscle cells | Hypertension [82], hypoxia-induced pulmonary hypertension [84], myocardial ischemia/reperfusion injury, acute myocardial infarction [82], obesity, regulation of insulin sensitivity [85] | + [83] |
| hsa-miR-125b-5p | 11q24.1 21q21.1 [86] | Endothelial cells [89], cardiomyocytes [90] | Acute ischemic stroke [87], acute myocardial infarction [88,90] | |
| hsa-miR-126-3p | 9q34.3 [91] | Endothelial cells [21], vascular smooth muscle cells [95] | Acute myocardial infarction [93], T2DM [94] | + [21,95] |
| hsa-miR-133a-3p | 18q11.2 20q13.33 [96] | Heart | Heart failure [98], myocardial fibrosis in hypertrophic cardiomyopathy [58,97], arrhythmogenesis in the hypertrophic and failing hearts [99,100], coronary artery calcification [102] | + [99,100] |
| hsa-miR-146a-5p | 5q33.3 [103,104] | Myocardium, brain | Angiogenesis [105], hypoxia, ischemia/reperfusion-induced cardiac injury [107], coronary atherosclerosis, coronary heart disease in patients with subclinical hypothyroidism [108], acute ischemic stroke, acute cerebral ischemia [106] | + [107] |
| hsa-miR-181a-5p | 1q32.1 9q33.3 [109] | Monocytes, adipocytes, hepatocytes | Atherosclerosis [109], T1DM [113], T2DM [109,111], obesity [109,110,111], metabolic syndrome, coronary artery disease [110], insulin resistance [111], non-alcoholic fatty liver disease [112], ischaemic stroke, transient ischaemic attack, acute myocardial infarction [114,115] | |
| hsa-miR-195-5p | 17p13.1 [116] | Aorta, abdominal aorta | Cardiac hypertrophy, heart failure [29,118], abdominal aortic aneurysms [119], aortic stenosis [120] | + [29,118] |
| hsa-miR-210-3p | 11p15.5 | Endothelial cells, cardiomyocytes [125], skeletal muscle [124] | Hypoxia [39], atherosclerotic plaque formation [25,123,126], heart failure [127], cerebral ischemia [128] | |
| hsa-miR-342-3p | 14q32.2 | Endothelial cells | Obesity [129], T1DM [130,133], T2DM [130,131,132], GDM [130], endothelial dysfunction [134] |
| Normal Pregnancies with Normal Clinical Findings (n = 50) | Normal Pregnancies with Abnormal Clinical Findings (n = 38) | PE (n = 133) | FGR (n = 34) | GH (n = 54) | p-Value 1 | p-Value 2 | p-Value 3 | p-Value 4 | |
|---|---|---|---|---|---|---|---|---|---|
| At follow-up | |||||||||
| Age (years) | 5 (3–11) | 5 (3–11) | 5 (3–11) | 4 (3–10) | 4.5 (3–10) | 1.000 | 1.000 | 1.000 | 1.000 |
| Height (cm) | 115 (98–144.5) | 118.5 (100–153) | 114 (97–155) | 106.5 (93–152) | 111.5 (96–159.5) | 1.000 | 1.000 | 0.020 | 1.000 |
| Weight (kg) | 20.35 (14–37) | 22.3 (14.7–40.8) | 19.4 (11.85–54.9) | 16.25 (12–37) | 19.6 (14–47.5) | 1.000 | 1.000 | 0.002 | 1.000 |
| BMI (kg/m2) | 15.43 (13.22–18.09) | 15.87 (13.3–20) | 14.91 (12.34–22.81) | 14.18 (12.7–19.24) | 15.35 (13.42–19.7) | 1.000 | 1.000 | 0.004 | 1.000 |
| Systolic BP (mmHg) | 98 (84–115) | 104 (89–123) | 99 (84–132) | 97 (82–123) | 99 (80–129) | 0.001 | 1.000 | 1.000 | 0.487 |
| Diastolic BP (mmHg) | 60 (38–68) | 64.4 (43–81) | 61 (41–88) | 60 (42–75) | 61.5 (49–83) | 0.028 | 0.545 | 1.000 | 1.000 |
| Heart rate (n/min) | 90 (67–110) | 90.5 (51–120) | 92 (64–117) | 96 (62–112) | 94.5 (65–129) | 1.000 | 1.000 | 1.000 | 1.000 |
| During gestation | |||||||||
| Maternal age at delivery (years) | 32.5 (26–40) | 32 (25–43) | 32 (21–44) | 32 (22–41) | 32 (27–51) | 1.000 | 1.000 | 1.000 | 1.000 |
| GA at delivery (weeks) | 39.86 (37.71–41.57) | 39.93 (37.86–41.86) | 35.79 (26–41.72) | 35.64 (28–41) | 38.63 (33.43–41.28) | 1.000 | <0.001 | <0.001 | 0.002 |
| Mode of delivery | 0.429 | <0.001 | <0.001 | <0.001 | |||||
| Vaginal | 46 (92.00 %) | 33 (68.84%) | 8 (14.3 %) | 7 (20.59%) | 24 (44.44%) | ||||
| CS | 4 (8.00 %) | 5 (13.16%) | 48 (85.7 %) | 27 (79.41%) | 30 (55.56%) | ||||
| Fetal birth weight (g) | 3425 (2730–4220) | 3295 (2530–4450) | 2370 (660–4490) | 1870 (650-3010) | 3140 (1040-4310) | 1.000 | <0.001 | <0.001 | 0.113 |
| Fetal sex | 0.217 | 0.055 | 0.470 | 0.414 | |||||
| Boy | 29 (58.00%) | 17 (44.74%) | 56 (42.11%) | 17 (50.00%) | 27 (50.00%) | ||||
| Girl | 21 (42.00 %) | 21 (55.26%) | 77 (57.89%) | 17 (50.00%) | 27 (50.00%) | ||||
| Primiparity | 0.140 | 0.001 | <0.001 | 0.362 | |||||
| Yes | 29 (58.00%) | 16 (42.11%) | 108 (81.20%) | 33 (97.06%) | 36 (66.67 %) | ||||
| No | 21 (42.00%) | 22 (57.89%) | 25 (18.80 %) | 1 (2.94 %) | 18 (33.33 %) | ||||
| Birth order of index pregnancy | 0.158 | 0.168 | 0.009 | 0.602 | |||||
| 1st | 25 (50.00%) | 12 (31.58%) | 86 (64.66%) | 28 (82.35) | 28 (51.85%) | ||||
| 2nd | 18 (36.00%) | 14 (36.84%) | 27 (20.30%) | 2 (5.88%) | 14 (25.93%) | ||||
| 3rd | 5 (10.00%) | 10 (26.32%) | 13 (9.77 %) | 2 (5.88%) | 9 (16.66 %) | ||||
| 4th+ | 2 (4.00 %) | 2 (5.26%) | 7 (5.26 %) | 2 (5.88%) | 3 (5.56 %) | ||||
| Infertility treatment | 0.726 | 0.001 | 0.007 | 0.117 | |||||
| Yes | 2 (4.00%) | 1 (2.63%) | 34 (25.56%) | 8 (23.53 %) | 7 (12.96%) | ||||
| No | 48 (96.00%) | 37 (97.37%) | 99 (74.44%) | 26 (76.47 %) | 47 (87.04%) | ||||
| Assay Name | miRBase ID | NCBI Location Chromosome | microRNA Sequence |
|---|---|---|---|
| hsa-miR-1 | hsa-miR-1-3p | Chr20: 61151513-61151583 [+] | 5′-UGGAAUGUAAAGAAGUAUGUAU-3′ |
| hsa-miR-16 | hsa-miR-16-5p | Chr13: 50623109-50623197 [−] | 5′-UAGCAGCACGUAAAUAUUGGCG- 3′ |
| hsa-miR-17 | hsa-miR-17-5p | Chr13: 92002859-92002942 [+] | 5′-CAAAGUGCUUACAGUGCAGGUAG-3′ |
| hsa-miR-20a | hsa-miR-20a-5p | Chr13: 92003319-92003389 [+] | 5′-UAAAGUGCUUAUAGUGCAGGUAG-3′ |
| hsa-miR-20b | hsa-miR-20b-5p | ChrX: 133303839-133303907 [−] | 5′-CAAAGUGCUCAUAGUGCAGGUAG-3′ |
| hsa-miR-21 | hsa-miR-21-5p | Chr17: 57918627-57918698 [+] | 5′-UAGCUUAUCAGACUGAUGUUGA-3′ |
| hsa-miR-23a | hsa-miR-23a-3p | Chr19: 13947401-13947473 [−] | 5′-AUCACAUUGCCAGGGAUUUCC-3′ |
| hsa-miR-24 | hsa-miR-24-3p | Chr19: 13947101-13947173 [−] | 5′-UGGCUCAGUUCAGCAGGAACAG-3′ |
| hsa-miR-26a | hsa-miR-26a-5p | Chr3: 38010895-38010971 [+] | 5′-UUCAAGUAAUCCAGGAUAGGCU-3′ |
| hsa-miR-29a | hsa-miR-29a-3p | Chr7: 130561506-130561569 [−] | 5′-UAGCACCAUCUGAAAUCGGUUA-3′ |
| hsa-miR-92a | hsa-miR-92a-3p | Chr13: 92003568-92003645 [+] | 5′-UAUUGCACUUGUCCCGGCCUGU-3′ |
| hsa-miR-100 | hsa-miR-100-5p | Chr11: 122022937-122023016 [−] | 5′-AACCCGUAGAUCCGAACUUGUG-3′ |
| hsa-miR-103 | hsa-miR-103a-3p | Chr20: 3898141-3898218 [+] | 5′-AGCAGCAUUGUACAGGGCUAUGA-3′ |
| hsa-miR-125b | hsa-miR-125b-5p | Chr21: 17962557-17962645 [+] | 5′-UCCCUGAGACCCUAACUUGUGA-3′ |
| hsa-miR-126 | hsa-miR-126-3p | Chr9: 139565054-139565138 [+] | 5′-UCGUACCGUGAGUAAUAAUGCG-3′ |
| hsa-miR-130b | hsa-miR-130b-3p | Chr22: 22007593-22007674 [+] | 5′-CAGUGCAAUGAUGAAAGGGCAU-3′ |
| hsa-miR-133a | hsa-miR-133a-3p | Chr20: 61162119-61162220 [+] | 5′-UUUGGUCCCCUUCAACCAGCUG-3′ |
| hsa-miR-143 | hsa-miR-143-3p | Chr5: 148808481-148808586 [+] | 5′-UGAGAUGAAGCACUGUAGCUC-3′ |
| hsa-miR-145 | hsa-miR-145-5p | Chr5: 148810209-148810296 [+] | 5′-GUCCAGUUUUCCCAGGAAUCCCU-3′ |
| hsa-miR-146a | hsa-miR-146a-5p | Chr5: 159912359-159912457 [+] | 5′-UGAGAACUGAAUUCCAUGGGUU-3′ |
| hsa-miR-155 | hsa-miR-155-5p | Chr21: 26946292-26946356 [+] | 5′-UUAAUGCUAAUCGUGAUAGGGGU-3′ |
| hsa-miR-181a | hsa-miR-181a-5p | Chr9: 127454721-127454830 [+] | 5′-AACAUUCAACGCUGUCGGUGAGU-3′ |
| hsa-miR-195 | hsa-miR-195-5p | Chr17: 6920934-6921020 [−] | 5′-UAGCAGCACAGAAAUAUUGGC-3′ |
| hsa-miR-199a | hsa-miR-199a-5p | Chr19: 10928102-10928172 [−] | 5′-CCCAGUGUUCAGACUACCUGUUC-3′ |
| hsa-miR-210 | hsa-miR-210-3p | Chr11: 568089-568198 [−] | 5′-CUGUGCGUGUGACAGCGGCUGA-3′ |
| hsa-miR-221 | hsa-miR-221-3p | ChrX: 45605585-45605694 [−] | 5′-AGCUACAUUGUCUGCUGGGUUUC-3′ |
| hsa-miR-342-3p | hsa-miR-342-3p | Chr14: 100575992-100576090 [+] | 5′-UCUCACACAGAAAUCGCACCCGU-3′ |
| mmu-miR-499 | hsa-miR-499a-5p | Chr20: 33578179-33578300 [+] | 5′-UUAAGACUUGCAGUGAUGUUU-3′ |
| hsa-miR-574-3p | hsa-miR-574-3p | Chr4: 38869653-38869748 [+] | 5′-CACGCUCAUGCACACACCCACA-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hromadnikova, I.; Kotlabova, K.; Dvorakova, L.; Krofta, L.; Sirc, J. Postnatal Expression Profile of microRNAs Associated with Cardiovascular and Cerebrovascular Diseases in Children at the Age of 3 to 11 Years in Relation to Previous Occurrence of Pregnancy-Related Complications. Int. J. Mol. Sci. 2019, 20, 654. https://doi.org/10.3390/ijms20030654
Hromadnikova I, Kotlabova K, Dvorakova L, Krofta L, Sirc J. Postnatal Expression Profile of microRNAs Associated with Cardiovascular and Cerebrovascular Diseases in Children at the Age of 3 to 11 Years in Relation to Previous Occurrence of Pregnancy-Related Complications. International Journal of Molecular Sciences. 2019; 20(3):654. https://doi.org/10.3390/ijms20030654
Chicago/Turabian StyleHromadnikova, Ilona, Katerina Kotlabova, Lenka Dvorakova, Ladislav Krofta, and Jan Sirc. 2019. "Postnatal Expression Profile of microRNAs Associated with Cardiovascular and Cerebrovascular Diseases in Children at the Age of 3 to 11 Years in Relation to Previous Occurrence of Pregnancy-Related Complications" International Journal of Molecular Sciences 20, no. 3: 654. https://doi.org/10.3390/ijms20030654
APA StyleHromadnikova, I., Kotlabova, K., Dvorakova, L., Krofta, L., & Sirc, J. (2019). Postnatal Expression Profile of microRNAs Associated with Cardiovascular and Cerebrovascular Diseases in Children at the Age of 3 to 11 Years in Relation to Previous Occurrence of Pregnancy-Related Complications. International Journal of Molecular Sciences, 20(3), 654. https://doi.org/10.3390/ijms20030654