CGRP Induces Differential Regulation of Cytokines from Satellite Glial Cells in Trigeminal Ganglia and Orofacial Nociception
Abstract
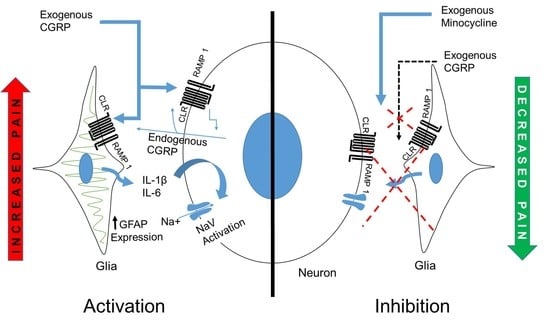
:1. Introduction
2. Results
2.1. Effect of Intra-Ganglionic Calcitonin Gene-Related Peptide (CGRP)
2.1.1. Intra-Ganglionic CGRP Mediates Orofacial Thermal Hyperalgesia
2.1.2. Intra-Ganglionic CGRP-Induced Thermal Hyperalgesia Is Accompanied by Satellite Glial Cell Activation in Trigeminal Ganglion (TG)
2.1.3. Intra-Ganglionic CGRP-Induced Thermal Hyperalgesia Is Accompanied by Differential Regulation of Cytokines in TG
2.1.4. CGRP Induces Differential Regulation of Various Cytokines from the Glial Cells
2.2. Effect of Injecting Minocycline (Min) Intra-Ganglionic 1 Hour before Injecting CGRP
2.2.1. Min Prevented the Pro-Nociceptive Effect of Intra-Ganglionic CGRP
2.2.2. Min Prevented the Pro-Nociceptive Effect of Intra-Ganglionic CGRP via Inhibition of Glial Activation
2.2.3. Min Induced Glial Inactivation Is Accompanied by Reduced Expression of Cytokines in TG
2.3. CGRP-Induced Neuronal Activation Is Prevented by Min
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Anesthesia
4.3. Intra-Ganglionic Drug Administration
4.4. Behavioral Assessment
4.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.6. Immunohistochemistry
4.7. Cytokine Measurement
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CGRP | Calcitonin gene-related peptide |
| DAPI | 4′,6-Diamidino-2-phenylindole dihydrochloride |
| GFAP | Anti-glial fibrillary acidic protein |
| GS | Glutamine synthetase |
| IG | Intraganglionic |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| IL-1RA | Interleukin-1 receptor antagonist |
| L/F | Reward licking events/face-contact events |
| MAPK | Mitogen activated protein kinase |
| Min | Minocycline |
| NaV | Voltage gated sodium channel |
| OPAD | Orofacial pain assessment device |
| PNS | Peripheral nervous system |
| RT-PCR | Reverse transcriptase polymerase chain reaction |
| SGC | Satellite glia cell |
| TNF-α | Tumor necrosis factor-α |
| TG | Trigeminal ganglia |
References
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Takahashi, M.; Matsumoto, S. Contribution of the activation of satellite glia in sensory ganglia to pathological pain. Neurosci. Biobehav. Rev. 2009, 33, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Seal, R.P. Illuminating the Gap: neuronal cross-talk within sensory ganglia and persistent pain. Neuron 2016, 91, 950–951. [Google Scholar] [CrossRef]
- Matsuka, Y.; Neubert, J.K.; Maidment, N.T.; Spigelman, I. Concurrent release of ATP and substance P within guinea pig trigeminal ganglia in vivo. Brain Res. 2001, 915, 248–255. [Google Scholar] [CrossRef]
- Matsuka, Y.; Ono, T.; Iwase, H.; Mitrirattanakul, S.; Omoto, K.S.; Cho, T.; Lam, Y.Y.; Snyder, B.; Spigelman, I. Altered ATP release and metabolism in dorsal root ganglia of neuropathic rats. Mol. Pain 2008, 4, 66. [Google Scholar] [CrossRef]
- Suadicani, S.O.; Cherkas, P.S.; Zuckerman, J.; Smith, D.N.; Spray, D.C.; Hanani, M. Bidirectional calcium signaling between satellite glial cells and neurons in cultured mouse trigeminal ganglia. Neuron Glia Biol. 2010, 6, 43–51. [Google Scholar] [CrossRef]
- Mitterreiter, J.G.; Ouwendijk, W.J.; Velzen, M.; Nierop, G.P.; Osterhaus, A.D.; Verjans, G.M. Satellite glial cells in human trigeminal ganglia have a broad expression of functional Toll-like receptors. Eur. J. Immunol. 2017, 47, 1181–1187. [Google Scholar] [CrossRef]
- Vause, C.V.; Durham, P.L. Calcitonin gene-related peptide differentially regulates gene and protein expression in trigeminal glia cells: findings from array analysis. Neurosci. Lett. 2010, 473, 163–167. [Google Scholar] [CrossRef]
- Thalakoti, S.; Patil, V.V.; Damodaram, S.; Vause, C.V.; Langford, L.E.; Freeman, S.E.; Durham, P.L. Neuron-glia signaling in trigeminal ganglion: Implications for migraine pathology. Headache 2007, 47, 1008–1023; discussion 1005–1024. [Google Scholar] [CrossRef]
- Uceyler, N.; Tscharke, A.; Sommer, C. Early cytokine expression in mouse sciatic nerve after chronic constriction nerve injury depends on calpain. Brain Behav. Immun. 2007, 21, 553–560. [Google Scholar] [CrossRef]
- Liu, L.; Yang, T.M.; Liedtke, W.; Simon, S.A. Chronic IL-1beta signaling potentiates voltage dependent sodium currents in trigeminal nociceptive neurons. J. Neurophysiol. 2006, 95, 1478–1490. [Google Scholar] [CrossRef] [PubMed]
- Binshtok, A.M.; Wang, H.; Zimmermann, K.; Amaya, F.; Vardeh, D.; Shi, L.; Brenner, G.J.; Ji, R.R.; Bean, B.P.; Woolf, C.J.; et al. Nociceptors are interleukin-1beta sensors. J. Neurosci. 2008, 28, 14062–14073. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Melemedjian, O.K.; Price, T.J.; Dussor, G. Sensitization of dural afferents underlies migraine-related behavior following meningeal application of interleukin-6 (IL-6). Mol. Pain 2012, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Dublin, P.; Hanani, M. Satellite glial cells in sensory ganglia: their possible contribution to inflammatory pain. Brain Behav. Immun. 2007, 21, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Cady, R.J.; Glenn, J.R.; Smith, K.M.; Durham, P.L. Calcitonin gene-related peptide promotes cellular changes in trigeminal neurons and glia implicated in peripheral and central sensitization. Mol. Pain 2011, 7. [Google Scholar] [CrossRef] [PubMed]
- Benemei, S.; Nicoletti, P.; Capone, J.G.; Geppetti, P. CGRP receptors in the control of pain and inflammation. Curr. Opin. Pharmacol. 2009, 9, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Assas, B.M.; Pennock, J.; Miyan, J.A. Calcitonin gene-related peptide is a key neurotransmitter in the neuro-immune axis. Front. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eftekhari, S.; Salvatore, C.A.; Calamari, A.; Kane, S.A.; Tajti, J.; Edvinsson, L. Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion. Neuroscience 2010, 169, 683–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Felice, M.; Ossipov, M.H.; Wang, R.; Lai, J.; Chichorro, J.; Meng, I.; Dodick, D.W.; Vanderah, T.W.; Dussor, G.; Porreca, F. Triptan-induced latent sensitization a possible basis for medication overuse headache. Ann. Neurol. 2010, 67, 325–337. [Google Scholar]
- Miller, S.; Liu, H.; Warfvinge, K.; Shi, L.; Dovlatyan, M.; Xu, C.; Edvinsson, L. Immunohistochemical localization of the calcitonin gene-related peptide binding site in the primate trigeminovascular system using functional antagonist antibodies. Neuroscience 2016, 328, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Winborn, C.S.; Marquez de Prado, B.; Russo, A.F. Sensitization of calcitonin gene-related peptide receptors by receptor activity-modifying protein-1 in the trigeminal ganglion. J. Neurosci. 2007, 27, 2693–2703. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, S.; Ossipov, M.H.; Johnson, K.W. The role of calcitonin gene–related peptide in peripheral and central pain mechanisms including migraine. Pain 2017, 158, 543–559. [Google Scholar] [CrossRef]
- Souza, G.R.; Talbot, J.; Lotufo, C.M.; Cunha, F.Q.; Cunha, T.M.; Ferreira, S.H. Fractalkine mediates inflammatory pain through activation of satellite glial cells. Proc. Natl. Acad. Sci. USA 2013, 110, 11193–11198. [Google Scholar] [CrossRef] [Green Version]
- Katagiri, A.; Shinoda, M.; Honda, K.; Toyofuku, A.; Sessle, B.J.; Iwata, K. Satellite glial cell P2Y12 receptor in the trigeminal ganglion is involved in lingual neuropathic pain mechanisms in rats. Mol. Pain 2012, 8, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Vit, J.P.; Jasmin, L.; Bhargava, A.; Ohara, P.T. Satellite glial cells in the trigeminal ganglion as a determinant of orofacial neuropathic pain. Neuron Glia Biol. 2006, 2, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Black, J.A.; Liu, S.; Tanaka, M.; Cummins, T.R.; Waxman, S.G. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain 2004, 108, 237–247. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [PubMed]
- Ceruti, S.; Villa, G.; Fumagalli, M.; Colombo, L.; Magni, G.; Zanardelli, M.; Fabbretti, E.; Verderio, C.; van den Maagdenberg, A.M.; Nistri, A.; et al. Calcitonin gene-related peptide-mediated enhancement of purinergic neuron/glia communication by the algogenic factor bradykinin in mouse trigeminal ganglia from wild-type and R192Q Cav2.1 Knock-in mice: implications for basic mechanisms of migraine pain. J. Neurosci. 2011, 31, 3638–3649. [Google Scholar] [CrossRef]
- De Corato, A.; Lisi, L.; Capuano, A.; Tringali, G.; Russo, C.D. Trigeminal satellite cells express functional calcitonin gene-related peptide receptors, whose activation enhances interleukin-1β pro-inflammatory effects. J. Neuroimmunol. 2011, 237, 39–46. [Google Scholar] [CrossRef]
- Capuano, A.; De Corato, A.; Lisi, L.; Tringali, G.; Navarra, P.; Russo, C.D. Proinflammatory-activated trigeminal satellite cells promote neuronal sensitization: Relevance for migraine pathology. Mol. Pain 2009, 5. [Google Scholar] [CrossRef]
- Takeda, M.; Tanimoto, T.; Kadoi, J.; Nasu, M.; Takahashi, M.; Kitagawa, J.; Matsumoto, S. Enhanced excitability of nociceptive trigeminal ganglion neurons by satellite glial cytokine following peripheral inflammation. Pain 2007, 129, 155–166. [Google Scholar] [CrossRef]
- Covasala, O.; Stirn, S.L.; Albrecht, S.; De Col, R.; Messlinger, K. Calcitonin gene-related peptide receptors in rat trigeminal ganglion do not control spinal trigeminal activity. J. Neurophysiol. 2012, 108, 431–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, A.D.; Tam, C.W.; Lazar, Z.; Shih, M.K.; Brain, S.D. The calcitonin gene-related peptide (CGRP) receptor antagonist BIBN4096BS blocks CGRP and adrenomedullin vasoactive responses in the microvasculature. Br. J. Pharmacol. 2004, 142, 1091–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassen, L.H.; Haderslev, P.A.; Jacobsen, V.B.; Iversen, H.K.; Sperling, B.; Olesen, J. CGRP may play a causative role in migraine. Cephalalgia 2002, 22, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Ledeboer, A.; Sloane, E.M.; Milligan, E.D.; Frank, M.G.; Mahony, J.H.; Maier, S.F.; Watkins, L.R. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain 2005, 115, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Chen, Y.L.; Li, A.H.; Lu, J.C.; Wang, H.L. Minocycline, a microglial inhibitor, blocks spinal CCL2-induced heat hyperalgesia and augmentation of glutamatergic transmission in substantia gelatinosa neurons. J. Neuroinflamm. 2014, 11. [Google Scholar] [CrossRef]
- Zanjani, T.M.; Sabetkasaei, M.; Mosaffa, N.; Manaheji, H.; Labibi, F.; Farokhi, B. Suppression of interleukin-6 by minocycline in a rat model of neuropathic pain. Eur. J. Pharmacol. 2006, 538, 66–72. [Google Scholar] [CrossRef]
- Altmayr, F.; Jusek, G.; Holzmann, B. The neuropeptide calcitonin gene-related peptide causes repression of tumor necrosis factor-alpha transcription and suppression of ATF-2 promoter recruitment in Toll-like receptor-stimulated dendritic cells. J. Biol. Chem. 2010, 285, 3525–3531. [Google Scholar] [CrossRef]
- Kristiansen, K.A.; Edvinsson, L. Neurogenic inflammation: A study of rat trigeminal ganglion. J. Headache Pain 2010, 11, 485–495. [Google Scholar] [CrossRef]
- Bowen, E.J.; Schmidt, T.W.; Firm, C.S.; Russo, A.F.; Durham, P.L. Tumor necrosis factor-α stimulation of calcitonin gene-related peptide expression and secretion from rat trigeminal ganglion neurons. J. Neurochem. 2006, 96, 65–77. [Google Scholar] [CrossRef]
- Perini, F.; D’Andrea, G.; Galloni, E.; Pignatelli, F.; Billo, G.; Alba, S.; Bussone, G.; Toso, V. Plasma cytokine levels in migraineurs and controls. Headache 2005, 45, 926–931. [Google Scholar] [CrossRef]
- Zhang, P.; Bi, R.Y.; Gan, Y.H. Glial interleukin-1β upregulates neuronal sodium channel 1.7 in trigeminal ganglion contributing to temporomandibular joint inflammatory hypernociception in rats. J. Neuroinflamm. 2018, 15, 117. [Google Scholar] [CrossRef] [Green Version]
- Yeomans, D.C.; Levinson, S.R.; Peters, M.C.; Koszowski, A.G.; Tzabazis, A.Z.; Gilly, W.F.; Wilson, S.P. Decrease in inflammatory hyperalgesia by herpes vector mediated knockdown of Nav1.7 sodium channels in primary afferents. Hum. Gene. Ther. 2005, 16, 271–277. [Google Scholar] [CrossRef]
- Nassar, M.A.; Stirling, L.C.; Forlani, G.; Baker, M.D.; Matthews, E.A.; Dickenson, A.H.; Wood, J.N. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc. Natl. Acad. Sci. USA 2004, 101, 12706–12711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, J.J.; Reimann, F.; Nicholas, A.K.; Thornton, G.; Roberts, E.; Springell, K.; Karbani, G.; Jafri, H.; Mannan, J.; Raashid, Y.; et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature 2006, 444, 894–898. [Google Scholar] [CrossRef]
- Dib-Hajj, S.D.; Rush, A.M.; Cummins, T.R.; Hisama, F.M.; Novella, S.; Tyrrell, L.; Marshall, L.; Waxman, S.G. Gain-of-function mutation in Nav1.7 in familial erythromelalgia induces bursting of sensory neurons. Brain 2005, 128, 1847–1854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asghar, M.S.; Becerra, L.; Larsson, H.B.W.; Borsook, D.; Ashina, M. Calcitonin gene-related peptide modulates heat nociception in the human brain—An fMRI study in healthy volunteers. PLoS ONE 2016, 11, e0150334. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lundeberg, T.; Yu, L. Antinociceptive effects of calcitonin gene related peptide injected into periaqueductal grey of rats with mononeuropathy. Brain. Res. 2000, 859, 358–360. [Google Scholar] [CrossRef]
- Xu, W.; Lundeberg, T.; Wang, Y.T.; Li, Y.; Yu, L.C. Antinociceptive effect of calcitonin gene-related peptide in the central nucleus of amygdala activating opioid receptors through amygdala-periaqueductal gray pathway. Neuroscience 2003, 118, 1015–1022. [Google Scholar] [CrossRef]
- Yu, L.C.; Hansson, P.; Lundeberg, T. The calcitonin gene-related peptide antagonist CGRP8-37 increases the latency to withdrawal responses in rats. Brain Res. 1994, 653, 223–230. [Google Scholar] [CrossRef]
- Yao, G.; Huang, Q.; Wang, M.; Yang, C.L.; Liu, C.F.; Yu, T.M. Behavioral study of a rat model of migraine induced by CGRP. Neurosci. Lett. 2017, 651, 134–139. [Google Scholar] [CrossRef]
- Recober, A.; Kuburas, A.; Zhang, Z.; Wemmie, J.A.; Anderson, M.G.; Russo, A.F. Role of calcitonin gene-related peptide in light-aversive behavior: Implications for migraine. J. Neurosci. 2009, 29, 8798–8804. [Google Scholar] [CrossRef] [PubMed]
- Recober, A.; Kaiser, E.A.; Kuburas, A.; Russo, A.F. Induction of multiple photophobic behaviors in a transgenic mouse sensitized to CGRP. Neuropharmacology 2010, 58, 156–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Prado, B.M.; Hammond, D.L.; Russo, A.F. Genetic enhancement of calcitonin gene-related peptide-induced central sensitization to mechanical stimuli in mice. Pain 2009, 10, 992–1000. [Google Scholar] [CrossRef]
- Rojewska, E.; Popiolek-Barczyk, K.; Jurga, A.M.; Makuch, W.; Przewlocka, B.; Mika, J. Involvement of pro- and antinociceptive factors in minocycline analgesia in rat neuropathic pain model. J. Neuroimmunol. 2014, 277, 57–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.C.; Lu, N.; Cui, Y.; Yang, T.; Zhao, Z.Q.; Xin, W.J.; Liu, X.G. Prevention of paclitaxel induced allodynia by minocycline: Effect on loss of peripheral nervefibers and infiltration of macrophages in rats. Mol. Pain 2010, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Bileviciute, I.; Stenfors, C.; Theodorsson, E.; Lundeberg, T. Unilateral injection of calcitonin gene related peptide (CGRP) induces bilateral oedema formationand release of CGRP-like immunoreactivity in the rat hindpaw. Br. J. Pharmacol. 1998, 125, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Kirihara, Y.; Takechi, M.; Kurosaki, K.; Kobayashi, Y.; Saito, Y.; Takeuchi, T. Effects of an anesthetic mixture of medetomidine, midazolam, and butorphanol in rats-strain difference and antagonism by atipamezole. Exp. Anim. 2016, 65, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Neubert, J.K.; Mannes, A.J.; Keller, J.; Wexel, M.; Iadarola, M.J.; Caudle, R.M. Peripheral targeting of the trigeminal ganglion via the infraorbital foramen as a therapeutic strategy. Brain Res. Protoc. 2005, 15, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.; Mills, R.H.; Nolan, T.A.; Jenkins, A.C.; Mustafa, G.; Lloyd, C.D.; Caudle, R.M.; Neubert, J.K. Use of the Operant Orofacial Pain Assessment Device (OPAD) to measure changes in nociceptive behavior. J. Vis. Exp. 2013, 76, e50336. [Google Scholar] [CrossRef]
- Peinnequin, A.; Mouret, C.; Birot, O.; Alonso, A.; Mathieu, J.; Clarençon, D.; Agay, D.; Chancerelle, Y.; Multon, E. Rat pro-inflammatory cytokine and cytokine related mRNA quantification by real-time polymerase chain reaction using SYBR green. BMC Immunol. 2004, 5. [Google Scholar] [CrossRef]
- Zhang, P.; Gan, Y. Prostaglandin E2 upregulated trigeminal ganglionic sodium channel 1.7 involving temporomandibular joint inflammatory pain in rats. Inflammation 2017, 40, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Prins, M.; Eriksson, C.; Wierinckx, A.; Bol, J.G.J.M.; Binnekade, R.; Tilders, F.J.H.; Dam, A.M.V. Interleukin-1β and interleukin-1 receptor antagonist appear in grey matter additionally to white matter lesions during experimental multiple sclerosis. PLoS ONE 2013, 8, e83835. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Li, K.; Gai, Z.; She, X.; Zhang, N.; Xu, C.; Chen, X.; An, G.; Ma, Q.; Wang, R. Chronic noise exposure acts cumulatively to exacerbate alzheimer’s disease-like amyloid-β pathology and neuroinflammation in the rat hippocampus. Sci. Rep. 2015, 5, 12943. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shan, Q.; Meng, Y.; Pan, J.; Yi, S. Mrpl10 and Tbp are suitable reference genes for peripheral nerve crush injury. Int. J. Mol. Sci. 2017, 18, 263. [Google Scholar] [CrossRef] [PubMed]
- Matsuka, Y.; Edmonds, B.; Mitrirattanakul, S.; Schweizer, F.E.; Spigelman, I. Two types of neurotransmitter release patterns in isolectin B4-positive and negative trigeminal ganglion neurons. Neuroscience 2007, 144, 665–674. [Google Scholar] [Green Version]










| Cytokine | Average Fold Change (n = 3) | SEM |
|---|---|---|
| MIG/CXCL9 | 6.81 | 2.84 |
| L-SELECTIN/CD62L/LECAM-1 | 4.64 | 2.55 |
| IL-3 | 4.08 | 1.25 |
| LIX | 3.81 | 2.12 |
| IL-2 | 3.10 | 0.64 |
| IL-6 | 2.78 | 0.40 |
| IL-17 | 2.71 | 0.90 |
| FRACTALKALINE | 2.69 | 1.40 |
| CNTF | 2.63 | 1.23 |
| MIP-1α/CCL-3 | 2.51 | 1.10 |
| IL-1α | 2.50 | 0.75 |
| IL-13 | 2.38 | 0.69 |
| IP-10/CXCL10 | 2.30 | 0.92 |
| IL-4 | 2.23 | 1.37 |
| GM-CSF | 1.98 | 1.51 |
| IL-1ra | 1.96 | 0.31 |
| CINC-2α/β | 1.90 | 0.75 |
| IL-1β | 1.86 | 0.35 |
| IL-10 | 1.75 | 0.77 |
| VEGF | 1.64 | 0.46 |
| IFN-ϒ | 1.21 | 0.36 |
| sICAM-1 | 1.17 | 0.15 |
| THYMUS CHEMOKINE/CXCL7 | 1.15 | 0.69 |
| CINC-3 | 1.15 | 0.40 |
| TIMP-1 | 1.13 | 0.10 |
| CINC-1 | 1.13 | 0.35 |
| RANTES/CCL5 | 0.95 | 0.11 |
| TNF-α | 0.93 | 0.45 |
| MIP-3α/CCL20 | 0.88 | 0.10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afroz, S.; Arakaki, R.; Iwasa, T.; Oshima, M.; Hosoki, M.; Inoue, M.; Baba, O.; Okayama, Y.; Matsuka, Y. CGRP Induces Differential Regulation of Cytokines from Satellite Glial Cells in Trigeminal Ganglia and Orofacial Nociception. Int. J. Mol. Sci. 2019, 20, 711. https://doi.org/10.3390/ijms20030711
Afroz S, Arakaki R, Iwasa T, Oshima M, Hosoki M, Inoue M, Baba O, Okayama Y, Matsuka Y. CGRP Induces Differential Regulation of Cytokines from Satellite Glial Cells in Trigeminal Ganglia and Orofacial Nociception. International Journal of Molecular Sciences. 2019; 20(3):711. https://doi.org/10.3390/ijms20030711
Chicago/Turabian StyleAfroz, Shaista, Rieko Arakaki, Takuma Iwasa, Masamitsu Oshima, Maki Hosoki, Miho Inoue, Otto Baba, Yoshihiro Okayama, and Yoshizo Matsuka. 2019. "CGRP Induces Differential Regulation of Cytokines from Satellite Glial Cells in Trigeminal Ganglia and Orofacial Nociception" International Journal of Molecular Sciences 20, no. 3: 711. https://doi.org/10.3390/ijms20030711
APA StyleAfroz, S., Arakaki, R., Iwasa, T., Oshima, M., Hosoki, M., Inoue, M., Baba, O., Okayama, Y., & Matsuka, Y. (2019). CGRP Induces Differential Regulation of Cytokines from Satellite Glial Cells in Trigeminal Ganglia and Orofacial Nociception. International Journal of Molecular Sciences, 20(3), 711. https://doi.org/10.3390/ijms20030711