MRI Relaxometry for Quantitative Analysis of USPIO Uptake in Cerebral Small Vessel Disease
Abstract
:1. Introduction
2. Results
2.1. Compliance, Tolerability and Symptoms
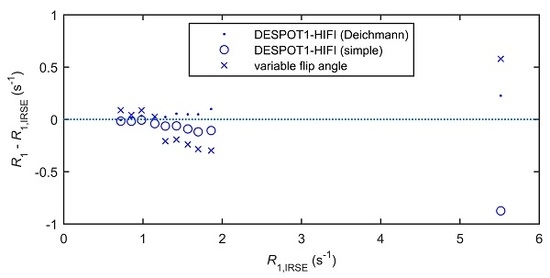
2.2. In Vitro Accuracy of R1 Mapping
2.3. Relaxation Rate Changes Following USPIO Administration
2.4. Blood-Normalised Relaxation Rate Changes
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. MRI and USPIO Administration
4.2.1. Patient Study
4.2.2. Phantom Validation
4.3. Image Processing and Analysis
4.3.1. R1 Mapping
4.3.2. R2* Mapping
4.3.3. Structural Image Processing
4.3.4. Quantitative Image Processing
4.4. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| USPIO | ultrasmall superparamagnetic particles of iron oxide |
| GM | grey matter |
| WM | white matter |
| WMH | white matter hyperintensities |
| CBV | cerebral blood volume |
| BBB | blood-brain barrier |
| SL | stroke lesion |
| ROI | region of interest |
| IR-sGRE | inversion-recovery prepared spoiled gradient echo |
| sGRE | spoiled gradient echo |
| MRI | magnetic resonance imaging |
References
- Aribisala, B.S.; Wiseman, S.; Morris, Z.; Valdes-Hernandez, M.C.; Royle, N.A.; Maniega, S.M.; Gow, A.J.; Corley, J.; Bastin, M.E.; Starr, J.; et al. Circulating inflammatory markers are associated with magnetic resonance imaging-visible perivascular spaces but not directly with white matter hyperintensities. Stroke 2014, 45, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Makin, S.J.; Hernandez, M.C.V.; Armitage, P.A.; Heye, A.K.; Chappell, F.M.; Munoz-Maniega, S.; Sakka, E.; Shuler, K.; Dennis, M.S.; et al. Blood-brain barrier failure as a core mechanism in cerebral small vessel disease and dementia: Evidence from a cohort study. Alzheimers Dement. 2017, 13, 634–643. [Google Scholar] [CrossRef]
- Wardlaw, J.; Smith, C.; Dichgans, M. Microbleeds in cerebral small vessel disease—Authors’ reply. Lancet Neurol. 2013, 12, 736–737. [Google Scholar] [CrossRef]
- Munoz Maniega, S.; Chappell, F.M.; Valdes Hernandez, M.C.; Armitage, P.A.; Makin, S.D.; Heye, A.K.; Thrippleton, M.J.; Sakka, E.; Shuler, K.; Dennis, M.S.; et al. Integrity of normal-appearing white matter: Influence of age, visible lesion burden and hypertension in patients with small-vessel disease. J. Cereb. Blood Flow Metab. 2017, 37, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Thrippleton, M.J.; Shi, Y.L.; Blair, G.; Hamilton, I.; Waiter, G.; Schwarzbauer, C.; Pernet, C.; Andrews, P.J.D.; Marshall, I.; Doubal, F.; et al. Cerebrovascular reactivity measurement in cerebral small vessel disease: Rationale and reproducibility of a protocol for MRI acquisition and image processing. Int. J. Stroke 2018, 13, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Heye, A.K.; Thrippleton, M.J.; Armitage, P.A.; Valdes Hernandez Mdel, C.; Makin, S.D.; Glatz, A.; Sakka, E.; Wardlaw, J.M. Tracer kinetic modelling for DCE-MRI quantification of subtle blood-brain barrier permeability. NeuroImage 2016, 125, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Neuwelt, E.A.; Hamilton, B.E.; Varallyay, C.G.; Rooney, W.R.; Edelman, R.D.; Jacobs, P.M.; Watnick, S.G. Ultrasmall superparamagnetic iron oxides (USPIOs): A future alternative magnetic resonance (MR) contrast agent for patients at risk for nephrogenic systemic fibrosis (NSF)? Kidney Int. 2009, 75, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Varallyay, C.G.; Nesbit, E.; Fu, R.; Gahramanov, S.; Moloney, B.; Earl, E.; Muldoon, L.L.; Li, X.; Rooney, W.D.; Neuwelt, E.A. High-resolution steady-state cerebral blood volume maps in patients with central nervous system neoplasms using ferumoxytol, a superparamagnetic iron oxide nanoparticle. J. Cereb. Blood Flow Metab. 2013, 33, 780–786. [Google Scholar] [CrossRef]
- Weinstein, J.S.; Varallyay, C.G.; Dosa, E.; Gahramanov, S.; Hamilton, B.; Rooney, W.D.; Muldoon, L.L.; Neuwelt, E.A. Superparamagnetic iron oxide nanoparticles: Diagnostic magnetic resonance imaging and potential therapeutic applications in neurooncology and central nervous system inflammatory pathologies, a review. J. Cereb. Blood Flow Metab. 2010, 30, 15–35. [Google Scholar] [CrossRef]
- MA3RS Study Investigators Aortic Wall Inflammation Predicts Abdominal Aortic Aneurysm Expansion, Rupture, and Need for Surgical Repair. Circulation 2017, 136, 787–797. [CrossRef]
- Stirrat, C.G.; Alam, S.R.; MacGillivray, T.J.; Gray, C.D.; Dweck, M.R.; Raftis, J.; Jenkins, W.S.; Wallace, W.A.; Pessotto, R.; Lim, K.H.; et al. Ferumoxytol-enhanced magnetic resonance imaging assessing inflammation after myocardial infarction. Heart 2017, 103, 1528–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deoni, S.C. High-resolution T1 mapping of the brain at 3T with driven equilibrium single pulse observation of T1 with high-speed incorporation of RF field inhomogeneities (DESPOT1-HIFI). J. Magn. Reson. Imaging 2007, 26, 1106–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rost, N.S.; Rahman, R.M.; Biffi, A.; Smith, E.E.; Kanakis, A.; Fitzpatrick, K.; Lima, F.; Worrall, B.B.; Meschia, J.F.; Brown, R.D., Jr.; et al. White matter hyperintensity volume is increased in small vessel stroke subtypes. Neurology 2010, 75, 1670–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staals, J.; Makin, S.D.; Doubal, F.N.; Dennis, M.S.; Wardlaw, J.M. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 2014, 83, 1228–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deichmann, R.; Good, C.D.; Josephs, O.; Ashburner, J.; Turner, R. Optimization of 3-D MP-RAGE sequences for structural brain imaging. NeuroImage 2000, 12, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.H.; Nighoghossian, N.; Wiart, M.; Desestret, V.; Cakmak, S.; Berthezene, Y.; Derex, L.; Louis-Tisserand, G.; Honnorat, J.; Froment, J.C.; et al. USPIO-Enhanced MRI of neuroinflammation at the sub-acute stage of ischemic stroke: Preliminary data. Cerebrovasc. Dis. 2007, 24, 544–546. [Google Scholar] [CrossRef] [PubMed]
- Nighoghossian, N.; Wiart, M.; Cakmak, S.; Berthezene, Y.; Derex, L.; Cho, T.H.; Nemoz, C.; Chapuis, F.; Tisserand, G.L.; Pialat, J.B.; et al. Inflammatory response after ischemic stroke—A USPIO-enhanced MRI study in patients. Stroke 2007, 38, 303–307. [Google Scholar] [CrossRef]
- Saleh, A.; Schroeter, M.; Jonkmanns, C.; Hartung, H.P.; Modder, U.; Jander, S. In vivo MRI of brain inflammation in human ischaemic stroke. Brain 2004, 127, 1670–1677. [Google Scholar] [CrossRef] [Green Version]
- Saleh, A.; Schroeter, M.; Ringelstein, A.; Hartung, H.P.; Siebler, M.; Modder, U.; Jander, S. Iron oxide particle-enhanced MRI suggests variability of brain inflammation at early stages after ischemic stroke. Stroke 2007, 38, 2733–2737. [Google Scholar] [CrossRef]
- Marques, J.P.; Kober, T.; Krueger, G.; van der Zwaag, W.; Van de Moortele, P.F.; Gruetter, R. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. NeuroImage 2010, 49, 1271–1281. [Google Scholar] [CrossRef] [Green Version]
- Stikov, N.; Boudreau, M.; Levesque, I.R.; Tardif, C.L.; Barral, J.K.; Pike, G.B. On the accuracy of T1 mapping: Searching for common ground. Magn. Reson. Med. 2015, 73, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.C.; Jain, V.; Li, C.; Giannetta, M.; Hurt, H.; Wehrli, F.W.; Wang, D.J. In vivo venous blood T1 measurement using inversion recovery true-FISP in children and adults. Magn. Reson. Med. 2010, 64, 1140–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarnykh, V.L. Actual flip-angle imaging in the pulsed steady state: A method for rapid three-dimensional mapping of the transmitted radiofrequency field. Magn. Reson. Med. 2007, 57, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Deistung, A.; Reichenbach, J.R. Quantitative susceptibility mapping (QSM) and R2(*) in the human brain at 3T: Evaluation of intra-scanner repeatability. Z. Med. Phys. 2017, 28, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Li, T.Q.; Gelderen, P.; Shmueli, K.; de Zwart, J.A.; Duyn, J.H. Susceptibility contrast in high field MRI of human brain as a function of tissue iron content. NeuroImage 2009, 44, 1259–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quarles, C.C.; Gochberg, D.F.; Gore, J.C.; Yankeelov, T.E. A theoretical framework to model DSC-MRI data acquired in the presence of contrast agent extravasation. Phys. Med. Biol. 2009, 54, 5749–5766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sourbron, S.; Heilmann, M.; Biffar, A.; Walczak, C.; Vautier, J.; Volk, A.; Peller, M. Bolus-tracking MRI with a simultaneous T1- and T2*-measurement. Magn. Reson. Med. 2009, 62, 672–681. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Doubal, F.; Armitage, P.; Chappell, F.; Carpenter, T.; Munoz Maniega, S.; Farrall, A.; Sudlow, C.; Dennis, M.; Dhillon, B. Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann. Neurol. 2009, 65, 194–202. [Google Scholar] [CrossRef]
- Tsialios, P.; Thrippleton, M.; Glatz, A.; Pernet, C. Evaluation of MRI sequences for quantitative T1 brain mapping. J. Phys. Conf. Ser. 2017, 931, 012038. [Google Scholar] [CrossRef] [Green Version]
- Brown, G.C.; Cowin, G.J.; Galloway, G.J. A USPIO doped gel phantom for R2* relaxometry. Magn. Reson. Mater. Phys. Biol. Med. 2017, 30, 15–27. [Google Scholar] [CrossRef]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 2002, 17, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Brix, G.; Schad, L.R.; Deimling, M.; Lorenz, W.J. Fast and precise T1 imaging using a TOMROP sequence. Magn. Reson. Imaging 1990, 8, 351–356. [Google Scholar] [CrossRef]
- Smith, S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002, 17, 143–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafael, C.; González, R.E.W. Digital Image Processing; Prentice Hall: Upper Saddle River, NJ, USA, 2007. [Google Scholar]
- Valdes Hernandez, M.D.C.; Chappell, F.M.; Munoz Maniega, S.; Dickie, D.A.; Royle, N.A.; Morris, Z.; Anblagan, D.; Sakka, E.; Armitage, P.A.; Bastin, M.E.; et al. Metric to quantify white matter damage on brain magnetic resonance images. Neuroradiology 2017, 59, 951–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdes Hernandez Mdel, C.; Armitage, P.A.; Thrippleton, M.J.; Chappell, F.; Sandeman, E.; Munoz Maniega, S.; Shuler, K.; Wardlaw, J.M. Rationale, design and methodology of the image analysis protocol for studies of patients with cerebral small vessel disease and mild stroke. Brain Behav. 2015, 5, e00415. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.; Johnson, G. DeltaR2 (*) gadolinium-diethylenetriaminepentacetic acid relaxivity in venous blood. Magn. Reson. Med. 2013, 69, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Bhagwandien, R.; Moerland, M.A.; Bakker, C.J.; Beersma, R.; Lagendijk, J.J. Numerical analysis of the magnetic field for arbitrary magnetic susceptibility distributions in 3D. Magn. Reson. Imaging 1994, 12, 101–107. [Google Scholar] [CrossRef]






| Characteristic | (N = 12) |
|---|---|
| Age (mean ± SD) | 74.1 ± 6.7 |
| Male | 67% (8) |
| Prior Stroke or TIA | 17% (2) |
| Diabetes | 17% (2) |
| Hypertension | 75% (9) |
| Hyperlipidaemia | 58% (7) |
| Ischaemic Heart Disease | 8% (1) |
| History of Smoking | 50% (6) |
| Alcohol Intake (units per week, median and range) | 9 (0–30) |
| NIHSS (median and range) | 2 (1–4) |
| Modified Rankin Score Post Stroke (median and range) | 1 (0–2) |
| Days Post-Stroke (median and range) | 48 (31–276) |
| WMH Volume (mL) (median and range) | 12.5 (3.2–46.9) |
| ROI | R1 (s−1) | R2* (s−1) | |||||
|---|---|---|---|---|---|---|---|
| Scan 1pre | Scan 1post | Scan 2 | Scan 1pre | Scan 1post | Scan 2 | Scan 3 | |
| blood | 0.526 (0.036) | 3.672 (0.629) *** | 4.665 (1.140) *** | - | - | - | - |
| WM | 1.090 (0.034) | 1.123 (0.022) ** | 1.129 (0.032) *** | 19.3 (0.7) | 22.1 (0.4) *** | 23.0 (0.8) *** | 19.3 (0.7) |
| GM | 0.825 (0.036) | 0.907 (0.022) *** | 0.908 (0.041) *** | 19.1 (1.3) | 24.9 (1.5) *** | 26.6 (2.2) *** | 19.3 (1.0) |
| WMH | 0.905 (0.075) | 0.983 (0.061) * | 0.967 (0.067) *** | 16.5 (1.1) | 19.7 (1.0) *** | 20.5 (1.6) *** | 16.6 (1.1) |
| SL | 0.926 (0.108) | 1.002 (0.116) * | 0.989 (0.102) *** | 19.0 (3.6) | 21.4 (2.5) ** | 23.9 (4.6) *** | 19.3 (3.7) |
| ROI | Scan 1post | Scan 2 | ||||
|---|---|---|---|---|---|---|
| β0 (s−1) | β1 | R2 | β0 (s−1) | β1 | R2 | |
| WM | 2.6 | 14.2 | 0.18 | 2.5 | 33.4 | 0.61 ** |
| GM | 4.7 | 14.9 | 0.06 | 1.7 | 69.2 | 0.70 *** |
| WMH | 2.1 | 18.7 | 0.75 * | 2.4 | 25.3 | 0.73 *** |
| SL | 1.8 | 47.2 | 0.34 | 1.3 | 56.9 | 0.64 ** |
| ROI | ∆R1,norm | |||
|---|---|---|---|---|
| Scan 1post | Scan 2 | Scan 1post | Scan 2 | |
| WM | 0.0084 (45) | 0.0087 (43) | 0.97 (16) | 0.93 (14) |
| GM | 0.0239 (21) *** | 0.0204 (16) *** | 1.86 (11) *** | 1.85 (14) *** |
| WMH | 0.0153 (68) | 0.0148 (50) * | 0.96 (22) | 0.97 (17) |
| SL | 0.0125 (34) | 0.0151 (38) * | 1.17 (26) | 1.21 (41) |
| Scan | Sequence | TR (ms) | TE (ms) | FA (°) | TI (ms) | FOV (mm) | Acquisition Matrix | Slices × Thickness (mm) | GRAPPA Factor | Time (m:ss) | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FLAIR | Axial 2D PROPELLER | 9100 | 125 | 130 | - | 240 | 256 | 48 × 3 | - | 4:53 | - |
| ME-sGRE | Axial 3D multi-echo spoiled gradient echo | 50 | 4.6 8.5 14.0 19.5 25.0 30.5 36.0 41.5 | 15 | - | 240 × 240 | 256 (AP) × 192 (LR) | 72 × 2 | 2 | 7:08 | 11.1% slice oversampling |
| DESPOT1-HIFI | Axial 3D IR-sGRE | 1190 | 2.3 | 5 | 1000 | 240 × 240 | 256 (AP) × 192 (LR) | 72 × 2 | - | 3:50 | 11.1% slice oversampling; echo-spacing = 4.5 ms for IR-sGRE |
| Axial 3D IR-sGRE 1 | 632 | 2.3 | 5 | 450 | 2:03 | ||||||
| Axial 3D sGRE | 5.7 | 2.5 | 12 | - | 1:29 | ||||||
| Axial 3D sGRE | 5.7 | 2.5 | 5 | - | 1:29 | ||||||
| Axial 3D sGRE | 5.7 | 2.5 | 3 | - | 1:29 | ||||||
| GRE | Axial 3D gradient echo | 40 | 20 | 15 | - | 240 × 240 | 320 (AP) × 256 (LR) | 48 × 3 | 2 | 5:35 | 25.0% slice oversampling |
| T1w | Sagittal IR-sGRE | 2300 | 2.98 | 9 | 1100 | 256 × 256 | 256 × 256 | 208 × 1 | 2 | 5:21 | 23.1% slice oversampling |
| T2w | Axial 2D PROPELLER | 11,400 | 120 | 90 | - | 240 | 384 | 48 × 3 | - | 4:24 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thrippleton, M.J.; Blair, G.W.; Valdes-Hernandez, M.C.; Glatz, A.; Semple, S.I.K.; Doubal, F.; Vesey, A.; Marshall, I.; Newby, D.E.; Wardlaw, J.M. MRI Relaxometry for Quantitative Analysis of USPIO Uptake in Cerebral Small Vessel Disease. Int. J. Mol. Sci. 2019, 20, 776. https://doi.org/10.3390/ijms20030776
Thrippleton MJ, Blair GW, Valdes-Hernandez MC, Glatz A, Semple SIK, Doubal F, Vesey A, Marshall I, Newby DE, Wardlaw JM. MRI Relaxometry for Quantitative Analysis of USPIO Uptake in Cerebral Small Vessel Disease. International Journal of Molecular Sciences. 2019; 20(3):776. https://doi.org/10.3390/ijms20030776
Chicago/Turabian StyleThrippleton, Michael J., Gordon W. Blair, Maria C. Valdes-Hernandez, Andreas Glatz, Scott I. K. Semple, Fergus Doubal, Alex Vesey, Ian Marshall, David E. Newby, and Joanna M. Wardlaw. 2019. "MRI Relaxometry for Quantitative Analysis of USPIO Uptake in Cerebral Small Vessel Disease" International Journal of Molecular Sciences 20, no. 3: 776. https://doi.org/10.3390/ijms20030776
APA StyleThrippleton, M. J., Blair, G. W., Valdes-Hernandez, M. C., Glatz, A., Semple, S. I. K., Doubal, F., Vesey, A., Marshall, I., Newby, D. E., & Wardlaw, J. M. (2019). MRI Relaxometry for Quantitative Analysis of USPIO Uptake in Cerebral Small Vessel Disease. International Journal of Molecular Sciences, 20(3), 776. https://doi.org/10.3390/ijms20030776