Supramolecular Gel as the Template for Catalysis, Inorganic Superstructure, and Pharmaceutical Crystallization
Abstract
:1. Introduction
2. Supramolecular Gels for Catalyzing Chemical Processes
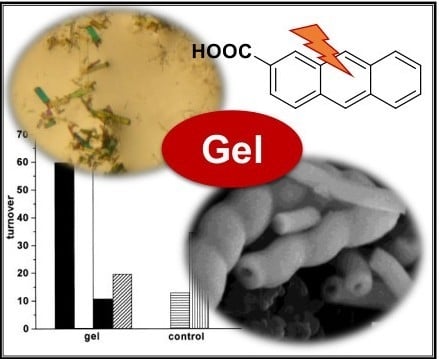
3. Supramolecular Gels for Catalyzing Photochemical Processes
4. Supramolecular Gels as the Surface for Fabricating Inorganic Superstructures
5. Supramolecular Gels as the Surface for Pharmaceutical Crystallization
6. Outlook
Conflicts of Interest
References
- Dawn, A.; Shiraki, T.; Haraguchi, S.; Tamaru, S.-I.; Shinkai, S. What kind of “soft materials” can we design from molecular gels? Chem. Asian J. 2011, 6, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.S.; Praveen, V.K.; Ajayaghosh, A. Functional π-gelators and their applications. Chem. Rev. 2014, 114, 1973–2129. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.D.; Steed, J.W. Gels with sense: Supramolecular materials that respond to heat, light and sound. Chem. Soc. Rev. 2016, 45, 6546–6596. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Yan, X.; Han, C.; Huang, F. Characterization of supramolecular gels. Chem. Soc. Rev. 2013, 42, 6697–6722. [Google Scholar] [CrossRef] [PubMed]
- Okesola, B.O.; Smith, D.K. Applying low-molecular weight supramolecular gelators in an environmental setting-selfassembled gels as smart materials for pollutant removal. Chem. Soc. Rev. 2016, 45, 4226–4251. [Google Scholar] [CrossRef]
- Draper, E.R.; Adams, D.J. Low-molecular-weight gels: the state of the art. Chemistry 2017, 3, 390–410. [Google Scholar] [CrossRef]
- Liu, M.; Ouyang, G.; Niu, D.; Sang, Y. Supramolecular gelatons: Towards the design of molecular gels. Org. Chem. Front. 2018, 5, 2885–2900. [Google Scholar] [CrossRef]
- Draper, E.R.; Adams, D.J. How should multicomponent supramolecular gels be characterised? Chem. Soc. Rev. 2018, 47, 3395–3405. [Google Scholar] [CrossRef] [Green Version]
- Mayr, J.; Saldıas, C.; Dıaz Dıaz, D. Release of small bioactive molecules from physical gels. Chem. Soc. Rev. 2018, 47, 1484–1515. [Google Scholar] [CrossRef]
- Murata, K.; Aoki, M.; Suzuki, T.; Harada, T.; Kawabata, H.; Komori, T.; Ohseto, F.; Ueda, K.; Shinkai, S. Thermal and light control of the sol-gel phase transition in cholesterol-based organic gels. Novel helical aggregation modes as detected by circular dichroism and electron microscopic observation. J. Am. Chem. Soc. 1994, 116, 6664–6676. [Google Scholar] [CrossRef]
- Snijder, C.S.; de Jong, J.C.; Meetsma, A.; van Bolhuis, F.; Feringa, B.L. A novel low molecular weight chiral gelator for apolar organic solvents. Chem. Eur. J. 1995, 1, 594–597. [Google Scholar] [CrossRef]
- Hanabusa, K.; Yamada, M.; Kimura, M.; Shirai, H. Prominent gelation and chiral aggregation of alkylamides derived from trans-1,2-diaminocyclohexane. Angew. Chem. Int. Ed. 1996, 35, 1949–1951. [Google Scholar] [CrossRef]
- Van Esch, J.H.; Schoonbeek, F.; de Loos, M.; Kooijman, H.; Spek, A.L.; Kellogg, R.M.; Feringa, B.L. Cyclic bis-urea compounds as gelators for organic solvents. Chem. Eur. J. 1999, 5, 937–950. [Google Scholar] [CrossRef]
- Shi, C.; Huang, Z.; Kilic, S.; Xu, J.; Enick, R.M.; Beckman, E.J.; Carr, A.J.; Melendez, R.E.; Hamilton, A.D. The gelation of CO2: A sustainable route to the creation of microcellular materials. Science 1999, 286, 1540–1543. [Google Scholar] [CrossRef] [PubMed]
- Luboradzki, R.; Gronwald, O.; Ikeda, M.; Shinkai, S.; Reinhoudt, D.N. An attempt to predict the gelation ability of hydrogen-bond-based gelators utilizing a glycoside library. Tetrahedron 2000, 56, 9595–9599. [Google Scholar] [CrossRef]
- Van Esch, J.H.; Feringa, B.L. New functional materials based on self-assembling organogels: From serendipity towards design. Angew. Chem. Int. Ed. 2000, 39, 2263–2266. [Google Scholar] [CrossRef]
- Dastidar, P. Supramolecular gelling agents: can they be designed? Chem. Soc. Rev. 2008, 37, 2699–2715. [Google Scholar] [CrossRef]
- Dawn, A.; Kumari, H. Low molecular weight supramolecular gels under shear: Rheology as the tool for elucidating structure–function correlation. Chem. Eur. J. 2018, 24, 762–776. [Google Scholar] [CrossRef]
- Malliaa, V.A.; Weiss, R.G. Correlations between thixotropic and structural properties of molecular gels with crystalline networks. Soft Matter 2016, 12, 3665–3676. [Google Scholar] [CrossRef]
- Dawn, A.; Shiraki, T.; Ichikawa, H.; Takada, A.; Takahashi, Y.; Tsuchiya, Y.; Lien, L.T.N.; Shinkai, S. Stereochemistry-dependent, mechanoresponsive supramolecular host assemblies for fullerenes: A guest-induced enhancement of thixotropy. J. Am. Chem. Soc. 2012, 134, 2161–2171. [Google Scholar] [CrossRef]
- Meyer, A.R.; Bender, C.R.; dos Santos, D.M.; Ziembowicz, F.I.; Frizzo, C.P.; Villetti, M.A.; Reichert, J.M.; Zanatta, N.; Bonacorso, H.G.; Martins, M.A.P. Effect of slight structural changes on the gelation properties of N-phenylstearamide supramolecular gels. Soft Matter 2018, 14, 6716–6727. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Sun, Z.; Tu, T. Novel supramolecular thixotropic metallohydrogels consisting of rare metal–organic nanoparticles: synthesis, characterization, and mechanism of aggregation. J. Phys. Chem. C 2013, 117, 25185–25194. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, Q.; He, T.; Li, Z.; Zhang, Y.; Yi, L. Multistimuli-responsive supramolecular gels: Design rationale, recent advances, and perspectives. ChemPhysChem 2014, 15, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Wang, X.; Yin, Z.; Jia, C.; Zhang, B.; Zhou, L.; Song, J. Novel two-component gels with multi-stimuli response: the gel–sol phase transition and color changes. J. Mater. Chem. C 2018, 6, 10192–10196. [Google Scholar] [CrossRef]
- Ren, Y.; Xie, S.; Grape, E.S.; Inge, A.K.; Ramstrom, O. Multistimuli-responsive enaminitrile molecular switches displaying H+-induced aggregate emission, metal ion-induced turn-on fluorescence, and organogelation properties. J. Am. Chem. Soc. 2018, 140, 13640–13643. [Google Scholar] [CrossRef] [PubMed]
- Borre, E.; Stumb, J.-F.; Bellemin-Laponnaz, S.; Mauro, M. Light-powered self-healable metallosupramolecular soft actuators. Angew. Chem. Int. Ed. 2016, 55, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yu, X.; Li, Y.; Ren, J.; Zhen, X. Robust, self-healing, and multistimuli-responsive supergelator for the visual recognition and separation of short-chain cycloalkanes and alkanes. ACS Appl. Mater. Interfaces 2017, 9, 13666–13675. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Praveen, V.K.; Ajayaghosh, A. The Chemistry and Applications of π-Gels. Annu. Rev. Mater. Res. 2016, 46, 235–262. [Google Scholar] [CrossRef]
- Diaz, D.D.; Saldias, C. Photon upconversion in supramolecular gels and synthetic application. Curr. Org. Chem. 2018, 22, 2223–2228. [Google Scholar] [CrossRef]
- Guerzo, A.D.; Olive, A.G.L.; Reichwagen, J.; Hopf, H.; Desvergne, J.-P. Energy transfer in self-assembled [n]-acene fibers involving ≥100 donors per acceptor. J. Am. Chem. Soc. 2005, 127, 17984–17985. [Google Scholar] [CrossRef]
- Sugiyasu, K.; Fujita, N.; Shinkai, S. Visible-light-harvesting organogel composed of cholesterol-based perylene derivatives. Angew. Chem. Int. Ed. 2004, 43, 1229–1233. [Google Scholar] [CrossRef] [PubMed]
- Praveen, V.K.; Ranjith, C.; Armaroli, N. White-light-emitting supramolecular gels. Angew. Chem. Int. Ed. 2014, 53, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Felip-León, C.; Díaz-Oltra, S.; Galindo, F.; Miravet, J.F. Chameleonic, light harvesting photonic gels based on orthogonal molecular fibrillization. Chem. Mater. 2016, 28, 7964–7972. [Google Scholar] [CrossRef]
- Duan, P.; Yanai, N.; Nagatomi, H.; Kimizuka, N. Photon upconversion in supramolecular gel matrixes: Spontaneous accumulation of light-harvesting donor–acceptor arrays in nanofibers and acquired air stability. J. Am. Chem. Soc. 2015, 137, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Gambhir, D.; Kumar, S.; Dey, G.; Krishnan, V.; Koner, R.R. Preferential intermolecular interactions lead to chiral recognition: enantioselective gel formation and collapse. Chem. Commun. 2018, 54, 11407–11410. [Google Scholar]
- Mukhopadhyay, P.; Iwashita, Y.; Shirakawa, M.; Kawano, S.-I.; Fujita, N.; Shinkai, S. Spontaneous colorimetric sensing of the positional isomers of dihydroxynaphthalene in a 1D organogel matrix. Angew. Chem. Int. Ed. 2006, 45, 1592–1595. [Google Scholar] [CrossRef]
- Chen, J.-F.; Liu, X.; Ma, J.-F.; Han, B.-B.; Ding, J.-D.; Lin, Q.; Yao, H.; Zhang, Y.-M.; Wei, T.-B. A pillar[5]arene-based multiple-stimuli responsive metal–organic gel was constructed for facile removal of mercury ions. Soft Matter 2017, 13, 5214–5218. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, Q.; Liu, M. Enantioselective recognition by chiral supramolecular gels. Chem. Asian J. 2016, 11, 2642–2649. [Google Scholar] [CrossRef]
- Lin, Q.; Lu, T.T.; Zhu, X.; Wei, T.B.; Li, H.; Zhang, Y.M. Rationally introduce multi-competitive binding interactions in supramolecular gels: a simple and efficient approach to develop multi-analyte sensor array. Chem. Sci. 2016, 7, 5341–5346. [Google Scholar] [CrossRef] [Green Version]
- Escuder, B.; Rodríguez-Llansola, F.; Miravet, J.F. Supramolecular gels as active media for organic reactions and catalysis. New J. Chem. 2010, 34, 1044–1054. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, Y.; Wu, J.; Liu, C.; Zhu, H.; Tu, T. Recent advances in supramolecular gels and catalysis. Chem. Asian J. 2018, 13, 712–729. [Google Scholar] [CrossRef] [PubMed]
- Tam, A.Y.-Y.; Yam, V.W.-W. Recent advances in metallogels. Chem. Soc. Rev. 2013, 42, 1540–1567. [Google Scholar]
- Dastidar, P.; Ganguly, S.; Sarkar, K. Metallogels from coordination complexes, organometallic, and coordination polymers. Chem. Asian J. 2016, 11, 2484–2498. [Google Scholar] [CrossRef] [PubMed]
- Häringa, M.; Díaz, D.D. Supramolecular metallogels with bulk self-healing properties prepared by in situ metal complexation. Chem. Commun. 2016, 52, 13068–13081. [Google Scholar] [Green Version]
- Winter, A.; Schubert, U.S. Synthesis and characterization of metallo-supramolecular polymers. Chem. Soc. Rev. 2016, 45, 5311–5357. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Lee, J.H.; Silverman, J.R.; John, G. Coordination polymer gels with important environmental and biological applications. Chem. Soc. Rev. 2013, 42, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ruiz, R.; Díaz, D.D. Photophysical and photochemical processes in 3D self-assembled gels as confined microenvironments. Soft Matter 2015, 11, 5180–5187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Inoue, Y. Supramolecular photochirogenesis. Chem. Soc. Rev. 2014, 43, 4123–4143. [Google Scholar] [CrossRef]
- van Bommel, K.J.C.; Friggeri, A.; Shinkai, S. Organic templates for the generation of inorganic materials. Angew. Chem. Int. Ed. 2003, 42, 980–999. [Google Scholar] [CrossRef]
- Kumar, D.K.; Steed, J.W. Supramolecular gel phase crystallization: orthogonal self-assembly under non-equilibrium conditions. Chem. Soc. Rev. 2014, 43, 2080–2088. [Google Scholar] [CrossRef] [Green Version]
- Cherney, A.H.; Kadunce, N.T.; Reisman, S.E. Enantioselective and enantiospecific transition-metal-catalyzed cross-coupling reactions of organometallic reagents to construct C–C bonds. Chem. Rev. 2015, 115, 9587–9652. [Google Scholar] [CrossRef] [PubMed]
- Ping, L.; Chung, D.S.; Bouffard, J.; Li, S.-G. Transition metal-catalyzed site- and regio-divergent C–H bond functionalization. Chem. Soc. Rev. 2017, 46, 4299–4328. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Su, Y.; Li, L.; Huang, H. Transition-metal catalysed C–N bond activation. Chem. Soc. Rev. 2016, 45, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Serrano, E.; Martin, R. Forging amides through metal-catalyzed C–C coupling with isocyanates. Eur. J. Org. Chem. 2018, 3051–3064. [Google Scholar] [CrossRef]
- Xing, B.; Choi, M.-F.; Xu, B. Design of coordination polymer gels as stable catalytic systems. Chem. Eur. J. 2002, 8, 5028–5032. [Google Scholar] [CrossRef]
- Liu, Y.-R.; He, L.; Zhang, J.; Wang, X.; Su, C.-Y. Evolution of spherical assemblies to fibrous networked Pd(II) metallogels from a pyridine-based tripodal ligand and their catalytic property. Chem. Mater. 2009, 21, 557–563. [Google Scholar] [CrossRef]
- Liao, Y.; He, L.; Huang, J.; Zhang, J.; Zhuang, L.; Shen, H.; Su, C.-Y. Magnetite nanoparticle-supported coordination polymer nanofibers: Synthesis and catalytic application in Suzuki-Miyaura coupling. ACS Appl. Mater. Interfaces 2010, 2, 2333–2338. [Google Scholar] [CrossRef]
- Slavık, P.; Kurka, D.W.; Smith, D.K. Palladium-scavenging self-assembled hybrid hydrogels–reusable highly-active green catalysts for Suzuki–Miyaura cross-coupling reactions. Chem. Sci. 2018, 9, 8673–8681. [Google Scholar] [CrossRef]
- Araujo, M.; Diaz-Oltra, S.; Escuder, B. Triazolyl-based molecular gels as ligands for autocatalytic ‘Click’ reactions. Chem. Eur. J. 2016, 22, 8676–8684. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, S.; Lee, J.Y.; Jung, J.H. A tetrazole-based metallogel induced with Ag+ ion and its silver nanoparticle in catalysis. Soft Matter 2012, 8, 6557–6563. [Google Scholar] [CrossRef]
- Paul, M.; Sarkar, K.; Dastidar, P. Metallogels derived from silver coordination polymers of C3-symmetric tris(pyridylamide) tripodal ligands: synthesis of Ag nanoparticles and catalysis. Chem. Eur. J. 2015, 21, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.; Assenmacher, W.; Peterlik, H.; Weisbarth, R.; Nieger, M.; Dotz, K.H. An air-stable organometallic low-molecular-mass gelator: Synthesis, aggregation, and catalytic application of a palladium pincer complex. Angew. Chem. Int. Ed. 2007, 46, 6368–6371. [Google Scholar] [CrossRef] [PubMed]
- Guler, M.O.; Stupp, S.I. A self-assembled nanofiber catalyst for ester hydrolysis. J. Am. Chem. Soc. 2007, 129, 12082–12083. [Google Scholar] [CrossRef] [PubMed]
- Tena-Solsona, M.; Nanda, J.; Díaz-Oltra, S.; Chotera, A.; Ashkenasy, G.; Escuder, B. Emergent catalytic behavior of self-assembled low molecular weight peptide-based aggregates and hydrogels. Chem. Eur. J. 2016, 22, 6687–6694. [Google Scholar] [CrossRef]
- Mazzier, D.; Carraro, F.; Crisma, M.; Rancan, M.; Toniolo, C.; Moretto, A. A terminally protected dipeptide: From crystal structure and self-assembly, through co-assembly with carbon-based materials, to a ternary catalyst for reduction chemistry in water. Soft Matter 2016, 12, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Gayen, K.; Basu, K.; Bairagi, D.; Castelletto, V.; Hamley, I.W.; Banerjee, A. Amino-acid-based metallo-hydrogel that acts like an esterase. ACS Appl. Bio Mater. 2018, 1, 1717–1724. [Google Scholar] [CrossRef]
- Dawn, A.; Fujita, N.; Haraguchi, S.; Sada, K.; Shinkai, S. An organogel system can control the stereochemical course of anthracene photodimerization. Chem. Commun. 2009, 2100–2102. [Google Scholar] [CrossRef]
- Dawn, A.; Fujita, N.; Haraguchi, S.; Sada, K.; Tamaru, S.-I.; Shinkai, S. Studies on a new class of organogelator containing 2-anthracenecarboxylic acid: Influence of gelator and solvent on stereochemistry of the photodimers. Org. Biomol. Chem. 2009, 7, 4378–4385. [Google Scholar] [CrossRef]
- Dawn, A.; Shiraki, T.; Haraguchi, S.; Sato, H.; Sada, K.; Shinkai, S. Transcription of chirality in the organogel systems dictates the enantiodifferentiating photodimerization of substituted anthracene. Chem. Eur. J. 2010, 16, 3676–3689. [Google Scholar] [CrossRef]
- Bhat, S.; Maitra, U. Hydrogels as reaction vessels: Acenaphthylene dimerization in hydrogels derived from bile acid analogues. Molecules 2007, 12, 2181–2189. [Google Scholar] [CrossRef]
- Bachl, J.; Hohenleutner, A.; Dhar, B.B.; Cativiela, C.; Maitra, U.; Ko¨nig, B.; Dıaz, D.D. Organophotocatalysis in nanostructured soft gel materials as tunable reaction vessels: comparison with homogeneous and micellar solutions. J. Mater. Chem. A 2013, 1, 4577–4588. [Google Scholar] [CrossRef]
- Häring, M.; Abramov, A.; Okumura, K.; Ghosh, I.; König, B.; Yanai, N.; Kimizuka, N.; Díaz, D.D. Air-sensitive photoredox catalysis performed under aerobic conditions in gel networks. J. Org. Chem. 2018, 83, 7928–7938. [Google Scholar]
- Ono, Y.; Nakashima, K.; Sano, M.; Kanekiyo, Y.; Inoue, K.; Hojo, J.; Shinkai, S. Organic gels are useful as a template for the preparation of hollow fiber silica. Chem. Commun. 1998, 1477–1478. [Google Scholar] [CrossRef]
- Ono, Y.; Nakashima, K.; Sano, M.; Hojo, J.; Shinkai, S. Template effect of cholesterol-based organogels on sol-gel polymerization creates novel silica with a helical structure. Chem. Lett. 1999, 1119–1120. [Google Scholar] [CrossRef]
- Ono, Y.; Nakashima, K.; Sano, M.; Hojo, J.; Shinkai, S. Organogels are useful as a template for the preparation of novel helical silica fibers. J. Mater. Chem. 2001, 11, 2412–2419. [Google Scholar] [CrossRef]
- Jung, J.H.; Ono, Y.; Shinkai, S. Novel preparation method for multi-layered, tubular silica using an azacrown-appended cholesterol as template and metaldeposition into the interlayer space. J. Chem. Soc. Perkin Trans. 2 1999, 1289–1291. [Google Scholar] [CrossRef]
- Jung, J.H.; Ono, Y.; Shinkai, S. Novel silica structures which are prepared by transcription of various superstructures formed in organogels. Langmuir 2000, 16, 1643–1649. [Google Scholar] [CrossRef]
- Jung, J.H.; Ono, Y.; Sakurai, K.; Sano, M.; Shinkai, S. Novel vesicular aggregates of crown-appended cholesterol derivatives which act as gelators of organic solvents and as templates for silica transcription. J. Am. Chem. Soc. 2000, 122, 8648–8653. [Google Scholar] [CrossRef]
- Jung, J.H.; Ono, Y.; Hanabusa, K.; Shinkai, S. Creation of both right-handed and left-handed silica structures by sol-gel transcription of organogel fibers comprised of chiral diaminocyclohexane derivatives. J. Am. Chem. Soc. 2000, 122, 5008–5009. [Google Scholar] [CrossRef]
- Kobayashi, S.; Hanabusa, K.; Hamasaki, N.; Kimura, M.; Shirai, H.; Shinkai, S. Preparation of TiO2 hollow-fibers using supramolecular assemblies. Chem. Mater. 2000, 12, 1523–1525. [Google Scholar] [CrossRef]
- Kobayashi, S.; Hamasaki, N.; Suzuki, M.; Kimura, M.; Shirai, H.; Hanabusa, K. Preparation of helical transition-metal oxide tubes using organogelators as structure-directing agents. J. Am. Chem. Soc. 2002, 124, 6550–6551. [Google Scholar] [CrossRef]
- Zhang, C.; Huo, H.; Li, Y.; Li, B.; Yang, Y. Preparation of helical CdS nanotubes using a sol–gel transcription approach. Mater. Lett. 2013, 102–103, 50–52. [Google Scholar] [CrossRef]
- Huo, H.; Wang, S.; Lin, S.; Li, Y.; Li, B.; Yang, Y. Chiral zirconia nanotubes prepared through a sol–gel transcription approach. J. Mater. Chem. A 2014, 2, 333–338. [Google Scholar] [CrossRef]
- Cai, H.; Wang, C.; Li, B.; Li, Y.; Yang, Y. Preparation and characterization of single-handed twisted platinum tubular nanoribbons. Mater. Lett. 2014, 133, 147–150. [Google Scholar] [CrossRef]
- Liu, D.; Li, B.; Guo, Y.; Li, Y.; Yang, Y. Inner surface chirality of single-handed twisted carbonaceous tubular nanoribbons. Chirality 2015, 27, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Tanaka, K.; Kato, Y.; Hanabusa, K. Metal oxide/TiO2 hybrid nanotubes fabricated through the organogel Route. Gels 2017, 3, 24. [Google Scholar] [CrossRef]
- Eren, E.D.; Tansik, G.; Tekinay, A.B.; Guler, M.O. Mineralized peptide nanofiber gels for enhanced osteogenic differentiation. ChemNanoMat 2018, 4, 837–845. [Google Scholar] [CrossRef]
- Daly, R.; Kotova, O.; Boese, M.; Gunnlaugsson, T.; Boland, J.J. Chemical nano-gardens: growth of salt nanowires from supramolecular self-assembly gels. Acs Nano 2013, 7, 4838–4845. [Google Scholar] [CrossRef]
- Scarpelli, F.; Ionescu, A.; Aiello, I.; La Deda, M.; Crispini, A.; Ghedini, M.; Brunelli, E.; Sesti, S.; Godbert, N. High order in a self-assembled iridium(III) complex gelator towards nanostructured IrO2 thin films. Chem. Asian J. 2017, 12, 2703–2710. [Google Scholar] [CrossRef]
- Lei, X.; Liu, T.; Tang, S. Fabrication of cactus rod-like mesoporous alumina with ionic liquid-supramolecular gelator as cotemplate. Cryst. Growth Des. 2018, 18, 4971–4977. [Google Scholar] [CrossRef]
- Nakagawa, M.; Kawai, T. Chirality-controlled syntheses of double-helical Au nanowires. J. Am. Chem. Soc. 2018, 140, 4991–4994. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Piepenbrock, M.-O.M.; Lloyd, G.O.; Clarke, N.; Howard, J.A.K.; Steed, J.W. Anion-switchable supramolecular gels for controlling pharmaceutical crystal growth. Nat. Chem. 2010, 2, 1037–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawn, A.; Andrew, K.S.; Yufit, D.S.; Hong, Y.; Reddy, J.P.; Jones, C.D.; Aguilar, J.A.; Steed, J.W. Supramolecular gel control of cisplatin crystallization: Identification of a new solvate form using a cisplatin-mimetic gelator. Cryst. Growth Des. 2015, 15, 4591–4599. [Google Scholar] [CrossRef]
- Foster, J.A.; Damodaran, K.K.; Maurin, A.; Day, G.M.; Thompson, H.P.G.; Cameron, G.J.; Bernal, J.C.; Steed, J.W. Pharmaceutical polymorph control in a drug-mimetic supramolecular gel. Chem. Sci. 2017, 8, 78–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.-R.; Bao, J.; Fan, X.; Dai, W.; Mei, X. pH-Switchable vitamin B9 gels for stoichiometry controlled spherical co-crystallization. Chem. Commun. 2016, 52, 13452–13455. [Google Scholar]
- Rahim, M.A.; Hata, Y.; Björnmalm, M.; Ju, Y.; Caruso, F. Supramolecular metal–phenolic gels for the crystallization of active pharmaceutical ingredients. Small 2018, 14, 1801202. [Google Scholar] [CrossRef] [PubMed]























© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawn, A. Supramolecular Gel as the Template for Catalysis, Inorganic Superstructure, and Pharmaceutical Crystallization. Int. J. Mol. Sci. 2019, 20, 781. https://doi.org/10.3390/ijms20030781
Dawn A. Supramolecular Gel as the Template for Catalysis, Inorganic Superstructure, and Pharmaceutical Crystallization. International Journal of Molecular Sciences. 2019; 20(3):781. https://doi.org/10.3390/ijms20030781
Chicago/Turabian StyleDawn, Arnab. 2019. "Supramolecular Gel as the Template for Catalysis, Inorganic Superstructure, and Pharmaceutical Crystallization" International Journal of Molecular Sciences 20, no. 3: 781. https://doi.org/10.3390/ijms20030781
APA StyleDawn, A. (2019). Supramolecular Gel as the Template for Catalysis, Inorganic Superstructure, and Pharmaceutical Crystallization. International Journal of Molecular Sciences, 20(3), 781. https://doi.org/10.3390/ijms20030781