Current Challenges and Perspectives for the Use of Aqueous Plant Extracts in the Management of Bacterial Infections: The Case-Study of Salmonella enterica Serovars
Abstract
:1. Introduction
2. Antimicrobial Potential of Aqueous Plant Extracts
3. Mechanism of Action of Plant Extracts. Where Do We Stand?
4. Looking for Structure-Antimicrobial Activity Relationship
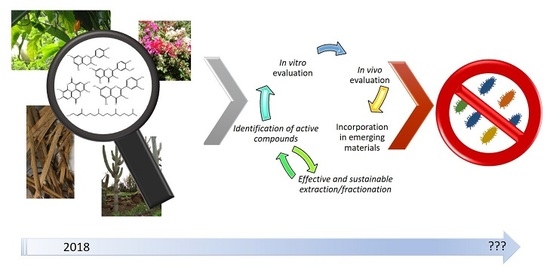
5. Current Challenges for Development of New Antimicrobials from Plant Extracts
6. Concluding Remarks and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Radulović, N.S.; Blagojević, P.D.; Stojanović-Radić, Z.Z.; Stojanović, N.M. Antimicrobial plant metabolites: Structural diversity and mechanism of action. Curr. Med. Chem. 2013, 20, 932–952. [Google Scholar]
- Kariuki, S.; Gordon, M.A.; Feasey, N.; Parry, C.M. Antimicrobial resistance and management of invasive Salmonella disease. Vaccine 2015, 33, C21–C29. [Google Scholar] [CrossRef] [PubMed]
- WHO Salmonella (Non-Typhoidal). Available online: http://www.who.int/news-room/fact-sheets/detail/salmonella-(non-typhoidal) (accessed on 10 December 2018).
- WHO List of Antibiotic Resistant Priority Pathogens. Available online: http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/ (accessed on 10 December 2018).
- Gyawali, R.; Hayek, S.A.; Ibrahim, S.A. Plant extracts as antimicrobials in food products. In Handbook of Natural Antimicrobials for Food Safety and Quality; Taylor, T., Ed.; Elsevier Ltd.: Cambridge, UK, 2015; pp. 49–68. ISBN 9781782420347. [Google Scholar]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef] [Green Version]
- Sunday, O.J.; Babatunde, S.K.; Ajiboye, A.E.; Adedayo, R.M.; Ajao, M.A.; Ajuwon, B.I. Evaluation of phytochemical properties and in-vitro antibacterial activity of the aqueous extracts of leaf, seed and root of Abrus precatorius Linn. against Salmonella and Shigella. Asian Pac. J. Trop. Biomed. 2016, 6, 755–759. [Google Scholar] [CrossRef]
- Boubaker, J.; Ben Mansour, H.; Ghedira, K.; Chekir Ghedira, L. Polar extracts from (Tunisian) Acacia salicina Lindl. study of the antimicrobial and antigenotoxic activities. BMC Complement. Altern. Med. 2012, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Obeidat, M.; Shatnawi, M.; Al-Alawi, M.; Al-zu`bi, E.; Al-Dmoor, H.; Al-Qudah, M.; El-Qudah, J.; Otri, I. Antimicrobial activity of crude extracts some plant leaves. Res. J. Microbiol. 2012, 7, 59–67. [Google Scholar] [CrossRef]
- Panda, S.K.; Thatoi, H.N.; Dutta, S.K. Antibacterial activity and phytochemical screening of leaf and bark extracts of Vitex negundo L. from similipal biosphere reserve, Orissa. J. Med. Plants Res. 2009, 3, 294–300. [Google Scholar] [CrossRef]
- Panda, S.K. Ethno-medicinal uses and screening of plants for antibacterial activity from Similipal Biosphere Reserve, Odisha, India. J. Ethnopharmacol. 2014, 151, 158–175. [Google Scholar] [CrossRef]
- Hammuel, C.; Yebpella, G.G.; Shallangwa, G.A.; Magomya, A.M.; Agbaji, A.S. Phytochemical and antimicrobial screening of methanol and aqueous extracts of Agave sisalana. Acta Pol. Pharm. Drug Res. 2011, 68, 535–539. [Google Scholar]
- Adeyemi, I.A.; Omonigbehin, A.E.; Stella, S.; Oluwatosin, O.; Jumoke, S. Antibacterial activity of extracts of Alchornea cordifolia (Schum and Thonn) Mull.Arg., Boerhavia diffusa (L) and Bridellia micranthal (Hoscht) Baill. used in traditional medicine in Nigeria on Helicobacter pylori and four diarrhoeage. African J. Biotechnol. 2008, 7, 3764–3767. [Google Scholar] [CrossRef]
- Onivogui, G.; Diaby, M.; Chen, X.; Zhang, H.; Kargbo, M.R.; Song, Y. Antibacterial and antifungal activities of various solvent extracts from the leaves and stem bark of Anisophyllea laurina R. Br ex Sabine used as traditional medicine in Guinea. J. Ethnopharmacol. 2015, 168, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Bhadauria, P.; Joseph, E.; Jadoun, Y.; Shukla, S. Inhibitory effect of different plant extracts on Salmonella Typhimurium growth. Indian J. Anim. Res. 2012, 46, 190–192. [Google Scholar]
- Loizzo, M.R.; Tundis, R.; Chandrika, U.G.; Abeysekera, A.M.; Menichini, F.; Frega, N.G. Antioxidant and antibacterial activities on foodborne pathogens of Artocarpus heterophyllus Lam. (Moraceae) leaves extracts. J. Food Sci. 2010, 75, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Abbasi, A.M.; Dastagir, G.; Nazir, A.; Shah, G.M.; Shah, M.M.; Shah, M.H. Ethnobotanical and antimicrobial study of some selected medicinal plants used in Khyber Pakhtunkhwa (KPK) as a potential source to cure infectious diseases. BMC Complement. Altern. Med. 2014, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- van Vuuren, S.F.; Nkwanyana, M.N.; de Wet, H. Antimicrobial evaluation of plants used for the treatment of diarrhoea in a rural community in northern Maputaland, KwaZulu-Natal, South Africa. BMC Complement. Altern. Med. 2015, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Iroha, I.R.; ILang, D.C.; Ayogu, T.E.; Oji, A.E.; Ugbo, E.C. Screening for anti-typhoid activity of some medicinal plants used in traditional medicine in Ebonyi state, Nigeria. Afr. J. Pharm. Pharmacol. 2010, 4, 860–864. [Google Scholar]
- Rajkumar, K.; Malathi, R. Phytochemical investigation GC-MS analysis and in vitro antimicrobial activity of Coleus forskohlii. Bangladesh J. Pharmacol. 2015, 10, 924–930. [Google Scholar] [CrossRef]
- Ahmed, T.; Kanwal, R.; Hassan, M.; Ayub, N.; Scholz, M.; McMinn, W. Coagulation and disinfection in water treatment using Moringa. Water Manag. 2010, 163, 381–388. [Google Scholar] [CrossRef]
- Ahmed, T.; Kanwal, R.; Hassan, M.; Ayub, N. Assessment of antibacterial activity of Colebrookia oppositifolia against waterborne pathogens isolated from drinking water of the Pothwar Region in Pakistan. Hum. Ecol. Risk Assess. An Int. J. 2009, 15, 401–415. [Google Scholar] [CrossRef]
- Fagbemi, J.F.; Ugoji, E.; Adenipekun, T.; Adelowotan, O. Evaluation of the antimicrobial properties of unripe banana (Musa sapientum L.), lemon grass (Cymbopogon citratus S.) and turmeric (Curcuma longa L.) on pathogens. African J. Biotechnol. 2009, 8, 1176–1182. [Google Scholar]
- Kaushik, P.; Goyal, P. In vitro evaluation of Datura innoxia (thorn-apple) for potential antibacterial activity. Indian J. Microbiol. 2008, 48, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Yakubu, A.I.; Mukhtar, M.D. In vitro antimicrobial activity of some phytochemical fractions of Euphorbia pulcherima L. (Poinsettia). J. Med. Plants Res. 2011, 5, 2470–2475. [Google Scholar]
- Taiwo, F.O.; Fidelis, A.A.; Oyedeji, O. Antibacterial activity and phytochemical profile of leaf extracts of Ficus abutilifolia. Br. J. Pharm. Res. 2016, 11, 1–10. [Google Scholar] [CrossRef]
- Habtamu, Y.; Eguale, T.; Wubete, A.; Sori, T. In vitro antimicrobial activity of selected Ethiopian medicinal plants against some bacteria of veterinary importance. African J. Microbiol. Res. 2010, 4, 1230–1234. [Google Scholar]
- Aiyegoro, O.A.; Afolayan, A.J.; Okoh, A. In vitro antibacterial time kill studies of leaves extracts of Helichrysum longifolium. J. Med. Plant Res. 2009, 3, 462–467. [Google Scholar]
- Frey, F.M.; Meyers, R. Antibacterial activity of traditional medicinal plants used by Haudenosaunee peoples of New York State. BMC Complement. Altern. Med. 2010, 10, 1–10. [Google Scholar] [CrossRef]
- Gull, I.; Sohail, M.; Shahbaz Aslam, M.; Amin Athar, M. Phytochemical, toxicological and antimicrobial evaluation of Lawsonia inermis extracts against clinical isolates of pathogenic bacteria. Ann. Clin. Microbiol. Antimicrob. 2013, 12, 36. [Google Scholar] [CrossRef]
- Jimoh, F.O.; Adedapo, A.A.; Afolayan, A.J. Comparison of the nutritional value and biological activities of the acetone, methanol and water extracts of the leaves of Solanum nigrum and Leonotis leonorus. Food Chem. Toxicol. 2010, 48, 964–971. [Google Scholar] [CrossRef]
- Saeidi, S.; Hassanpour, K.; Ghamgosha, M.; Heiat, M.; Taheri, R.A.; Mirhosseini, A.; Farnoosh, G. Antibacterial activity of ethyl acetate and aqueous extracts of Mentha longifolia L. and hydroalcoholic extract of Zataria multiflora Boiss. plants against important human pathogens. Asian Pac. J. Trop. Med. 2014, 7, S186–S189. [Google Scholar] [CrossRef]
- Serafini, M.R.; Santos, R.C.; Guimarães, A.G.; Dos Santos, J.P.; da Conceicão Santos, A.D.; Alves, I.A.; Gelain, D.P.; de Lima Nogueira, P.C.; Quintans-Júnior, L.J.; Bonjardim, L.R.; et al. Morinda citrifolia Linn leaf extract possesses antioxidant activities and reduces nociceptive behavior and leukocyte migration. J. Med. Food 2011, 14, 1159–1166. [Google Scholar] [CrossRef]
- Al_husnan, L.A.; Alkahtani, M.D.F. Impact of Moringa aqueous extract on pathogenic bacteria and fungi in vitro. Ann. Agric. Sci. 2016, 61, 247–250. [Google Scholar] [CrossRef]
- Junaid, S.A.; Abubakar, A.; Ofodile, A.; Olabode, A.; Cheonwu, G.; Okwori, A.; Adetunji, J. Evaluation of Securidaca longipenduculata leaf and root extracts for antimicrobial activities. Afr. J. Microbiol. Res. 2008, 2, 322–325. [Google Scholar]
- Junaid, S.; Olabode, A.; Onwuliri, F.; Okwori, A.; Agina, S. The antimicrobial properties of Ocimum gratissimum extracts on some selected bacterial gastrointestinal isolates. Afr. J. Biotechnol. 2006, 5, 2315–2321. [Google Scholar]
- Razwinani, M.; Tshikalange, T.; Motaung, S. Antimicrobial And Anti-Inflammatory Activities Of Pleurostylia Capensis Turcz (Loes) (Celastraceae). Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 452. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Shahzad, A.; Malik, A.; Anis, M. Antibacterial activity of aerial parts as well as in vitro raised calli of the medicinal plant Saraca asoca (Roxb.) de Wilde. Can. J. Microbiol. 2007, 53, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Ndamitso, M.M.; Mohammed, A.; Jimoh, T.O.; Idris, S.; Oyeleke, S.B.; Etsuyankpa, M.B. Phytochemical and antibacterial activity of Securidaca longepedunculata on selected pathogens. Afr. J. Microbiol. Res. 2013, 7, 5652–5656. [Google Scholar] [CrossRef]
- Doughari, J.H.; Okafor, N.B. Antibacterial activity of Senna siamae leaf extracts on Salmonella typhi. Afr. J. Microbiol. Res. 2008, 2, 42–46. [Google Scholar]
- Chattopadhyay, D.; Ojha, D.; Mukherjee, H.; Bag, P.; Vaidya, S.P.; Dutta, S. Validation of a traditional preparation against multi-drug resistant Salmonella Typhi and its protective efficacy in S. Typhimurium infected mice. Biomed. Pharmacother. 2018, 99, 286–289. [Google Scholar] [CrossRef]
- Al-Bayati, F.A.; Al-Mola, H.F. Antibacterial and antifungal activities of different parts of Tribulus terrestris L. growing in Iraq. J. Zhejiang Univ. Sci. B 2008, 9, 154–159. [Google Scholar] [CrossRef]
- Ceyhan, N.; Keskin, D.; Zorlu, Z.; Ugur, A. In-vitro antimicrobial activities of different extracts of grapevine leaves (Vitis vinifera L.) from West Anatolia against some pathogenic microorganisms. J. Pure Appl. Microbiol. 2012, 6, 1303–1308. [Google Scholar]
- Purfard, A.M.; Kavoosi, G. Chemical composition, radical scavenging, antibacterial and antifungal activities of Zataria multiflora bioss essential oil and aqueous extract. J. Food Saf. 2012, 32, 326–332. [Google Scholar] [CrossRef]
- Puntawong, S.; Okonogi, S.; Pringproa, K. In vitro antibacterial activity of Psidium guajava Linn. leaf extracts against pathogenic bacteria in pigs. Chiang Mai Univ. J. Nat. Sci. 2012, 11, 127–134. [Google Scholar]
- De Araújo, A.A.; Soares, L.A.L.; Assunção Ferreira, M.R.; De Souza Neto, M.A.; Da Silva, G.R.; De Araújo, R.F.; Guerra, G.C.B.; De Melo, M.C.N. Quantification of polyphenols and evaluation of antimicrobial, analgesic and anti-inflammatory activities of aqueous and acetone-water extracts of Libidibia ferrea, Parapiptadenia rigida and Psidium guajava. J. Ethnopharmacol. 2014, 156, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Ismail, M.; Muhammad, N.; Ali, F.; Ahmad, K.; Imran, M. Evaluation of the stem bark of Pistacia integerrima Stew ex Brandis for its antimicrobial and phytotoxic activities. J. Pharm. Pharmacol. 2011, 5, 1170–1174. [Google Scholar] [CrossRef]
- Silva, N.C.B.; Esquibel, M.A.; Santos, J.E.S.; Almeida, M.Z.; Sampaio, C.S.; Barros, T.F. In vitro antimicrobial activity of extracts from Abarema cochliacarpos (Gomes) Barneby and J.W. Grimes. African J. Microbiol. Res. 2010, 4, 1654–1658. [Google Scholar]
- Sani, D.; Sanni, S.; Ngulde, S.I. Phytochemical and antimicrobial screening of the stem aqueous extract of Anisopus mannii. J. Med. Plants Res. 2009, 3, 112–115. [Google Scholar]
- Nwadinigwe, A.O. Antimicrobial activities of methanol and aqueous extracts of the stem of Bryophyllum pinnatum Kurz (Crassulaceae). African J. Biotechnol. 2011, 10, 16342–16346. [Google Scholar] [CrossRef]
- Shilpakala Sainath, R.; Prathiba, J.; Malathi, R. Antimicrobial properties of the stem bark of Saraca indica (Caesalpiniaceae). Eur. Rev. Med. Pharmacol. Sci. 2009, 13, 371–374. [Google Scholar]
- Tanih, N.F.; Ndip, R.N. Evaluation of the acetone and aqueous extracts of mature stem bark of Sclerocarya birrea for antioxidant and antimicrobial properties. Evid. Based Complement. Altern. Med. 2012, 1–8. [Google Scholar] [CrossRef]
- Hassan, S.; Umar, R.; Lawal, M.; Bilbis, L.; Muhammad, B.; Dabai, Y. Evaluation of antibacterial activity and phytochemical analysis of root extracts of Boscia angustifolia. Afr. J. Biotechnol. 2006, 5, 1602–1607. [Google Scholar]
- Kahiya, C.; Zimudzi, C. Phytochemical, antimicrobial and cytotoxic evaluation of Indigofera serpentinicola. Bangladesh J. Pharmacol. 2015, 10, 166–172. [Google Scholar] [CrossRef]
- Sanusi, J.; Jibia, A.B.; Runka, J.Y.; Liadi, S.; Abubakar, A.A.; Zurmi, R.S. Antimicrobial activity of aqueous and ethanol extracts of violet plant (Securidaca longipedunculata Fres) on tested pathogenic bacteria. Int. J. Pharm. Sci. Res. 2015, 6, 3276–3284. [Google Scholar] [CrossRef]
- Kenny, O.; Smyth, T.J.; Walsh, D.; Kelleher, C.T.; Hewage, C.M.; Brunton, N.P. Investigating the potential of under-utilised plants from the Asteraceae family as a source of natural antimicrobial and antioxidant extracts. Food Chem. 2014, 161, 79–86. [Google Scholar] [CrossRef]
- Hossein Babaei, A.; Motamedifar, M.; Khalifat, S.; Mohammadi, A.; Zamani, K.; Motamedifar, A. In vitro study of Antibacterial Property and Cytotoxic Effects of Aqueous, Ethanolic, Methanolic, and Hydroalcoholic Extracts of Fenugreek seed. Pakistan J. Med. Heal. Sci. 2018, 12, 906–910. [Google Scholar]
- Gull, I.; Saeed, M.; Shaukat, H.; Aslam, S.M.; Samra, Z.; Athar, A.M. Inhibitory effect of Allium sativum and Zingiber officinale extracts on clinically important drug resistant pathogenic bacteria. Ann. Clin. Microbiol. Antimicrob. 2012, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Koffi-Nevry, R.; Kouassi, K.C.; Nanga, Z.Y.; Koussémon, M.; Loukou, G.Y. Antibacterial activity of two bell pepper extracts: Capsicum annuum L. and Capsicum frutescens. Int. J. Food Prop. 2012, 15, 961–971. [Google Scholar] [CrossRef]
- Nwodo, U.U.; Obiiyeke, G.E.; Chigor, V.N.; Okoh, A.I. Assessment of Tamarindus indica extracts for antibacterial activity. Int. J. Mol. Sci. 2011, 12, 6385–6396. [Google Scholar] [CrossRef]
- Stobnicka, A.; Gniewosz, M. Antimicrobial protection of minced pork meat with the use of Swamp Cranberry (Vaccinium oxycoccos L.) fruit and pomace extracts. J. Food Sci. Technol. 2018, 55, 62–71. [Google Scholar] [CrossRef]
- Muhammad Abubakar, E.-M. Antibacterial activity of crude extracts of Euphorbia hirta against some bacteria associated with enteric infections. J. Med. Plants Res. 2009, 3, 498–505. [Google Scholar] [CrossRef]
- El-Azzouny, M.M.; El-Demerdash, A.S.; Seadawy, H.G.; Abou-Khadra, S.H. Antimicrobial Effect of Garlic (Allium sativum) and Thyme (Zataria multiflora Boiss) Extracts on Some Food Borne Pathogens and Their Effect on Virulence Gene Expression. Cell. Mol. Biol. 2018, 64, 79–86. [Google Scholar] [CrossRef]
- Tala, D.S.; Gatsing, D.; Fodouop, S.P.C.; Fokunang, C.; Kengni, F.; Djimeli, M.N. In vivo anti-salmonella activity of aqueous extract of Euphorbia prostrata Aiton (Euphorbiaceae) and its toxicological evaluation. Asian Pac. J. Trop. Biomed. 2015, 5, 310–318. [Google Scholar] [CrossRef]
- Madani, A.; Jain, S.K. Anti-salmonella activity of Terminalia belerica: In vitro and in vivo studies. Indian J. Exp. Biol. 2008, 46, 817–821. [Google Scholar] [PubMed]
- Waihenya, R.K.; Mtambo, M.M.A.; Nkwengulila, G.; Minga, U.M. Efficacy of crude extract of Aloe secundiflora against Salmonella gallinarum in experimentally infected free-range chickens in Tanzania. J. Ethnopharmacol. 2002, 79, 317–323. [Google Scholar] [CrossRef]
- Pierre, S.; Fodouop, C.; Donald Tala, S.; Keilah, L.P.; Kodjio, N.; Didiane Yemele, M.; Herve Kamdje Nwabo, A.; Nji-Kah, B.; Tchoumboue, J.; Gatsing, D. Effects of Vitellaria paradoxa (C.F. Gaertn.) aqueous leaf extract administration on Salmonella typhimurium-infected rats. BMC Complement. Altern. Med. 2017, 17, 160. [Google Scholar] [CrossRef]
- Kengni, F.; Fodouop, S.P.C.; Tala, D.S.; Djimeli, M.N.; Fokunang, C.; Gatsing, D. Antityphoid properties and toxicity evaluation of Harungana madagascariensis Lam (Hypericaceae) aqueous leaf extract. J. Ethnopharmacol. 2016, 179, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Owais, M.; Sharad, K.S.; Shehbaz, A.; Saleemuddin, M. Antibacterial efficacy of Withania somnifera (ashwagandha) an indigenous medicinal plant against experimental murine salmonellosis. Phytomedicine 2005, 12, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Barkaoui, T.; Kacem, R.; Guesmi, F.; Blell, A.; Landoulsi, A. Article evaluation of antibacterial and antioxidant properties of Urtica urens extract tested by experimental animals. Int. J. Pharmacol. 2017, 13, 332–339. [Google Scholar] [CrossRef]
- Kim, S.; Fung, D.Y.C. Antibacterial Effect of Water-Soluble Arrowroot (Puerariae radix) Tea Extracts on Foodborne Pathogens in Ground Beef and Mushroom Soup. J. Food Prot. 2004, 67, 1953–1956. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.-H. Antibacterial activity and synergistic antibacterial potential of biosynthesized silver nanoparticles against foodborne pathogenic bacteria along with its anticandidal and antioxidant effects. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Calatayud, M.; López-de-Dicastillo, C.; López-Carballo, G.; Vélez, D.; Hernández Muñoz, P.; Gavara, R. Active films based on cocoa extract with antioxidant, antimicrobial and biological applications. Food Chem. 2013, 139, 51–58. [Google Scholar] [CrossRef]
- Steenackers, H.; Hermans, K.; Vanderleyden, J.; De Keersmaecker, S.C.J. Salmonella biofilms: An overview on occurrence, structure, regulation and eradication. Food Res. Int. 2012, 45, 502–531. [Google Scholar] [CrossRef]
- Vijayan, S.R.; Santhiyagu, P.; Singamuthu, M.; Kumari Ahila, N.; Jayaraman, R.; Ethiraj, K. Synthesis and characterization of silver and gold nanoparticles using aqueous extract of seaweed, Turbinaria conoides, and their antimicrofouling activity. Sci. World J. 2014, 2014, 938272. [Google Scholar] [CrossRef] [PubMed]
- Kulshreshtha, G.; Borza, T.; Rathgeber, B.; Stratton, G.S.; Thomas, N.A.; Critchley, A.; Hafting, J.; Prithiviraj, B. Red seaweeds Sarcodiotheca gaudichaudii and Chondrus crispus down regulate virulence factors of Salmonella Enteritidis and induce immune responses in Caenorhabditis elegans. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Anti-swarming and -biofilm activities of rose phenolic extract during simulated in vitro gastrointestinal digestion. Food Control 2016, 64, 189–195. [Google Scholar] [CrossRef]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Review. J. Nat. Remedies 2009, 9, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Oey, M.; Malhotra, P. Azithromycin and ceftriaxone combination treatment for relapsed Salmonella Paratyphi A bacteraemia. J. Travel Med. 2016, 23, tav032. [Google Scholar] [CrossRef] [PubMed]
- Tyers, M.; Wright, G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019, 17. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.; Khan, A.; Borghetto, I.; Kazmi, S.U.; Rubino, S.; Paglietti, B. Synergistic antimicrobial activity of Camellia sinensis and Juglans regia against multidrug-resistant bacteria. PLoS One 2015, 10, e0118431. [Google Scholar] [CrossRef]
- Suresh, G.; Das, R.K.; Kaur Brar, S.; Rouissi, T.; Avalos Ramirez, A.; Chorfi, Y.; Godbout, S. Alternatives to antibiotics in poultry feed: molecular perspectives. Crit. Rev. Microbiol. 2018, 44, 318–335. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant polyphenols as antioxidant and antibacterial agents for shelf-life extension of meat and meat products: Classification, structures, sources, and action mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef]
- Kortman, G.A.M.; Boleij, A.; Swinkels, D.W.; Tjalsma, H. Iron availability increases the pathogenic potential of Salmonella Typhimurium and other enteric pathogens at the intestinal epithelial interface. PLoS ONE 2012, 7, e29968. [Google Scholar] [CrossRef] [PubMed]
- Savoia, D. Plant-derived antimicrobial compounds: alternatives to antibiotics. Future Microbiol. 2012, 7, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Nazir, M.; Ali, M.S.; Hussain, H.; Lee, Y.S.; Riaz, N.; Jabbar, A. Antimicrobial natural products: an update on future antibioticdrug candidates. Nat. Prod. Rep. 2010, 27, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Rempe, C.S.; Lenaghan, S.C.; Burris, K.P.; Stewart, C.N. Metabolomic analysis of the mechanism of action of yerba mate aqueous extract on Salmonella enterica serovar Typhimurium. Metabolomics 2017, 13, 16. [Google Scholar] [CrossRef]
- Heck, C.I.; de Mejia, E.G. Yerba Mate Tea (Ilex paraguariensis): A Comprehensive Review on Chemistry, Health Implications, and Technological Considerations. J. Food Sci. 2007, 72, R138–R151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cetin-Karaca, H.; Newman, M.C. Antimicrobial efficacy of plant phenolic compounds against Salmonella and Escherichia Coli. Food Biosci. 2015, 11, 8–16. [Google Scholar] [CrossRef]
- Li, G.; Yan, C.; Xu, Y.; Feng, Y.; Wu, Q.; Lv, X.; Yang, B.; Wang, X.; Xia, X. Punicalagin inhibits Salmonella virulence factors and has anti-quorum-sensing potential. Appl. Environ. Microbiol. 2014, 80, 6204–6211. [Google Scholar] [CrossRef]
- Rempe, C.S.; Burris, K.P.; Lenaghan, S.C.; Stewart, C.N., Jr. The potential of systems biology to discover antibacterial mechanisms of plant phenolics. Front. Microbiol. 2017, 8, 422. [Google Scholar] [CrossRef]
- Kilani-Jaziri, S.; Bhouri, W.; Skandrani, I.; Limem, I.; Chekir-Ghedira, L.; Ghedira, K. Phytochemical, antimicrobial, antioxidant and antigenotoxic potentials of Cyperus rotundus extracts. S. Afr. J. Bot. 2011, 77, 767–776. [Google Scholar] [CrossRef]
- Nuamsetti, T.; Dechayuenyong, P.; Tantipaibulvut, S. Antibacterial activity of pomegranate fruit peels and arils. ScienceAsia 2012, 38, 319–322. [Google Scholar] [CrossRef]
- Coruh, I.; Gormez, A.; Ercisli, S.; Sengul, M. Total phenolic content, antioxidant, and antibacterial activity of Rumex crispus Grown Wild in Turkey. Pharm. Biol. 2008, 46, 634–638. [Google Scholar] [CrossRef]
- Şeker Karatoprak, G.; İlgün, S.; Koşar, M. Phenolic composition, anti-inflammatory, antioxidant, and antimicrobial activities of Alchemilla mollis (Buser) Rothm. Chem. Biodivers. 2017, 14, e1700150. [Google Scholar] [CrossRef] [PubMed]
- Merkl, R.; Hrádková, I.; Filip, V.; Šmidrkal, J. Antimicrobial and antioxidant properties of phenolic acids alkyl esters. Czech J. Food Sci. 2010, 28, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Antibacterial activities of phenolic benzaldehydes and benzoic acids against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2003, 66, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Arima, H.; Ashida, H.; Danno, G.-I. Rutin-enhanced antibacterial activities of flavonoids against Bacillus cereus and Salmonella enteritidis. Struct. Flavonol Flavanone Biosci. Biotechnol. Biochem 2002, 66, 1009–1014. [Google Scholar] [CrossRef]
- Puupponen-Pimia, R.; Nohynek, L.; Meier, C.; Kahkonen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.-M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costabile, A.; Sanghi, S.; Martin-Pelaez, S.; Mueller-Harvey, I.; Gibson, G.R.; Rastall, R.A.; Klinder, A. Inhibition of Salmonella Typhimurium by tannins in vitro. J. Food Agric. Environ. 2011, 9, 119–124. [Google Scholar]
- Tian, F.; Li, B.; Ji, B.; Zhang, G.; Luo, Y. Identification and structure–activity relationship of gallotannins separated from Galla chinensis. LWT - Food Sci. Technol. 2009, 42, 1289–1295. [Google Scholar] [CrossRef]
- Ríos, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef]
- Coates, A.R.M.; Hu, Y. Targeting non-multiplying organisms as a way to develop novel antimicrobials. Trends Pharmacol. Sci. 2008, 29, 143–150. [Google Scholar] [CrossRef]
- Junio, H.A.; Sy-Cordero, A.A.; Ettefagh, K.A.; Burns, J.T.; Micko, K.T.; Graf, T.N.; Richter, S.J.; Cannon, R.E.; Oberlies, N.H.; Cech, N.B. Synergy-directed fractionation of botanical medicines: A case study with goldenseal ( Hydrastis canadensis ). J. Nat. Prod. 2011, 74, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, J.J.; Todd, D.A.; Egan, J.M.; Raja, H.A.; Oberlies, N.H.; Kvalheim, O.M.; Cech, N.B. Biochemometrics for natural products research: Comparison of data analysis approaches and application to identification of bioactive compounds. J. Nat. Prod. 2016, 79, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Alminger, M.; Aura, A.M.; Bohn, T.; Dufour, C.; El, S.N.; Gomes, A.; Karakaya, S.; Martínez-Cuesta, M.C.; Mcdougall, G.J.; Requena, T.; Santos, C.N. In vitro models for studying secondary plant metabolite digestion and bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2014, 13, 413–436. [Google Scholar] [CrossRef]
- Ayaz, M.; Junaid, M.; Ullah, F.; Sadiq, A.; Ovais, M.; Ahmad, W.; Ahmad, S.; Zeb, A. Chemical profiling, antimicrobial and insecticidal evaluations of Polygonum hydropiper L. BMC Complement. Altern. Med. 2016, 16, 502. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.H.; Faizi, S.; Kazmi, S.U. Antibacterial activity in spices and local medicinal plants against clinical isolates of Karachi, Pakistan. Pharm. Biol. 2011, 49, 833–839. [Google Scholar] [CrossRef] [Green Version]
- Kalaycıoğlu, Z.; Torlak, E.; Akın-Evingür, G.; Özen, İ.; Erim, F.B. Antimicrobial and physical properties of chitosan films incorporated with turmeric extract. Int. J. Biol. Macromol. 2017, 101, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Kengni, F.; Tala, D.; Djimeli, M.; Fodouop, S.; Kodjio, N.; Magnifouet, H.; Gatsing, D. In vitro antimicrobial activity of Harungana madagascriensis and Euphorbia prostrata extracts against some pathogenic Salmonella sp. Int. J. Biol. Chem. Sci. 2013, 7, 1106. [Google Scholar] [CrossRef]
- Lamas, A.; Miranda, J.; Vázquez, B.; Cepeda, A.; Franco, C.; Lamas, A.; Miranda, J.M.; Vázquez, B.; Cepeda, A.; Franco, C.M. An evaluation of alternatives to nitrites and sulfites to inhibit the growth of Salmonella enterica and Listeria monocytogenes in meat products. Foods 2016, 5, 74. [Google Scholar] [CrossRef]
- Lamas, A.; Paz-Mendez, A.M.; Regal, P.; Vazquez, B.; Miranda, J.M.; Cepeda, A.; Franco, C.M. Food preservatives influence biofilm formation, gene expression and small RNAs in Salmonella enterica. LWT 2018, 97, 1–8. [Google Scholar] [CrossRef]
 ”antimicrobial Salmonella extract” OR “antibacterial Salmonella extract”, and
”antimicrobial Salmonella extract” OR “antibacterial Salmonella extract”, and  ”antimicrobial extract” OR “antibacterial extract” in topic, from 2006 to 2018, via Web of ScienceTM.
”antimicrobial extract” OR “antibacterial extract” in topic, from 2006 to 2018, via Web of ScienceTM.
 ”antimicrobial Salmonella extract” OR “antibacterial Salmonella extract”, and
”antimicrobial Salmonella extract” OR “antibacterial Salmonella extract”, and  ”antimicrobial extract” OR “antibacterial extract” in topic, from 2006 to 2018, via Web of ScienceTM.
”antimicrobial extract” OR “antibacterial extract” in topic, from 2006 to 2018, via Web of ScienceTM.

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, S.A.O.; Martins, C.; Pereira, C.; Silvestre, A.J.D.; Rocha, S.M. Current Challenges and Perspectives for the Use of Aqueous Plant Extracts in the Management of Bacterial Infections: The Case-Study of Salmonella enterica Serovars. Int. J. Mol. Sci. 2019, 20, 940. https://doi.org/10.3390/ijms20040940
Santos SAO, Martins C, Pereira C, Silvestre AJD, Rocha SM. Current Challenges and Perspectives for the Use of Aqueous Plant Extracts in the Management of Bacterial Infections: The Case-Study of Salmonella enterica Serovars. International Journal of Molecular Sciences. 2019; 20(4):940. https://doi.org/10.3390/ijms20040940
Chicago/Turabian StyleSantos, Sónia A. O., Cátia Martins, Carla Pereira, Armando J. D. Silvestre, and Sílvia M. Rocha. 2019. "Current Challenges and Perspectives for the Use of Aqueous Plant Extracts in the Management of Bacterial Infections: The Case-Study of Salmonella enterica Serovars" International Journal of Molecular Sciences 20, no. 4: 940. https://doi.org/10.3390/ijms20040940
APA StyleSantos, S. A. O., Martins, C., Pereira, C., Silvestre, A. J. D., & Rocha, S. M. (2019). Current Challenges and Perspectives for the Use of Aqueous Plant Extracts in the Management of Bacterial Infections: The Case-Study of Salmonella enterica Serovars. International Journal of Molecular Sciences, 20(4), 940. https://doi.org/10.3390/ijms20040940