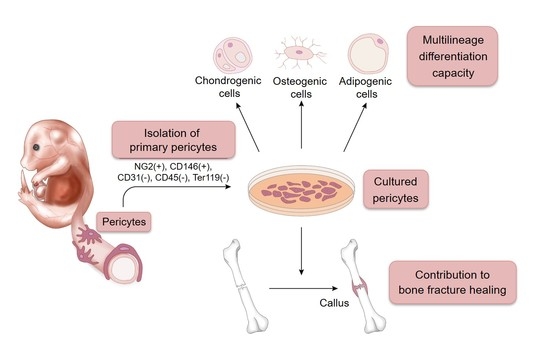
Pericytes as a Source of Osteogenic Cells in Bone Fracture Healing
Abstract
:1. Introduction
2. Results
2.1. A Novel Method for Isolating and Culturing Primary Pericytes
2.2. Immortalization of Primary Pericytes and Multilineage Differentiation Capacity In Vitro
2.3. Pericytes Differentiate into Osteogenic Cells In Vivo
2.4. Contribution of Implanted Pericytes to Bone Fracture Healing
2.5. Endogenous Pericytes also Contribute to Bone Fracture Healing In Vivo
3. Discussion
4. Materials and Methods
4.1. Pericyte Isolation and Culture
4.2. Immortalization of Pericytes
4.3. Osteogenic Differentiation Assay
4.4. Adipogenic Differentiation Assay
4.5. Chondrogenic Differentiation Assay
4.6. Experimental Animals
4.7. Bone Fracture Model and Pericyte Implantation
4.8. Quantitative Real-Time PCR (qPCR)
4.9. Histological and Immunohistochemical Analysis
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meling, T.; Harboe, K.; Søreide, K. Incidence of traumatic long-bone fractures requiring in-hospital management: A prospective age- and gender-specific analysis of 4890 fractures. Injury 2009, 40, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A. Enhancement of fracture-healing. J. Bone Jt. Surg. 1995, 77, 940–956. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells—Current trends and future prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef] [PubMed]
- Granero-Moltó, F.; Weis, J.A.; Miga, M.I.; Landis, B.; Myers, T.J.; O’Rear, L.; Longobardi, L.; Jansen, E.D.; Mortlock, D.P.; Spagnoli, A. Regenerative Effects of Transplanted Mesenchymal Stem Cells in Fracture Healing. Stem Cells 2009, 27, 1887–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar-Vazquez, R.; Carballo-Molina, O.A.; Collazo-Navarrete, O.; Guerrero-Rangel, M.; Saucedo-Campos, A.D.; Barrera-Lechuga, P.; Lopez-Marure, R.; Caceres-Cortes, J.R. Osteogenesis of human vascular endothelial cells in culture. Rev. Investig. Clin. Organo Hosp. Enfermedades Nutr. 2008, 60, 496–501. [Google Scholar] [PubMed]
- Lazard, Z.W.; Olmsted-Davis, E.A.; Salisbury, E.A.; Gugala, Z.; Sonnet, C.; Davis, E.L.; Beal, E.I.; Ubogu, E.E.; Davis, A.R. Osteoblasts Have a Neural Origin in Heterotopic Ossification. Clin. Orthop. Relat. Res. 2015, 473, 2790–2806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, K.R.; Kang, I.-H.; Baliga, U.; Xiong, Y.; Chatterjee, S.; Moore, E.; Parthiban, B.; Thyagarajan, K.; Borke, J.L.; Mehrotra, S.; et al. Hematopoietic Stem Cells as a Novel Source of Dental Tissue Cells. Sci. Rep. 2018, 8, 8026. [Google Scholar] [CrossRef] [PubMed]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.-W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, T.N.; Tozawa, Y.; Deutsch, U.; Wolburg-Buchholz, K.; Fujiwara, Y.; Gendron-Maguire, M.; Gridley, T.; Wolburg, H.; Risau, W.; Qin, Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 1995, 376, 70. [Google Scholar] [CrossRef] [PubMed]
- Maisonpierre, P.C.; Suri, C.; Jones, P.F.; Bartunkova, S.; Wiegand, S.J.; Radziejewski, C.; Compton, D.; McClain, J.; Aldrich, T.H.; Papadopoulos, N.; et al. Angiopoietin-2, a Natural Antagonist for Tie2 That Disrupts in vivo Angiogenesis. Science 1997, 277, 55. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Tang, Y.J.; Wei, Q.; Hirata, M.; Weng, A.; Han, I.; Okawa, A.; Takeda, S.; Whetstone, H.; Nadesan, P.; et al. Mesenchymal Tumors Can Derive from Ng2/Cspg4-Expressing Pericytes with β-Catenin Modulating the Neoplastic Phenotype. Cell Rep. 2016, 16, 917–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-S.; Lue, T.F. Defining Vascular Stem Cells. Stem Cells Dev. 2013, 22, 1018–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Covas, D.T.; Panepucci, R.A.; Fontes, A.M.; Silva, W.A.; Orellana, M.D.; Freitas, M.C.C.; Neder, L.; Santos, A.R.D.; Peres, L.C.; Jamur, M.C.; et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp. Hematol. 2008, 36, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Fuoco, C.; Sangalli, E.; Vono, R.; Testa, S.; Sacchetti, B.; Latronico, M.V.G.; Bernardini, S.; Madeddu, P.; Cesareni, G.; Seliktar, D.; et al. 3D hydrogel environment rejuvenates aged pericytes for skeletal muscle tissue engineering. Front. Physiol. 2014, 5, 203. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.C.; Tucker, H.A.; Bunnell, B.A.; Andreeff, M.; Schober, W.; Gaynor, A.S.; Strickler, K.L.; Lin, S.; Lacey, M.R.; O’Connor, K.C. Cell-Surface Expression of Neuron-Glial Antigen 2 (NG2) and Melanoma Cell Adhesion Molecule (CD146) in Heterogeneous Cultures of Marrow-Derived Mesenchymal Stem Cells. Tissue Eng. Part A 2013, 19, 2253–2266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, T.S.; Gavina, M.; Chen, C.-W.; Sun, B.; Teng, P.-N.; Huard, J.; Deasy, B.M.; Zimmerlin, L.; Péault, B. Placental perivascular cells for human muscle regeneration. Stem Cells Dev. 2011, 20, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, J.C.; Over, P.; Turner, M.E.; Thompson, R.L.; Foka, H.G.; Chen, W.C.W.; Péault, B.; Gridelli, B.; Schmelzer, E. Perivascular Mesenchymal Progenitors in Human Fetal and Adult Liver. Stem Cells Dev. 2012, 21, 3258–3269. [Google Scholar] [CrossRef] [PubMed]
- Tsang, W.P.; Shu, Y.; Kwok, P.L.; Zhang, F.; Lee, K.K.H.; Tang, M.K.; Li, G.; Chan, K.M.; Chan, W.-Y.; Wan, C. CD146+ human umbilical cord perivascular cells maintain stemness under hypoxia and as a cell source for skeletal regeneration. PLoS ONE 2013, 8, e76153. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.W.; Saparov, A.; Corselli, M.; Crisan, M.; Zheng, B.; Péault, B.; Huard, J. Isolation of blood-vessel-derived multipotent precursors from human skeletal muscle. J. Vis. Exp. 2014, e51195. [Google Scholar] [CrossRef] [PubMed]
- Tawonsawatruk, T.; West, C.C.; Murray, I.R.; Soo, C.; Péault, B.; Simpson, A.H.R.W. Adipose derived pericytes rescue fractures from a failure of healing—Non-union. Sci. Rep. 2016, 6, 22779. [Google Scholar] [CrossRef] [PubMed]
- Baily, J.E.; Chen, W.C.W.; Khan, N.; Murray, I.R.; González Galofre, Z.N.; Huard, J.; Péault, B. Isolation of Perivascular Multipotent Precursor Cell Populations from Human Cardiac Tissue. J. Vis. Exp. 2016. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; Zhang, X.; Crisan, M.; Hardy, W.R.; Liang, P.; Meyers, C.A.; Lobo, S.; Lagishetty, V.; Childers, M.K.; Asatrian, G.; et al. Isolation and characterization of canine perivascular stem/stromal cells for bone tissue engineering. PLoS ONE 2017, 12, e0177308. [Google Scholar] [CrossRef] [PubMed]
- Birbrair, A.; Zhang, T.; Wang, Z.M.; Messi, M.L.; Enikolopov, G.N.; Mintz, A.; Delbono, O. Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev. 2013, 22, 2298–2314. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, P.S.; Bose, B. Hepatic perivascular mesenchymal stem cells with myogenic properties. J. Tissue Eng. Regen. Med. 2017, 12, e1297–e1310. [Google Scholar] [CrossRef] [PubMed]
- Crouch, E.E.; Doetsch, F. FACS isolation of endothelial cells and pericytes from mouse brain microregions. Nat. Protoc. 2018, 13, 738. [Google Scholar] [CrossRef] [PubMed]
- Guimarães-Camboa, N.; Cattaneo, P.; Sun, Y.; Moore-Morris, T.; Gu, Y.; Dalton, N.D.; Rockenstein, E.; Masliah, E.; Peterson, K.L.; Stallcup, W.B.; et al. Pericytes of Multiple Organs Do Not Behave as Mesenchymal Stem Cells In Vivo. Cell Stem Cell 2017, 20, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Brookes, O.; Battaglia, G. Pericytes from Mesenchymal Stem Cells as a model for the blood-brain barrier. Sci. Rep. 2017, 7, 39676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva Meirelles, L.; Bellagamba, B.C.; Camassola, M.; Nardi, N.B. Mesenchymal stem cells and their relationship to pericytes. Front. Biosci. 2016, 21, 130–156. [Google Scholar] [CrossRef]
- Birbrair, A.; Zhang, T.; Wang, Z.-M.; Messi, M.L.; Mintz, A.; Delbono, O. Pericytes at the intersection between tissue regeneration and pathology. Clin. Sci. 2015, 128, 81–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundberg, C.; Ivarsson, M.; Gerdin, B.; Rubin, K. Pericytes as collagen-producing cells in excessive dermal scarring. Lab. Investig. J. Tech. Methods Pathol. 1996, 74, 452–466. [Google Scholar]
- Lin, S.-L.; Kisseleva, T.; Brenner, D.A.; Duffield, J.S. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am. J. Pathol. 2008, 173, 1617–1627. [Google Scholar] [CrossRef] [PubMed]
- Dulauroy, S.; Di Carlo, S.E.; Langa, F.; Eberl, G.; Peduto, L. Lineage tracing and genetic ablation of ADAM12+ perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat. Med. 2012, 18, 1262. [Google Scholar] [CrossRef] [PubMed]
- Göritz, C.; Dias, D.O.; Tomilin, N.; Barbacid, M.; Shupliakov, O.; Frisén, J. A Pericyte Origin of Spinal Cord Scar Tissue. Science 2011, 333, 238. [Google Scholar] [CrossRef] [PubMed]
- Birbrair, A.; Zhang, T.; Wang, Z.-M.; Messi, M.L.; Mintz, A.; Delbono, O. Pericytes: Multitasking cells in the regeneration of injured, diseased, and aged skeletal muscle. Front. Aging Neurosci. 2014, 6, 245. [Google Scholar] [CrossRef] [PubMed]
- Dellavalle, A.; Sampaolesi, M.; Tonlorenzi, R.; Tagliafico, E.; Sacchetti, B.; Perani, L.; Innocenzi, A.; Galvez, B.G.; Messina, G.; Morosetti, R.; et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat. Cell Biol. 2007, 9, 255. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.L.; Hausman, G.J.; Campion, D.R. Response of Pericytes to Thermal Lesion in the Inguinal Fat Pad of 10-Day-Old Rats. Cells Tissues Organs 1982, 114, 41–57. [Google Scholar] [CrossRef]
- Hashimoto, K.; Ochi, H.; Sunamura, S.; Kosaka, N.; Mabuchi, Y.; Fukuda, T.; Yao, K.; Kanda, H.; Ae, K.; Okawa, A.; et al. Cancer-secreted hsa-miR-940 induces an osteoblastic phenotype in the bone metastatic microenvironment via targeting ARHGAP1 and FAM134A. Proc. Natl. Acad. Sci. USA 2018, 115, 2204–2209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Bergles, D.E.; Nishiyama, A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development 2008, 135, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Hill, R.A.; Dietrich, D.; Komitova, M.; Suzuki, R.; Nishiyama, A. Age-dependent fate and lineage restriction of single NG2 cells. Development 2011, 138, 745–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madisen, L.; Zwingman, T.A.; Sunkin, S.M.; Oh, S.W.; Zariwala, H.A.; Gu, H.; Ng, L.L.; Palmiter, R.D.; Hawrylycz, M.J.; Jones, A.R.; et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010, 13, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Hanada, R.; Kimura, A.; Abe, T.; Matsumoto, T.; Iwasaki, M.; Inose, H.; Ida, T.; Mieda, M.; Takeuchi, Y.; et al. Central control of bone remodeling by neuromedin U. Nat. Med. 2007, 13, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Takeda, S.; Xu, R.; Ochi, H.; Sunamura, S.; Sato, T.; Shibata, S.; Yoshida, Y.; Gu, Z.; Kimura, A.; et al. Sema3A regulates bone-mass accrual through sensory innervations. Nature 2013, 497, 490–493. [Google Scholar] [CrossRef] [PubMed]





| Isolation Method | Markers for Sorting | Species | Tissue | Year | Ref. |
|---|---|---|---|---|---|
| FACS * | CD146+, CD34−, CD45−, CD56− | Human | Placenta | 2011 | [17] |
| FACS | CD146+, CD34−, CD45−, CD56− | Human | Hepatic tissue | 2012 | [18] |
| Magnetic beads | CD146+ | Human | Umbilical cord | 2013 | [19] |
| FACS | CD146+, CD34−, CD45−, CD56− | Human | Skeletal muscle | 2014 | [20] |
| FACS | CD146+, CD31−, CD34−, CD45− | Human | Adipose | 2016 | [21] |
| FACS | CD146+, CD34−, CD45−, CD56−, CD144− | Human | Cardiac tissue | 2016 | [22] |
| FACS | CD146+, CD34−, CD45− | Human | Canine adipose | 2017 | [23] |
| FACS | NG2/DsRed+, Nestin/GFP+/− | Mouse | Skeletal muscle | 2017 | [24] |
| FACS | CD146+, CD34−, CD45−, CD56− | Mouse | Hepatic tissue | 2018 | [25] |
| FACS | CD13+, CD31−, CD41−, CD45− | Mouse | Brain | 2018 | [26] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Supakul, S.; Yao, K.; Ochi, H.; Shimada, T.; Hashimoto, K.; Sunamura, S.; Mabuchi, Y.; Tanaka, M.; Akazawa, C.; Nakamura, T.; et al. Pericytes as a Source of Osteogenic Cells in Bone Fracture Healing. Int. J. Mol. Sci. 2019, 20, 1079. https://doi.org/10.3390/ijms20051079
Supakul S, Yao K, Ochi H, Shimada T, Hashimoto K, Sunamura S, Mabuchi Y, Tanaka M, Akazawa C, Nakamura T, et al. Pericytes as a Source of Osteogenic Cells in Bone Fracture Healing. International Journal of Molecular Sciences. 2019; 20(5):1079. https://doi.org/10.3390/ijms20051079
Chicago/Turabian StyleSupakul, Sopak, Kenta Yao, Hiroki Ochi, Tomohito Shimada, Kyoko Hashimoto, Satoko Sunamura, Yo Mabuchi, Miwa Tanaka, Chihiro Akazawa, Takuro Nakamura, and et al. 2019. "Pericytes as a Source of Osteogenic Cells in Bone Fracture Healing" International Journal of Molecular Sciences 20, no. 5: 1079. https://doi.org/10.3390/ijms20051079
APA StyleSupakul, S., Yao, K., Ochi, H., Shimada, T., Hashimoto, K., Sunamura, S., Mabuchi, Y., Tanaka, M., Akazawa, C., Nakamura, T., Okawa, A., Takeda, S., & Sato, S. (2019). Pericytes as a Source of Osteogenic Cells in Bone Fracture Healing. International Journal of Molecular Sciences, 20(5), 1079. https://doi.org/10.3390/ijms20051079