Transcriptomics and Immunological Analyses Reveal a Pro-Angiogenic and Anti-Inflammatory Phenotype for Decidual Endothelial Cells
Abstract
:1. Introduction
2. Results
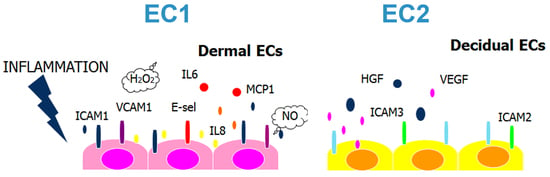
2.1. Decidual Endothelial Cells Express a Different Profile of Angiogenic Factors and Adhesion Molecules Compared to Endothelial Cell Isolated from Dermal Skin
2.2. DECs Respond Feebly to Vasoactive Stimuli Leading to Vascular Leakage Compared to ADMECs
2.3. DECs Produce Lower Levels of Oxygen-Derived Reactive Molecules than ADMECs in Response to TNF-α and Histamine Challenge
2.4. DECs, Compared to ADMECs, Are Weak Responders to TNF-α Stimulation with Respect to Chemokine Secretion, Adhesion Molecule Expression and Leukocyte Recruitment
2.5. IFN-γ-stimulated DECs Are Potent Recruiters of Natural Killer and Regulatory T Cells
3. Discussion
4. Materials and Methods
4.1. Cell Isolation and Culture
4.2. Immunofluorescence
4.3. RNA Isolation, cDNA Synthesis and Quantitative Real-Time Polymerase-Chain Reaction (RT-qPCR)
4.4. Microarray
4.5. Growth Factors and Chemokines Detection
4.6. Migration Assays
4.7. Detection of Adhesion Molecules on ECs
4.8. Endothelial Leakage
4.9. Measurement of Total H2O2 Production
4.10. Statistic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADMECs | Adult Dermal Microvascular Endothelial cells |
| AU | Arbitrary Units |
| BK | Bradykinin |
| BSA | Bovine Serum Albumin |
| C1q | Complement component 1q |
| CCL | Chemokine (C–C motif) Ligand |
| CD | Cluster of Differentiation |
| cDNA | Complementary DNA |
| CM | Conditioned Medium |
| CQ | Comparative Quantification |
| CXCL | Chemokine (C–X–C motif) Ligand |
| DECs | Decidual endothelial cells |
| ECs | Endothelial cells |
| ELISA | Enzyme-Linked ImmunoSorbent Assay |
| E-Selectin | Endothelial–Selectin |
| FCS | Foetal Calf Serum |
| FITC | Fluorescein Isothiocyanate |
| FoxP3 | Forkhead box P3 |
| GEO | Gene Expression Omnibus |
| GEP | Gene Expression |
| HEVs | High Endothelial Venules |
| HGF | Hepatocyte Growth Factor |
| HIS | Histamine |
| HRP | Horseradish Peroxidase |
| HUVECs | Human Umbilical Vein Endothelial Cells |
| ICAM | Intercellular Adhesion Molecule |
| IFN-γ | Interferon gamma |
| IGF-1 | Insulin-like Growth Factor-1 |
| IGFBP3 | Insulin-like Growth Factor-Binding Protein 3 |
| IgG | Immunoglobulin G |
| IL | Interleukin |
| IP-10 | Interferon gamma-induced Protein 10 |
| LM | Lympho-Monocytes |
| LPS | Lipopolysaccharide |
| mAb | Monoclonal Antibody |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MIG | Monokine Induced by Gamma |
| MIP-1α | Macrophage Inflammatory Proteins-1α |
| mRNA | Messenger RNA |
| NK | Natural Killer |
| PAF | Platelet-Activating Factor |
| PE | Phycoerythrin |
| RANTES | Regulated on Activation, Normal T cell Expressed and Secreted |
| ROS | Reactive Oxygen Species |
| RT | Room Temperature |
| RT-qPCR | Quantitative real-time polymerase-chain reaction |
| SOD | Superoxide Dismutase |
| TLR4 | Toll-Like Receptor 4 |
| TNF-α | Tumor Necrosis Factor α |
| Treg | Regulatory T |
| TW | Transwell |
| VCAM-1 | Vascular Cell Adhesion Protein-1 |
| VE-cadherin | Vascular endothelial cadherin |
| VEGF-A | Vascular Endothelial Growth Factor A |
| vWF | von Willebrand Factor |
References
- Mantovani, A.; Bussolino, F.; Dejana, E. Cytokine regulation of endothelial cell function. FASEB J. 1992, 6, 2591–2599. [Google Scholar] [CrossRef] [PubMed]
- Garlanda, C.; Dejana, E. Heterogeneity of endothelial cells. Specific markers. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Dejana, E.; Fiocchi, C. Immune regulation by microvascular endothelial cells: Directing innate and adaptive immunity, coagulation, and inflammation. J. Immunol. 2007, 178, 6017–6022. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. Endothelial cell heterogeneity. Cold Spring Harb. Perspect. Med. 2012, 2, a006429. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Kupper, T.S. Inflammatory skin diseases, T cells, and immune surveillance. N. Engl. J. Med. 1999, 341, 1817–1828. [Google Scholar] [CrossRef]
- Nourshargh, S.; Alon, R. Leukocyte migration into inflamed tissues. Immunity 2014, 41, 694–707. [Google Scholar] [CrossRef]
- Gellersen, B.; Brosens, I.A.; Brosens, J.J. Decidualization of the human endometrium: Mechanisms, functions, and clinical perspectives. Semin. Reprod. Med. 2007, 25, 445–453. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Sargent, I.L.; Borzychowski, A.M.; Redman, C.W. NK cells and human pregnancy--an inflammatory view. Trends Immunol. 2006, 27, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Bulla, R.; Fischetti, F.; Bossi, F.; Tedesco, F. Feto-maternal immune interaction at the placental level. Lupus 2004, 13, 625–629. [Google Scholar] [CrossRef]
- Zucchetto, A.; Benedetti, D.; Tripodo, C.; Bomben, R.; Dal Bo, M.; Marconi, D.; Bossi, F.; Lorenzon, D.; Degan, M.; Rossi, F.M.; et al. CD38/CD31, the CCL3 and CCL4 chemokines, and CD49d/vascular cell adhesion molecule-1 are interchained by sequential events sustaining chronic lymphocytic leukemia cell survival. Cancer Res. 2009, 69, 4001–4009. [Google Scholar] [CrossRef]
- Groger, M.; Holnthoner, W.; Maurer, D.; Lechleitner, S.; Wolff, K.; Mayr, B.B.; Lubitz, W.; Petzelbauer, P. Dermal microvascular endothelial cells express the 180-kDa macrophage mannose receptor in situ and in vitro. J. Immunol. 2000, 165, 5428–5434. [Google Scholar] [CrossRef] [PubMed]
- Bulla, R.; Agostinis, C.; Bossi, F.; Rizzi, L.; Debeus, A.; Tripodo, C.; Radillo, O.; De Seta, F.; Ghebrehiwet, B.; Tedesco, F. Decidual endothelial cells express surface-bound C1q as a molecular bridge between endovascular trophoblast and decidual endothelium. Mol. Immunol. 2008, 45, 2629–2640. [Google Scholar] [CrossRef] [Green Version]
- Goebeler, M.; Yoshimura, T.; Toksoy, A.; Ritter, U.; Brocker, E.B.; Gillitzer, R. The chemokine repertoire of human dermal microvascular endothelial cells and its regulation by inflammatory cytokines. J. Investig. Dermatol. 1997, 108, 445–451. [Google Scholar] [CrossRef]
- Pober, J.S.; Cotran, R.S. Cytokines and endothelial cell biology. Physiol. Rev. 1990, 70, 427–451. [Google Scholar] [CrossRef]
- Santoni, A.; Carlino, C.; Stabile, H.; Gismondi, A. Mechanisms underlying recruitment and accumulation of decidual NK cells in uterus during pregnancy. Am. J. Reprod. Immunol. 2008, 59, 417–424. [Google Scholar] [CrossRef]
- Chaouat, G.; Petitbarat, M.; Bulla, R.; Dubanchet, S.; Valdivia, K.; Ledee, N.; Steffen, T.; Jensenius, J.C.; Tedesco, F. Early regulators in abortion and implications for a preeclampsia model. J. Reprod. Immunol. 2009, 82, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Burrows, T.D.; King, A.; Loke, Y.W. Expression of adhesion molecules by endovascular trophoblast and decidual endothelial cells: Implications for vascular invasion during implantation. Placenta 1994, 15, 21–33. [Google Scholar] [CrossRef]
- Hecht, J.L.; Zsengeller, Z.K.; Spiel, M.; Karumanchi, S.A.; Rosen, S. Revisiting decidual vasculopathy. Placenta 2016, 42, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, R.H.; Dunk, C.E.; Lye, S.J.; Aplin, J.D.; Harris, L.K.; Jones, R.L. Extravillous Trophoblast and Endothelial Cell Crosstalk Mediates Leukocyte Infiltration to the Early Remodeling Decidual Spiral Arteriole Wall. J. Immunol. 2017, 198, 4115–4128. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.A.; Greaves, E.; Critchley, H.O.; Saunders, P.T. Estrogen-dependent regulation of human uterine natural killer cells promotes vascular remodelling via secretion of CCL2. Hum. Reprod. 2015, 30, 1290–1301. [Google Scholar] [CrossRef] [Green Version]
- Piao, L.; Chen, C.P.; Yeh, C.C.; Basar, M.; Masch, R.; Cheng, Y.C.; Lockwood, C.J.; Schatz, F.; Huang, S.J. Chinese herbal medicine for miscarriage affects decidual micro-environment and fetal growth. Placenta 2015, 36, 559–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agostinis, C.; Bulla, R.; Tripodo, C.; Gismondi, A.; Stabile, H.; Bossi, F.; Guarnotta, C.; Garlanda, C.; De Seta, F.; Spessotto, P.; et al. An alternative role of C1q in cell migration and tissue remodeling: Contribution to trophoblast invasion and placental development. J. Immunol. 2010, 185, 4420–4429. [Google Scholar] [CrossRef]
- Spessotto, P.; Dri, P.; Bulla, R.; Zabucchi, G.; Patriarca, P. Human eosinophil peroxidase enhances tumor necrosis factor and hydrogen peroxide release by human monocyte-derived macrophages. Eur. J. Immunol. 1995, 25, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Pontillo, A.; Girardelli, M.; Agostinis, C.; Masat, E.; Bulla, R.; Crovella, S. Bacterial LPS differently modulates inflammasome gene expression and IL-1beta secretion in trophoblast cells, decidual stromal cells, and decidual endothelial cells. Reprod. Sci. 2013, 20, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Masat, E.; Gasparini, C.; Agostinis, C.; Bossi, F.; Radillo, O.; De Seta, F.; Tamassia, N.; Cassatella, M.A.; Bulla, R. RelB activation in anti-inflammatory decidual endothelial cells: A master plan to avoid pregnancy failure? Sci. Rep. 2015, 5, 14847. [Google Scholar] [CrossRef]
- Goddard, L.M.; Iruela-Arispe, M.L. Cellular and molecular regulation of vascular permeability. Thromb. Haemost. 2013, 109, 407–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bossi, F.; Peerschke, E.I.; Ghebrehiwet, B.; Tedesco, F. Cross-talk between the complement and the kinin system in vascular permeability. Immunol. Lett. 2011, 140, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Schremmer-Danninger, E.; Nagler, D.K.; Miska, K.; Flaig, M.J.; Faussner, A.; Fink, E.; Raggi, M.C.; Jochum, M.; Rehbock, J. Kinin receptors in stimulated and characterized decidua tissue-derived cells. Int. Immunopharmacol. 2007, 7, 103–112. [Google Scholar] [CrossRef]
- Bossi, F.; Fischetti, F.; Regoli, D.; Durigutto, P.; Frossi, B.; Gobeil, F., Jr.; Ghebrehiwet, B.; Peerschke, E.I.; Cicardi, M.; Tedesco, F. Novel pathogenic mechanism and therapeutic approaches to angioedema associated with C1 inhibitor deficiency. J. Allergy Clin. Immunol. 2009, 124, 1303–1310 e4. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, F.; Pausa, M.; Nardon, E.; Introna, M.; Mantovani, A.; Dobrina, A. The cytolytically inactive terminal complement complex activates endothelial cells to express adhesion molecules and tissue factor procoagulant activity. J. Exp. Med. 1997, 185, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Lash, G.E.; Otun, H.A.; Innes, B.A.; Kirkley, M.; De Oliveira, L.; Searle, R.F.; Robson, S.C.; Bulmer, J.N. Interferon-gamma inhibits extravillous trophoblast cell invasion by a mechanism that involves both changes in apoptosis and protease levels. FASEB J. 2006, 20, 2512–2518. [Google Scholar] [CrossRef] [PubMed]
- Carlino, C.; Stabile, H.; Morrone, S.; Bulla, R.; Soriani, A.; Agostinis, C.; Bossi, F.; Mocci, C.; Sarazani, F.; Tedesco, F.; et al. Recruitment of circulating NK cells through decidual tissues: A possible mechanism controlling NK cell accumulation in the uterus during early pregnancy. Blood 2008, 111, 3108–3115. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.E.; Cartwright, J.E.; Begum, R.; Laing, K.; Thilaganathan, B.; Whitley, G.S. Trophoblast-induced changes in C-x-C motif chemokine 10 expression contribute to vascular smooth muscle cell dedifferentiation during spiral artery remodeling. Arterioscler. Thromb. Vasc. Biol. 2013, 33, e93–e101. [Google Scholar] [CrossRef]
- McCurdy, R.D.; McGrath, J.J.; Mackay-Sim, A. Validation of the comparative quantification method of real-time PCR analysis and a cautionary tale of housekeeping gene selection. Gene Ther. Mol. Biol. 2008, 12, 15–24. [Google Scholar]
- Eisen, M.B.; Spellman, P.T.; Brown, P.O.; Botstein, D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 1998, 95, 14863–14868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledee, N.; Munaut, C.; Serazin, V.; Perrier d’Hauterive, S.; Lombardelli, L.; Logiodice, F.; Wainer, R.; Gridelet, V.; Chaouat, G.; Frankenne, F.; et al. Performance evaluation of microbead and ELISA assays for follicular G-CSF: A non-invasive biomarker of oocyte developmental competence for embryo implantation. J. Reprod. Immunol. 2010, 86, 126–132. [Google Scholar] [CrossRef]
- Bossi, F.; Rizzi, L.; Bulla, R.; Debeus, A.; Tripodo, C.; Picotti, P.; Betto, E.; Macor, P.; Pucillo, C.; Wurzner, R.; et al. C7 is expressed on endothelial cells as a trap for the assembling terminal complement complex and may exert anti-inflammatory function. Blood 2009, 113, 3640–3648. [Google Scholar] [CrossRef] [Green Version]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agostinis, C.; Masat, E.; Bossi, F.; Ricci, G.; Menegazzi, R.; Lombardelli, L.; Zito, G.; Mangogna, A.; Degan, M.; Gattei, V.; et al. Transcriptomics and Immunological Analyses Reveal a Pro-Angiogenic and Anti-Inflammatory Phenotype for Decidual Endothelial Cells. Int. J. Mol. Sci. 2019, 20, 1604. https://doi.org/10.3390/ijms20071604
Agostinis C, Masat E, Bossi F, Ricci G, Menegazzi R, Lombardelli L, Zito G, Mangogna A, Degan M, Gattei V, et al. Transcriptomics and Immunological Analyses Reveal a Pro-Angiogenic and Anti-Inflammatory Phenotype for Decidual Endothelial Cells. International Journal of Molecular Sciences. 2019; 20(7):1604. https://doi.org/10.3390/ijms20071604
Chicago/Turabian StyleAgostinis, Chiara, Elisa Masat, Fleur Bossi, Giuseppe Ricci, Renzo Menegazzi, Letizia Lombardelli, Gabriella Zito, Alessandro Mangogna, Massimo Degan, Valter Gattei, and et al. 2019. "Transcriptomics and Immunological Analyses Reveal a Pro-Angiogenic and Anti-Inflammatory Phenotype for Decidual Endothelial Cells" International Journal of Molecular Sciences 20, no. 7: 1604. https://doi.org/10.3390/ijms20071604
APA StyleAgostinis, C., Masat, E., Bossi, F., Ricci, G., Menegazzi, R., Lombardelli, L., Zito, G., Mangogna, A., Degan, M., Gattei, V., Piccinni, M. -P., Kishore, U., & Bulla, R. (2019). Transcriptomics and Immunological Analyses Reveal a Pro-Angiogenic and Anti-Inflammatory Phenotype for Decidual Endothelial Cells. International Journal of Molecular Sciences, 20(7), 1604. https://doi.org/10.3390/ijms20071604