The Effect of Molecular Weight on the Antibacterial Activity of N,N,N-Trimethyl Chitosan (TMC)
Abstract
:1. Introduction
2. Results
2.1. Synthesis
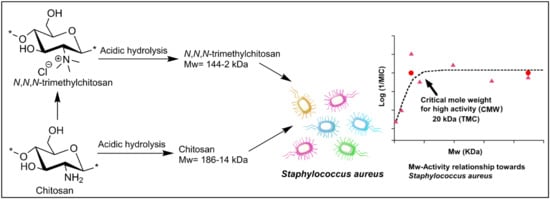
2.2. Acidic Hydrolysis
2.3. Structural Characterization of Hydrolyzed Products
2.4. Mw-Antibacterial Property Relationship
3. Discussion
3.1. Synthesis and Acid Degradation
3.2. Mw-Antibacterial Property Relationship
4. Materials and Methods
4.1. Materials
4.2. Synthesis
N,N,N-Trimethyl Chitosan
4.3. Hydrolysis
Hydrolysis of TMC and Chitosan
4.4. Characterization
- (1)
- Degree of acetylation,
- (2)
- DS for N,N,N-trimethylation,
4.5. Molecular Weight Determination
4.6. Antibacterial Tests
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Einbu, A.; Varum, K.M. Depolymerization and de-N-acetylation of chitin oligomers in hydrochloric acid. Biomacromolecules 2007, 8, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.S.; Shen, D.F. Effect of reaction temperature and reaction time on the preparation of low-molecular-weight chitosan using phosphoric acid. Carbohyd. Polym. 2002, 49, 393–396. [Google Scholar] [CrossRef]
- Varum, K.M.; Ottoy, M.H.; Smidsrod, O. Acid hydrolysis of chitosans. Carbohyd. Polym. 2001, 46, 89–98. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N.; Choudhari, Y.M. Chitooligosaccharides: Synthesis, Characterization and Applications. Polym. Sci. Ser. A 2011, 53, 583–612. [Google Scholar] [CrossRef]
- Kulikov, S.; Tikhonov, V.; Blagodatskikh, I.; Bezrodnykh, E.; Lopatin, S.; Khairullin, R.; Philippova, Y.; Abramchuk, S. Molecular weight and pH aspects of the efficacy of oligochitosan against methicillin-resistant Staphylococcus aureus (MRSA). Carbohyd. Polym. 2012, 87, 545–550. [Google Scholar] [CrossRef]
- Kulikov, S.N.; Lisovskaya, S.A.; Zelenikhin, P.V.; Bezrodnykh, E.A.; Shakirova, D.R.; Blagodatskikh, M.V.; Tikhonov, V.E. Antifungal activity of oligochitosans (short chain chitosans) against some Candida species and clinical isolates of Candida albicans: Molecular weight-activity relationship. Eur. J. Med. Chem. 2014, 74, 169–178. [Google Scholar] [CrossRef]
- Chang, S.H.; Lin, H.T.V.; Wu, G.J.; Tsai, G.J. pH Effects on solubility, zeta potential, and correlation between antibacterial activity and molecular weight of chitosan. Carbohyd. Polym. 2015, 134, 74–81. [Google Scholar] [CrossRef]
- Li, K.C.; Xing, R.G.; Liu, S.; Qin, Y.K.; Yu, H.H.; Li, P.C. Size and pH effects of chitooligomers on antibacterial activity against Staphylococcus aureus. Int. J. Biol. Macromol. 2014, 64, 302–305. [Google Scholar] [CrossRef] [PubMed]
- No, H.K.; Park, N.Y.; Lee, S.H.; Hwang, H.J.; Meyers, S.P. Antibacterial activities of chitosans and chitosan oligomers with different molecular weights on spoilage bacteria isolated from tofu. J. Food Sci. 2002, 67, 1511–1514. [Google Scholar] [CrossRef]
- Mellegard, H.; Strand, S.P.; Christensen, B.E.; Granum, P.E.; Hardy, S.P. Antibacterial activity of chemically defined chitosans: Influence of molecular weight, degree of acetylation and test organism. Int. J. Food Microbiol. 2011, 148, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Sellimi, S.; Rinaudo, M.; Jellouli, K.; Nasri, M. Influence of acetylation degree and molecular weight of homogeneous chitosans on antibacterial and antifungal activities. Int. J. Food Microbiol. 2014, 185, 57–63. [Google Scholar] [CrossRef]
- Omura, Y.; Shigemoto, M.; Akiyama, T.; Saimoto, H.; Shigemasa, Y.; Nakamura, I.; Tsuchido, T. Atimicrobial Activity of Chtiosan wtih Different Derees of aceylation and Moelcular Weights. Biocontrol. Sci. 2003, 8, 25–30. [Google Scholar] [CrossRef]
- Sahariah, P.; Masson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure-Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Rúnarsson, Ö.V.; Holappa, J.; Nevalainen, T.; Hjálmarsdóttir, M.; Järvinen, T.; Loftsson, T.; Einarsson, J.M.; Jónsdóttir, S.; Valdimarsdótti, M.; Másson, M. Antibacterial activity of methylated chitosan and chitooligomer derivatives: Synthesis and structure activity relationships. Eur. Polym. J. 2007, 43, 2660–2671. [Google Scholar] [CrossRef]
- Rúnarsson, Ö.V.; Holappa, J.; Malainer, C.; Steinsson, H.; Hjálmarsdóttir, M.; Nevalainen, T.; Másson, M. Antibacterial Activity of N-Quaternary Chitosan Derivatives: Synthesis, Characterization, and Structure Activity Relationship Investigations (SAR). Eur. Polym. J. 2010, 46, 1251–1267. [Google Scholar] [CrossRef]
- Sahariah, P.; Gaware, V.S.; Lieder, R.; Jónsdóttir, S.; Hjálmarsdóttir, M.Á.; Sigurjonsson, O.E.; Másson, M. The Effect of Substituent, Degree of Acetylation and Positioning of the Cationic Charge on the Antibacterial Activity of Quaternary Chitosan Derivatives. Mar. Drugs 2014, 12, 4635–4658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.T.; Xing, R.G.; Liu, S.; Yu, H.H.; Li, K.C.; Qin, Y.K.; Li, P.C. Molecular weight and pH effects of aminoethyl modified chitosan on antibacterial activity in vitro. Int. J. Biol. Macromol. 2012, 50, 918–924. [Google Scholar] [CrossRef]
- Sahariah, P.; Snorradottir, B.S.; Hjalmarsdottir, M.A.; Sigurjonsson, O.E.; Masson, M. Experimental design for determining quantitative structure activity relationship for antibacterial chitosan derivatives. J. Mater. Chem. B 2016, 4, 4762–4770. [Google Scholar] [CrossRef]
- Sahariah, P.; Sorensen, K.K.; Hjalmarsdottir, M.A.; Sigurjonsson, O.E.; Jensen, K.J.; Masson, M.; Thygesen, M.B. Antimicrobial peptide shows enhanced activity and reduced toxicity upon grafting to chitosan polymers. Chem. Commun. 2015, 51, 11611–11614. [Google Scholar] [CrossRef]
- Rúnarsson, Ö.V.; Holappa, J.; Jónsdóttir, S.; Steinsson, H.; Másson, M. N-Selective ‘one pot’ Synthesis of Highly N-Substituted Trimethyl Chitosan (TMC). Carbohyd. Polym. 2008, 74, 740–744. [Google Scholar] [CrossRef]
- Skjak-Braek, G.; Anthonsen, T.; Sandford, P.A. Chitin and Chitosan: Sources, Chemistry, Biochemistry, Phusical Properties and Applications; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1989. [Google Scholar]
- Domard, A.; Cartier, N. Glucosamine Oligomers.1. Preparation and Characterization. Int. J. Biol. Macromol. 1989, 11, 297–302. [Google Scholar] [CrossRef]
- Knill, C.J.; Kennedy, J.F.; Mistry, J.; Miraftab, M.; Smart, G.; Groocock, M.R.; Williams, H.J. Acid hydrolysis of commercial chitosans. J. Chem. Technol. Biotechnol. 2005, 80, 1291–1296. [Google Scholar] [CrossRef]
- Horton, D.; Philips, K.D. Nitrous-acid Deamination of Glycosides and Acetates of 2-Amino-2-Deoxy-d-Glucose. Carbohyd. Res. 1973, 30, 367–374. [Google Scholar] [CrossRef]
- Nagasawa, K.; Tanoura, N. Reaction between carbohydrates and sulfuric-acid. 2. depolymerization and sulfation of chitosan by sulfuric-acid. Chem. Pharm. Bull. 1972, 20, 157–162. [Google Scholar] [CrossRef]
- Defaye, J.; Gadelle, A.; Pedersen, C. A convenient access to beta-(1-4)-linked amino-2-deoxy-d-glucopyranosyl fluoride oligosaccharides and beta-(1-4)-linked 2-amino-2-deoxy-d-glucopyranosyl oligosaccharides by fluorolysis and fluorohydrolysis of chitosan. Carbohyd. Res. 1994, 261, 267–277. [Google Scholar] [CrossRef]
- Tsao, C.T.; Chang, C.H.; Lin, Y.Y.; Wu, M.F.; Han, J.L.; Hsieh, K.H. Kinetic study of acid depolymerization of chitosan and effects of low molecular weight chitosan on erythrocyte rouleaux formation. Carbohyd. Res. 2011, 346, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Benediktsdóttir, B.E.; Baldursson, Ó.; Másson, M. Challenges in evaluation of chitosan and trimethylated chitosan (TMC) as mucosal permeation enhancers: From synthesis to in vitro application. J. Control. Release 2014, 173, 18–31. [Google Scholar] [CrossRef]
- Stawski, D.; Sahariah, P.; Hjalmarsdottir, M.; Wojciechowska, D.; Puchalski, M.; Masson, M. N,N,N-trimethyl chitosan as an efficient antibacterial agent for polypropylene and polylactide nonwovens. J. Text. Inst. 2017, 108, 1041–1049. [Google Scholar] [CrossRef]






| Reaction Time (h) | Acid Hydrolysis of TMC | Acid Hydrolysis of Chitosan | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 M HCl (60 °C) | Conc HCl (30 °C) | 1 M HCl (60 °C) | Conc HCl (30 °C) | |||||
| Mw (kDa) | Dispersity | Mw (kDa) | Dispersity | Mw (kDa) | Dispersity | Mw (kDa) | Dispersity | |
| 0.25 | 144 | 2.05 | 98 | 1.93 | 128 | 1.44 | 78 | 1.75 |
| 0.5 | 126 | 2.22 | 72 | 1.83 | 116 | 1.43 | 65 | 1.65 |
| 1 | 112 | 2.06 | 48 | 1.40 | 106 | 1.32 | 57 | 1.61 |
| 2 | 104 | 2.24 | 37 | 1.49 | 96 | 1.35 | 50 | 1.55 |
| 4 | 103 | 2.26 | 26 | 1.34 | 82 | 1.62 | 48 | 1.55 |
| 8 | 95 | 2.51 | 21 | 1.38 | 79 | 1.28 | 34 | 1.40 |
| 24 | 89 | 1.94 | 10 | 2.01 | 61 | 1.47 | 27 | 1.39 |
| 48 | 85 | 2.51 | 8 | 1.44 | 56 | 1.51 | 24 | 1.23 |
| 72 | 79 | 2.26 | 6 | 1.43 | 54 | 1.42 | 18 | 1.17 |
| 120 | 66 | 1.92 | 3 | 1.30 | 50 | 1.35 | 18 | 1.13 |
| 216 | 60 | 1.90 | 3 | 1.44 | 35 | 1.40 | 14 | 1.07 |
| 384 | – | – | 2 | 1.07 | – | – | 10 | 1.05 |
| 528 | – | – | 2 | 1.05 | – | – | 4 | 1.05 |
| 884 | – | – | – | – | – | – | 3 | 1.02 |
| 1388 | – | – | – | – | – | – | 2 | 1.03 |
| TMC | CHITOSAN | ||
|---|---|---|---|
| Mw (kDa) | MIC (g/mL) | Mw (kDa) | MIC (g/mL) |
| 186 | 32 × 10−6 | 225 | 4.1 × 10−3 |
| 144 | 32 × 10−6 | 128 | 4.1 × 10−3 |
| 136 | 32 × 10−6 | 96 | 4.1 × 10−3 |
| 114 | 64 × 10−6 | 82 | 4.1 × 10−3 |
| 104 | 32 × 10−6 | 79 | 4.1 × 10−3 |
| 103 | 32 × 10−6 | 71 | 4.1 × 10−3 |
| 98 | 32 × 10−6 | 65 | 4.1 × 10−3 |
| 88 | 32 × 10−6 | 57 | 4.1 × 10−3 |
| 69 | 16 × 10−6 | 54 | 4.1 × 10−3 |
| 66 | 32 × 10−6 | 50 | 4.1 × 10−3 |
| 60 | 64 × 10−6 | 35 | 8.2 × 10−3 |
| 48 | 32 × 10−6 | 27 | 8.2 × 10−3 |
| 37 | 32 × 10−6 | 18 | 8.2 × 10−3 |
| 26 | 64 × 10−6 | 14 | 8.2 × 10−3 |
| 21 | 32 × 10−6 | 10 | 8.2 × 10−3 |
| 10 | 128 × 10−6 | 4 | ≥32.8 × 10−3 |
| 8 | 256 × 10−6 | 3 | ≥32.8× 10−3 |
| 6 | 512 × 10−6 | 2 | ≥32.8 × 10−3 |
| 4 | 4096 × 10−6 | 0.19 (glucosamine) | ≥32.768 × 10−3 |
| 3 | ≥32,768 × 10−6 | ||
| Gentamicin (positive control) | 0.5 × 10−6 | 1 × 10−6 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahariah, P.; Cibor, D.; Zielińska, D.; Hjálmarsdóttir, M.Á.; Stawski, D.; Másson, M. The Effect of Molecular Weight on the Antibacterial Activity of N,N,N-Trimethyl Chitosan (TMC). Int. J. Mol. Sci. 2019, 20, 1743. https://doi.org/10.3390/ijms20071743
Sahariah P, Cibor D, Zielińska D, Hjálmarsdóttir MÁ, Stawski D, Másson M. The Effect of Molecular Weight on the Antibacterial Activity of N,N,N-Trimethyl Chitosan (TMC). International Journal of Molecular Sciences. 2019; 20(7):1743. https://doi.org/10.3390/ijms20071743
Chicago/Turabian StyleSahariah, Priyanka, Dorota Cibor, Dorota Zielińska, Martha Á. Hjálmarsdóttir, Dawid Stawski, and Már Másson. 2019. "The Effect of Molecular Weight on the Antibacterial Activity of N,N,N-Trimethyl Chitosan (TMC)" International Journal of Molecular Sciences 20, no. 7: 1743. https://doi.org/10.3390/ijms20071743
APA StyleSahariah, P., Cibor, D., Zielińska, D., Hjálmarsdóttir, M. Á., Stawski, D., & Másson, M. (2019). The Effect of Molecular Weight on the Antibacterial Activity of N,N,N-Trimethyl Chitosan (TMC). International Journal of Molecular Sciences, 20(7), 1743. https://doi.org/10.3390/ijms20071743