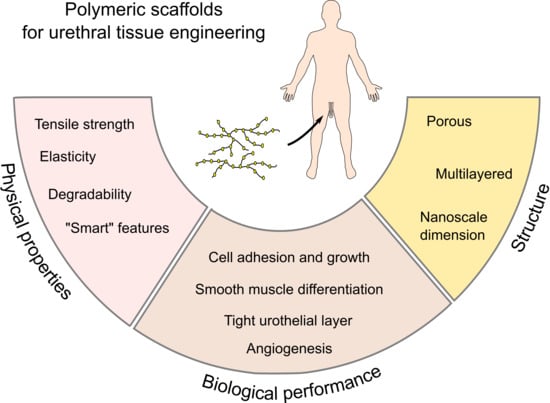
From Acellular Matrices to Smart Polymers: Degradable Scaffolds that are Transforming the Shape of Urethral Tissue Engineering
Abstract
:1. Introduction
2. Structural and Functional Properties of the Male Urethra
3. Polymeric Biomaterials in Urethral Tissue Engineering
4. In Vivo Performance of Biodegradable Scaffolds for Urethral Reconstruction
5. Future Directions: Hybrid and Smart Polymers
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Lazzeri, M.; Sansalone, S.; Guazzoni, G.; Barbagli, G. Incidence, Causes, and Complications of Urethral Stricture Disease. Eur. Urol. Suppl. 2016, 15, 2–6. [Google Scholar] [CrossRef]
- Keays, M.A.; Dave, S. Current hypospadias management: Diagnosis, surgical management, and long-term patient-centred outcomes. Can. Urol. Assoc. J. 2017, 11, S48–S53. [Google Scholar] [CrossRef] [Green Version]
- Chapple, C.; Andrich, D.; Atala, A.; Barbagli, G.; Cavalcanti, A.; Kulkarni, S.; Mangera, A.; Nakajima, Y. SIU/ICUD Consultation on Urethral Strictures: The Management of Anterior Urethral Stricture Disease Using Substitution Urethroplasty. Urology 2014, 83, S31–S47. [Google Scholar] [CrossRef]
- Snodgrass, W.; Bush, N. Primary hypospadias repair techniques: A review of the evidence. Urol. Ann. 2016, 8, 403–408. [Google Scholar] [CrossRef]
- Fenton, A.S.; Morey, A.F.; Aviles, R.; Garcia, C.R. Anterior urethral strictures: Etiology and characteristics. Urology 2005, 65, 1055–1058. [Google Scholar] [CrossRef]
- Mundy, A.R.; Andrich, D.E. Urethral strictures. BJU Int. 2011, 107, 6–26. [Google Scholar] [CrossRef]
- Caldamone, A.A.; Edstrom, L.E.; Koyle, M.A.; Rabinowitz, R.; Hulbert, W.C. Buccal mucosal grafts for urethral reconstruction. Urology 1998, 51, 15–19. [Google Scholar] [CrossRef]
- Barbagli, G.; Palminteri, E.; Guazzoni, G.; Montorsi, F.; Turini, D.; Lazzeri, M. Bulbar urethroplasty using buccal mucosa grafts placed on the ventral, dorsal or lateral surface of the urethra: Are results affected by the surgical technique? J. Urol. 2005, 174, 955–958. [Google Scholar] [CrossRef]
- Ding, J.; Li, Q.; Li, S.; Li, F.; Zhou, C.; Zhou, Y.; Hu, J.; Xie, L.; Cao, Y.; Zhang, S. Ten years’ experience for hypospadias repair: Combined buccal mucosa graft and local flap for urethral reconstruction. Urol. Int. 2014, 93, 454–459. [Google Scholar] [CrossRef]
- Markiewicz, M.R.; DeSantis, J.L.; Margarone, J.E.; Pogrel, M.A.; Chuang, S.-K. Morbidity Associated with Oral Mucosa Harvest for Urological Reconstruction: An Overview. J. Oral Maxillofac. Surg. 2008, 66, 739–744. [Google Scholar] [CrossRef]
- Harris, C.R.; Osterberg, E.C.; Sanford, T.; Alwaal, A.; Gaither, T.W.; McAninch, J.W.; McCulloch, C.E.; Breyer, B.N. National Variation in Urethroplasty Cost and Predictors of Extreme Cost: A Cost Analysis with Policy Implications. Urology 2016, 94, 246–254. [Google Scholar] [CrossRef] [Green Version]
- Abbas, T.O.; Mahdi, E.; Hasan, A.; AlAnsari, A.; Pennisi, C.P. Current Status of Tissue Engineering in the Management of Severe Hypospadias. Front. Pediatr. 2018, 5, 283. [Google Scholar] [CrossRef]
- De Kemp, V.; De Graaf, P.; Fledderus, J.O.; Bosch, J.L.H.R.; De Kort, L.M.O. Tissue engineering for human urethral reconstruction: Systematic review of recent literature. PLoS ONE 2015, 10, e0118653. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Xu, Y.-M.; Fu, Q.; Zhu, W.-D.; Cui, L.; Chen, J. Evaluation of the biocompatibility and mechanical properties of naturally derived and synthetic scaffolds for urethral reconstruction. J. Biomed. Mater. Res. Part A 2010, 94A, 317–325. [Google Scholar] [CrossRef]
- Beiko, D.T.; Knudsen, B.E.; Watterson, J.D.; Cadieux, P.A.; Reid, G.; Denstedt, J.D. Urinary tract biomaterials. J. Urol. 2004, 171, 2438–2444. [Google Scholar] [CrossRef]
- de Graaf, P.; van der Linde, E.M.; Rosier, P.F.W.M.; Izeta, A.; Sievert, K.-D.; Bosch, J.L.H.R.; de Kort, L.M.O. Systematic Review to Compare Urothelium Differentiation with Urethral Epithelium Differentiation in Fetal Development, as a Basis for Tissue Engineering of the Male Urethra. Tissue Eng. Part B Rev. 2017, 23, 257–267. [Google Scholar] [CrossRef]
- Da Silva, E.A.; Sampaio, F.J.B.; Ortiz, V.; Cardoso, L.E.M. Regional differences in the extracellular matrix of the human spongy urethra as evidenced by the composition of glycosaminoglycans. J. Urol. 2002, 167, 2183–2187. [Google Scholar] [CrossRef]
- De Graaf, P.; Ramadan, R.; Linssen, E.C.; Staller, N.A.; Hendrickx, A.P.A.; Pigot, G.L.S.; Meuleman, E.J.H.; Bouman, M.; Özer, M.; Bosch, J.L.H.R.; et al. The multilayered structure of the human corpus spongiosum. Histol. Histopathol. 2018, 33, 1335–1345. [Google Scholar] [PubMed]
- Coenen, A.M.J.; Bernaerts, K.V.; Harings, J.A.W.; Jockenhoevel, S.; Ghazanfari, S. Elastic materials for tissue engineering applications: Natural, synthetic, and hybrid polymers. Acta Biomater. 2018, 79, 60–82. [Google Scholar] [CrossRef]
- Augsburger, H.R. Elastic fibre system of the female canine urethra. Histochemical identification of elastic, elaunin and oxytalan fibres. Anat. Histol. Embryol. 1997, 26, 297–302. [Google Scholar] [CrossRef]
- Kim, R.J.; Kerns, J.M.; Liu, S.; Nagel, T.; Zaszczurynski, P.; Lin, D.L.; Damaser, M.S. Striated muscle and nerve fascicle distribution in the female raturethral sphincter. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2007, 290, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.H.; Wang, T.-J.; Tseng, G.-F.; Lee, Y.F.; Huang, Y.-S.; Chen, J.-R.; Cheng, C.-L. The Distribution of Muscles Fibers and Their Types in the Female Rat Urethra: Cytoarchitecture and Three-Dimensional Reconstruction. Anat. Rec. 2013, 296, 1640–1649. [Google Scholar] [CrossRef] [Green Version]
- Jankowski, R.J.; Prantil, R.L.; Fraser, M.O.; Chancellor, M.B.; de Groat, W.C.; Huard, J.; Vorp, D.A. Development of an experimental system for the study of urethral biomechanical function. Am. J. Physiol. Physiol. 2004, 286, F225–F232. [Google Scholar] [CrossRef]
- Watanabe, H.; Yamamoto, T.Y. Autonomic innervation of the muscles in the wall of the bladder and proximal urethra of male rats. J. Anat. 1979, 128, 873–886. [Google Scholar]
- Male Reproductive System. Available online: http://histology.medicine.umich.edu/slides/male-reproductive-system (accessed on 20 December 2018).
- Shirozu, H.; Koyanagi, T.; Takashima, T.; Horimoto, N.; Akazawa, K.; Nakano, H. Penile tumescence in the human fetus at term—A preliminary report. Early Hum. Dev. 1995, 41, 159–166. [Google Scholar] [CrossRef]
- Ohel, G.; Haddad, S.; Samueloff, A. Fetal Urine Production and Micturition and Fetal Behavioral State. Am. J. Perinatol. 1995, 12, 91–92. [Google Scholar] [CrossRef]
- Walter, J.S.; Wheeler, J.S.; Morgan, C.; Zaszczurynski, P.; Plishka, M. Measurement of total urethral compliance in females with stress incontinence. Neurourol. Urodyn. 1993, 12, 273–276. [Google Scholar] [CrossRef]
- Mijailovich, S.M.; Sullivan, M.P.; Yalla, S.V.; Venegas, J.G. Effect of urethral compliance on the steady state p-Q relationships assessed with a mechanical analog of the male lower urinary tract. Neurourol. Urodyn. 2007, 26, 234–246. [Google Scholar] [CrossRef]
- Thind, P.; Lose, G.; Colstrup, H. How to measure urethral elastance in a simple way. Elastance: Definition, determination and implications. Urol. Res. 1991, 19, 241–244. [Google Scholar] [CrossRef]
- Yalla, S.V.; Burros, H.M. Conduit and regional compliance of female canine urethra. Urology 1974, 4, 155–161. [Google Scholar] [CrossRef]
- Husmann, D.A.; Rathbun, S.R. Long-Term Followup of Visual Internal Urethrotomy for Management of Short (Less Than 1 Cm) Penile Urethral Strictures Following Hypospadias Repair. J. Urol. 2006, 176, 1738–1741. [Google Scholar] [CrossRef]
- Lalla, M.; Gregersen, H.; Olsen, L.H.; Jørgensen, T.M. In Vivo Biomechanical Assessment of Anterior Rabbit Urethra After Repair of Surgically Created Hypospadias. J. Urol. 2010, 184, 675–682. [Google Scholar] [CrossRef]
- Hammouda, H.M.; El-Ghoneimi, A.; Bagli, D.J.; McLorie, G.A.; Khoury, A.E. Tubularized Incised Plate Repair: Functional Outcome After Intermediate Followup. J. Urol. 2003, 169, 331–333. [Google Scholar] [CrossRef]
- Eassa, W.; Brzezinski, A.; Capolicchio, J.P.; Jednak, R.; El-Sherbiny, M. How do asymptomatic toilet-trained children void following tubularized incised-plate hypospadias repair? Can. Urol. Assoc. J. 2012, 6, 238–242. [Google Scholar] [CrossRef] [Green Version]
- Lalla, M.; Riis, C.; Jørgensen, C.S.; Danielsen, C.C.; Jørgensen, T.M. A biomechanical, histological and biochemical study in an experimental rabbit hypospadias repair model using scanning acoustic microscopy. J. Pediatr. Urol. 2011, 7, 404–411. [Google Scholar] [CrossRef]
- Gabrich, P.N.; Vasconcelos, J.S.P.; Damião, R.; Silva, E.A. Penile anthropometry in Brazilian children and adolescents. J. Pediatr. 2007, 83, 441–446. [Google Scholar] [CrossRef]
- Simsek, A.; Aldamanhori, R.; Chapple, C.R.; MacNeil, S. Overcoming scarring in the urethra: Challenges for tissue engineering. Asian J. Urol. 2018, 5, 69–77. [Google Scholar] [CrossRef]
- Liu, J.S.; Bury, M.I.; Fuller, N.J.; Sturm, R.M.; Ahmad, N.; Sharma, A.K. Bone Marrow Stem/Progenitor Cells Attenuate the Inflammatory Milieu Following Substitution Urethroplasty. Sci. Rep. 2016, 6, 35638. [Google Scholar] [CrossRef] [Green Version]
- Birder, L.; Andersson, K.-E. Urothelial Signaling. Physiol. Rev. 2013, 93, 653–680. [Google Scholar] [CrossRef] [Green Version]
- Akkad, T.; Pelzer, A.E.; Mitterberger, M.; Rehder, P.; Leonhartsberger, N.; Bartsch, G.; Strasser, H. Influence of intravesical potassium on pelvic floor activity in women with recurrent urinary tract infections: Comparative urodynamics might lead to enhanced detection of dysfunctional voiding. BJU Int. 2007, 100, 1071–1074. [Google Scholar] [CrossRef]
- Rajasekaran, M.; Stein, P.; Parsons, C.L. Toxic factors in human urine that injure urothelium. Int. J. Urol. 2006, 13, 409–414. [Google Scholar] [CrossRef] [Green Version]
- Adamowicz, J.; Kloskowski, T.; Tworkiewicz, J.; Pokrywczyńska, M.; Drewa, T. Urine Is a Highly Cytotoxic Agent: Does It Influence Stem Cell Therapies in Urology? Transplant. Proc. 2012, 44, 1439–1441. [Google Scholar] [CrossRef]
- Li, H.; Xu, Y.; Xie, H.; Li, C.; Song, L.; Feng, C.; Zhang, Q.; Xie, M.; Wang, Y.; Lv, X. Epithelial-differentiated adipose-derived stem cells seeded bladder acellular matrix grafts for urethral reconstruction: An animal model. Tissue Eng. Part A 2014, 20, 774–784. [Google Scholar] [CrossRef]
- Ribeiro-Filho, L.A.; Sievert, K.-D. Acellular matrix in urethral reconstruction. Adv. Drug Deliv. Rev. 2015, 82–83, 38–46. [Google Scholar] [CrossRef]
- Denstedt, J.; Atala, A. Biomaterials and Tissue Engineering in Urology; Woodhead Publishing: Sawston, UK, 2009; ISBN 9781845696375. [Google Scholar]
- Bisson, I.; Hilborn, J.; Wurm, F.; Meyrat, B.; Frey, P. Human urothelial cells grown on collagen adsorbed to surface-modified polymers. Urology 2002, 60, 176–180. [Google Scholar] [CrossRef]
- Azevedo, H.S.; Reis, R.L. Understanding the Enzymatic Degradation of Biodegradable Polymers and Strategies to Control Their Degradation Rate; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Babensee, J.E.; Anderson, J.M.; McIntire, L.V.; Mikos, A.G. Host response to tissue engineered devices. Adv. Drug Deliv. Rev. 1998, 33, 111–139. [Google Scholar] [CrossRef]
- Knecht, S.; Erggelet, C.; Endres, M.; Sittinger, M.; Kaps, C.; Stüssi, E. Mechanical testing of fixation techniques for scaffold-based tissue-engineered grafts. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 83, 50–57. [Google Scholar] [CrossRef]
- Perkins, L.E.L.E.L.; Kossuth, M.B.B.; Fox, J.C.C.; Rapoza, R.J.J. Paving the way to a bioresorbable technology: Development of the absorb BRS program. Catheter. Cardiovasc. Interv. 2016, 88, 1–9. [Google Scholar] [CrossRef]
- Moon, J.J.; West, J.L. Vascularization of engineered tissues: Approaches to promote angio-genesis in biomaterials. Curr. Top. Med. Chem. 2008, 8, 300–310. [Google Scholar]
- Nooeaid, P.; Salih, V.; Beier, J.P.; Boccaccini, A.R. Osteochondral tissue engineering: Scaffolds, stem cells and applications. J. Cell. Mol. Med. 2012, 16, 2247–2270. [Google Scholar] [CrossRef]
- Carmagnola, I.; Ranzato, E.; Chiono, V. Scaffold functionalization to support a tissue biocompatibility. In Functional 3D Tissue Engineering Scaffolds; Elsevier: Amsterdam, The Netherlands, 2018; pp. 255–277. [Google Scholar]
- Versteegden, L.R.M.; de Jonge, P.K.J.D.; IntHout, J.; van Kuppevelt, T.H.; Oosterwijk, E.; Feitz, W.F.J.; de Vries, R.B.M.; Daamen, W.F. Tissue Engineering of the Urethra: A Systematic Review and Meta-analysis of Preclinical and Clinical Studies. Eur. Urol. 2017, 72, 594–606. [Google Scholar] [CrossRef]
- Qi, N.; Li, W.; Tian, H. A Systematic Review of Animal and Clinical Studies on the Use of Scaffolds for Urethral Repair. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016, 36, 111–117. [Google Scholar] [CrossRef]
- Davis, N.F.; Cunnane, E.M.; O’Brien, F.J.; Mulvihill, J.J.; Walsh, M.T. Tissue engineered extracellular matrices (ECMs) in urology: Evolution and future directions. Surgeon 2018, 16, 55–65. [Google Scholar] [CrossRef]
- Badylak, S.F.; Lantz, G.C.; Coffey, A.; Geddes, L.A. Small intestinal submucosa as a large diameter vascular graft in the dog. J. Surg. Res. 1989, 47, 74–80. [Google Scholar] [CrossRef]
- Zhang, Y.; Kropp, B.P.; Moore, P.; Cowan, R.; Furness, P.D.; Kolligian, M.E.; Frey, P.; Cheng, E.Y. Coculture of bladder urothelial and smooth muscle cells on small intestinal submucosa: Potential applications for tissue engineering technology. J. Urol. 2000, 164, 928–934, discussion 934–935. [Google Scholar] [CrossRef]
- Fiala, R.; Vidlar, A.; Vrtal, R.; Belej, K.; Student, V. Porcine Small Intestinal Submucosa Graft for Repair of Anterior Urethral Strictures. Eur. Urol. 2007, 51, 1702–1708. [Google Scholar] [CrossRef]
- Palminteri, E.; Berdondini, E.; Colombo, F.; Austoni, E. Small Intestinal Submucosa (SIS) Graft Urethroplasty: Short-term Results. Eur. Urol. 2007, 51, 1695–1701. [Google Scholar] [CrossRef]
- Palminteri, E.; Berdondini, E.; Fusco, F.; De Nunzio, C.; Salonia, A. Long-term Results of Small Intestinal Submucosa Graft in Bulbar Urethral Reconstruction. Urology 2012, 79, 695–701. [Google Scholar] [CrossRef]
- Campodonico, F.; Benelli, R.; Michelazzi, A.; Ognio, E.; Toncini, C.; Maffezzini, M. Bladder Cell Culture on Small Intestinal Submucosa as Bioscaffold: Experimental Study on Engineered Urothelial Grafts. Eur. Urol. 2004, 46, 531–537. [Google Scholar] [CrossRef]
- Badylak, S.F.; Kropp, B.; McPherson, T.; Liang, H.; Snyder, P.W. Small Intestinal Submucosa: A Rapidly Resorbed Bioscaffold for Augmentation Cystoplasty in a Dog Model. Tissue Eng. 1998, 4, 379–387. [Google Scholar] [CrossRef]
- Kubricht, W.S.; Williams, B.J.; Eastham, J.A.; Venable, D.D. Tensile strength of cadaveric fascia lata compared to small intestinal submucosa using suture pull through analysis. J. Urol. 2001, 165, 486–490. [Google Scholar] [CrossRef]
- Weiser, A.C.; Franco, I.; Herz, D.B.; Silver, R.I.; Reda, E.F. Single Layered Small Intestinal Submucosa in the Repair of Severe Chordee and Complicated Hypospadias. J. Urol. 2003, 170, 1593–1595. [Google Scholar] [CrossRef]
- Hayn, M.H.; Bellinger, M.F.; Schneck, F.X. Small Intestine Submucosa as a Corporal Body Graft in the Repair of Severe Chordee. Urology 2009, 73, 277–279. [Google Scholar] [CrossRef]
- Zhang, L.; Du, A.; Li, J.; Pan, M.; Han, W.; Xiao, Y. Development of a cell-seeded modified small intestinal submucosa for urethroplasty. Heliyon 2016, 2, e00087. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.J.; Meng, J.; Oberpenning, F.; Atala, A. Bladder augmentation using allogenic bladder submucosa seeded with cells. Urology 1998, 51, 221–225. [Google Scholar] [CrossRef]
- Orabi, H.; Safwat, A.S.; Shahat, A.; Hammouda, H.M. The use of small intestinal submucosa graft for hypospadias repair: Pilot study. Arab J. Urol. 2013, 11, 415–420. [Google Scholar] [CrossRef]
- Hauser, S.; Bastian, P.J.; Fechner, G.; Müller, S.C. Small intestine submucosa in urethral stricture repair in a consecutive series. Urology 2006, 68, 263–266. [Google Scholar] [CrossRef]
- Nuininga, J.E.; van Moerkerk, H.; Hanssen, A.; Hulsbergen, C.A.; Oosterwijk-Wakka, J.; Oosterwijk, E.; de Gier, R.P.E.; Schalken, J.A.; van Kuppevelt, T.; Feitz, W.F.J. Rabbit urethra replacement with a defined biomatrix or small intestinal submucosa. Eur. Urol. 2003, 44, 266–271. [Google Scholar] [CrossRef]
- Chun, S.Y.; Kim, B.S.; Kwon, S.Y.; Park, S.I.; Song, P.H.; Yoo, E.S.; Kim, B.W.; Kwon, T.G.; Kim, H.T. Urethroplasty using autologous urethral tissue-embedded acellular porcine bladder submucosa matrix grafts for the management of long-segment urethral stricture in a rabbit model. J. Korean Med. Sci. 2015, 30, 301–307. [Google Scholar] [CrossRef]
- Huang, J.-W.; Xie, M.-K.; Zhang, Y.; Wei, G.-J.; Li, X.; Li, H.-B.; Wang, J.-H.; Zhu, W.-D.; Li, C.; Xu, Y.-M.; et al. Reconstruction of Penile Urethra With the 3-Dimensional Porous Bladder Acellular Matrix in a Rabbit Model. Urology 2014, 84, 1499–1505. [Google Scholar] [CrossRef]
- Li, C.; Xu, Y.-M.; Song, L.-J.; Fu, Q.; Cui, L.; Yin, S. Urethral Reconstruction Using Oral Keratinocyte Seeded Bladder Acellular Matrix Grafts. J. Urol. 2008, 180, 1538–1542. [Google Scholar] [CrossRef]
- Atala, A.; Guzman, L.; Retik, A.B. A novel inert collagen matrix for hypospadias repair. J. Urol. 1999, 162, 1148–1151. [Google Scholar] [CrossRef]
- Gu, G.-L.; Xia, S.-J.; Zhang, J.; Liu, G.-H.; Yan, L.; Xu, Z.-H.; Zhu, Y.-J. Tubularized Urethral Replacement Using Tissue-Engineered Peritoneum-Like Tissue in a Rabbit Model. Urol. Int. 2012, 89, 358–364. [Google Scholar] [CrossRef]
- Roth, C.C.; Kropp, B.P. Recent advances in urologic tissue engineering. Curr. Urol. Rep. 2009, 10, 119–125. [Google Scholar] [CrossRef]
- Xie, M.; Song, L.; Wang, J.; Fan, S.; Zhang, Y.; Xu, Y. Evaluation of stretched electrospun silk fibroin matrices seeded with urothelial cells for urethra reconstruction. J. Surg. Res. 2013, 184, 774–781. [Google Scholar] [CrossRef]
- Xie, M.; Xu, Y.; Song, L.; Wang, J.; Lv, X.; Zhang, Y. Tissue-engineered buccal mucosa using silk fibroin matrices for urethral reconstruction in a canine model. J. Surg. Res. 2014, 188, 1–7. [Google Scholar] [CrossRef]
- Hudson, S.M. Silk polymers: Material science and biotechnology, D. Kaplan, W. Adams, B. Farmer and C. Viney, eds. American Chemical Society, ACS Symposium Series 544 (1944). 370 pp. Polym. Adv. Technol. 1995, 6, 717. [Google Scholar] [CrossRef]
- Cunniff, P.M.; Fossey, S.A.; Auerbach, M.A.; Song, J.W.; Kaplan, D.L.; Adams, W.W.; Eby, R.K.; Mahoney, D.; Vezie, D.L. Mechanical and thermal properties of dragline silk from the spider Nephila clavipes. Polym. Adv. Technol. 1994, 5, 401–410. [Google Scholar] [CrossRef]
- Wang, Y.; Rudym, D.D.; Walsh, A.; Abrahamsen, L.; Kim, H.-J.; Kim, H.S.; Kirker-Head, C.; Kaplan, D.L. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials 2008, 29, 3415–3428. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Panilaitis, B.; Altman, G.H.; Chen, J.; Jin, H.J.; Karageorgiou, V.; Kaplan, D.L. Macrophage responses to silk. Biomaterials 2003, 24, 3079–3085. [Google Scholar] [CrossRef]
- Chung, Y.G.; Tu, D.; Franck, D.; Gil, E.S.; Algarrahi, K.; Adam, R.M.; Kaplan, D.L.; Estrada, C.R.; Mauney, J.R. Acellular bi-layer silk fibroin scaffolds support tissue regeneration in a rabbit model of onlay urethroplasty. PLoS ONE 2014, 9, e91592. [Google Scholar] [CrossRef]
- Ribeiro, C.; Sencadas, V.; Correia, D.M.; Lanceros-Méndez, S. Piezoelectric polymers as biomaterials for tissue engineering applications. Colloids Surf. B Biointerfaces 2015, 136, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, M.R.; San Román, J. Introduction to smart polymers and their applications. In Smart Polymers and their Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–11. [Google Scholar]
- Knipe, J.M.; Peppas, N.A. Multi-responsive hydrogels for drug delivery and tissue engineering applications. Regen. Biomater. 2014, 1, 57–65. [Google Scholar] [CrossRef]
- Kowalski, P.S.; Bhattacharya, C.; Afewerki, S.; Langer, R. Smart Biomaterials: Recent Advances and Future Directions. ACS Biomater. Sci. Eng. 2018, 4, 3809–3817. [Google Scholar] [CrossRef]
- Anderson, D.G.; Burdick, J.A.; Langer, R. MATERIALS SCIENCE: Smart Biomaterials. Science 2004, 305, 1923–1924. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Matsunaga, Y.T. Thermo-responsive polymers and their application as smart biomaterials. J. Mater. Chem. B 2017, 5, 4307–4321. [Google Scholar] [CrossRef]
- Jochum, F.D.; Theato, P. Temperature- and light-responsive smart polymer materials. Chem. Soc. Rev. 2013, 42, 7468–7483. [Google Scholar] [CrossRef]
- Tsuda, Y.; Shimizu, T.; Yamato, M.; Kikuchi, A.; Sasagawa, T.; Sekiya, S.; Kobayashi, J.; Chen, G.; Okano, T. Cellular control of tissue architectures using a three-dimensional tissue fabrication technique. Biomaterials 2007, 28, 4939–4946. [Google Scholar] [CrossRef]
- Shiroyanagi, Y.; Yamato, M.; Yamazaki, Y.; Toma, H.; Okano, T. Transplantable Urothelial Cell Sheets Harvested Noninvasively from Temperature-Responsive Culture Surfaces by Reducing Temperature. Tissue Eng. 2003, 9, 1005–1012. [Google Scholar] [CrossRef]
- Rickert, D.; Moses, M.A.; Lendlein, A.; Kelch, S.; Franke, R.-P. The importance of angiogenesis in the interaction between polymeric biomaterials and surrounding tissue. Clin. Hemorheol. Microcirc. 2003, 28, 175–181. [Google Scholar]
- Correia, C.O.; Leite, Á.J.; Mano, J.F. Chitosan/bioactive glass nanoparticles scaffolds with shape memory properties. Carbohydr. Polym. 2015, 123, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Tzoneva, R.; Seifert, B.; Behl, M.; Lendlein, A. Elastic multiblock copolymers for vascular regeneration: Protein adsorption and hemocompatibility. Clin. Hemorheol. Microcirc. 2012, 52, 337–348. [Google Scholar]
- Hardy, J.G.; Palma, M.; Wind, S.J.; Biggs, M.J. Responsive Biomaterials: Advances in Materials Based on Shape-Memory Polymers. Adv. Mater. 2016, 28, 5717–5724. [Google Scholar] [CrossRef] [PubMed]
- Roelofs, L.A.J.; Oosterwijk, E.; Kortmann, B.B.M.; Daamen, W.F.; Tiemessen, D.M.; Brouwer, K.M.; Eggink, A.J.; Crevels, A.J.; Wijnen, R.M.H.; van Kuppevelt, T.H.; et al. Bladder Regeneration Using a Smart Acellular Collagen Scaffold with Growth Factors VEGF, FGF2 and HB-EGF. Tissue Eng. Part A 2016, 22, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Jin, Y.; Sun, Y.; Wang, D.; Sun, J.; Wang, Z.; Zhang, W.; Jiang, X. A strategy for depositing different types of cells in three dimensions to mimic tubular structures in tissues. Adv. Mater. 2012, 24, 890–896. [Google Scholar] [CrossRef]
- Smolar, J.; Salemi, S.; Horst, M.; Sulser, T.; Eberli, D. Stem Cells in Functional Bladder Engineering. Transfus. Med. Hemother. 2016, 43, 328–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardy, J.G.; Cornelison, R.C.; Sukhavasi, R.C.; Saballos, R.J.; Vu, P.; Kaplan, D.L.; Schmidt, C.E. Electroactive Tissue Scaffolds with Aligned Pores as Instructive Platforms for Biomimetic Tissue Engineering. Bioengineering 2015, 2, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, L.; Guo, B.; Shao, Y.; Ma, P.X. Electroactive biodegradable polyurethane significantly enhanced Schwann cells myelin gene expression and neurotrophin secretion for peripheral nerve tissue engineering. Biomaterials 2016, 87, 18–31. [Google Scholar] [CrossRef]
- StÖlting, M.N.L.; Arnold, A.S.; Haralampieva, D.; Handschin, C.; Sulser, T.; Eberli, D. Magnetic stimulation supports muscle and nerve regeneration after trauma in mice. Muscle Nerve 2016, 53, 598–607. [Google Scholar] [CrossRef] [Green Version]
- Orabi, H.; Bouhout, S.; Morissette, A.; Rousseau, A.; Chabaud, S.; Bolduc, S. Tissue engineering of urinary bladder and urethra: Advances from bench to patients. Sci. World J. 2013, 2013, 154564. [Google Scholar] [CrossRef] [PubMed]
- Dodat, H.; Landry, J.-L.; Szwarc, C.; Culem, S.; Murat, F.-J.; Dubois, R. Spongioplasty and separation of the corpora cavernosa for hypospadias repair. BJU Int. 2003, 91, 528–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhat, A.; Sabharwal, K.; Bhat, M.; Saran, R.; Singla, M.; Kumar, V. Outcome of tubularized incised plate urethroplasty with spongioplasty alone as additional tissue cover: A prospective study. Indian J. Urol. 2014, 30, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, S.; Delaage, P.-H.; Bargy, F. Anatomical basis of surgical repair of hypospadias by spongioplasty. Surg. Radiol. Anat. 2000, 22, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Mauney, J.R.; Adam, R.M. Dynamic reciprocity in cell–scaffold interactions. Adv. Drug Deliv. Rev. 2015, 82–83, 77–85. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, T.O.; Yalcin, H.C.; Pennisi, C.P. From Acellular Matrices to Smart Polymers: Degradable Scaffolds that are Transforming the Shape of Urethral Tissue Engineering. Int. J. Mol. Sci. 2019, 20, 1763. https://doi.org/10.3390/ijms20071763
Abbas TO, Yalcin HC, Pennisi CP. From Acellular Matrices to Smart Polymers: Degradable Scaffolds that are Transforming the Shape of Urethral Tissue Engineering. International Journal of Molecular Sciences. 2019; 20(7):1763. https://doi.org/10.3390/ijms20071763
Chicago/Turabian StyleAbbas, Tariq O., Huseyin C. Yalcin, and Cristian P. Pennisi. 2019. "From Acellular Matrices to Smart Polymers: Degradable Scaffolds that are Transforming the Shape of Urethral Tissue Engineering" International Journal of Molecular Sciences 20, no. 7: 1763. https://doi.org/10.3390/ijms20071763
APA StyleAbbas, T. O., Yalcin, H. C., & Pennisi, C. P. (2019). From Acellular Matrices to Smart Polymers: Degradable Scaffolds that are Transforming the Shape of Urethral Tissue Engineering. International Journal of Molecular Sciences, 20(7), 1763. https://doi.org/10.3390/ijms20071763