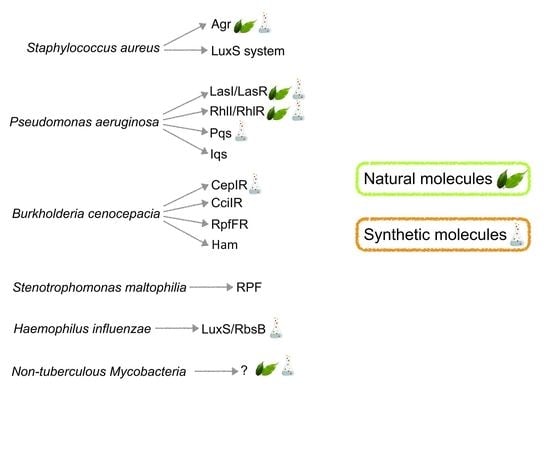
Quorum Sensing as Antivirulence Target in Cystic Fibrosis Pathogens
Abstract
:1. Introduction
2. Staphylococcus aureus
2.1. S. aureus Infections in cystic fibrosis
2.2. Quorum Sensing Systems of S. aureus
2.2.1. The Agr System
2.2.2. The LuxS System
2.3. Molecules Targeting QS in S. aureus
2.3.1. Natural Molecules
Generic QS Inhibitors
Biofilm Inhibitors
Agr Inhibitors
δ-Toxin Production Inhibitors
2.3.2. Synthetic Molecules
Generic QS Inhibitors
Biofilm Inhibitors
Agr Inhibitors
SarA Inhibitors
3. Pseudomonas aeruginosa
3.1. P. aeruginosa Infections in cystic fibrosis
3.2. Quorum Sensing Systems of P. aeruginosa
3.3. Molecules Targeting QS in P. aeruginosa
3.3.1. Natural Products
3.3.2. Synthetic Molecules
Inhibitor of LasIR QS System
3.3.3. Inhibitors of rhl Quorum Sensing System
3.3.4. Inhibition of PqsR
Inhibitors of PQS Biosynthesis
3.3.5. “PAN-INHIBITORS” of QS
4. Burkholderia cepacia
4.1. B. cepacia Infections in cystic fibrosis
4.2. Quorum Sensing Systems of B. cepacia
4.3. Molecules Targeting QS in B. cenocepacia
4.3.1. Natural Molecules
4.3.2. Synthetic Molecules
5. Emerging CF Pathogens
5.1. Stenotrophomonas maltophilia
5.1.1. S. maltophilia Infections in cystic fibrosis
5.1.2. Quorum Sensing Systems of S. maltophilia
5.1.3. Molecules Targeting QS in S. maltophilia
5.2. Haemophilus Influenzae
5.2.1. H. influenzae Infections in cystic fibrosis
5.2.2. Quorum Sensing Systems of H. influenzae
5.2.3. Molecules Targeting QS in H. influenzae
5.3. Non-Tuberculous Mycobacteria (NTM)
5.3.1. NTM Infections in cystic fibrosis
5.3.2. Quorum Sensing Systems of NTM
5.3.3. Molecules Targeting QS in NTM
6. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AHL | Acyl Homoserine Lactone |
| CF | Cystic Fibrosis |
| DSF | Diffusible Signal Factor |
| NTM | Non-tuberculous Mycobacteria |
| QS | Quorum sensing |
| QSI | Quorum sensing inhibitor |
References
- Riordan, J.R.; Rommens, J.M.; Kerem, B.; Alon, N.; Rozmahel, R.; Grzelczak, Z.; Zielenski, J.; Lok, S.; Plavsic, N.; Chou, J.L. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science 1989, 245, 1066–1073. [Google Scholar] [CrossRef]
- Döring, G.; Gulbins, E. Cystic fibrosis and innate immunity: How chloride channel mutations provoke lung disease. Cell Microbiol. 2009, 11, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.L.; Burns, J.L.; Ramsey, B.W. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003, 168, 918–951. [Google Scholar] [CrossRef] [PubMed]
- Lipuma, J.J. The changing microbial epidemiology in cystic fibrosis. Clin. Microbiol. Rev. 2010, 23, 299–323. [Google Scholar] [CrossRef] [PubMed]
- Munguia, J.; Nizet, V. Pharmacological Targeting of the Host-Pathogen Interaction: Alternatives to Classical Antibiotics to Combat Drug-Resistant Superbugs. Trends Pharmacol. Sci. 2017, 38, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Dickey, S.W.; Cheung, G.Y.C.; Otto, M. Different drugs for bad bugs: Antivirulence strategies in the age of antibiotic resistance. Nat. Rev. Drug Discov. 2017, 16, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Defoirdt, T. Quorum-Sensing Systems as Targets for Antivirulence Therapy. Trends Microbiol. 2018, 26, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef]
- Ji, G.; Pei, W.; Zhang, L.; Qiu, R.; Lin, J.; Benito, Y.; Lina, G.; Novick, R.P. Staphylococcus intermedius produces a functional agr autoinducing peptide containing a cyclic lactone. J. Bacteriol. 2005, 187, 3139–3150. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Gamby, S.; Zheng, Y.; Sintim, H.O. Small molecule inhibitors of AI-2 signaling in bacteria: State-of-the-art and future perspectives for anti-quorum sensing agents. Int. J. Mol. Sci. 2013, 14, 17694–17728. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Zhang, L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 2015, 6, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Boon, C.; Eberl, L.; Zhang, L.H. Differential modulation of Burkholderia cenocepacia virulence and energy metabolism by the quorum-sensing signal BDSF and its synthase. J. Bacteriol. 2009, 191, 7270–7278. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wu, J.; Eberl, L.; Zhang, L.H. Structural and functional characterization of diffusible signal factor family quorum-sensing signals produced by members of the Burkholderia cepacia complex. Appl. Environ. Microbiol. 2010, 76, 4675–4683. [Google Scholar] [CrossRef] [PubMed]
- LaSarre, B.; Federle, M.J. Exploiting quorum sensing to confuse bacterial pathogens. Microbiol. Mol. Biol. Rev. 2013, 77, 73–111. [Google Scholar] [CrossRef] [PubMed]
- Monte, J.; Abreu, A.C.; Borges, A.; Simões, L.C.; Simões, M. Antimicrobial Activity of Selected Phytochemicals against Escherichia coli and Staphylococcus aureus and Their Biofilms. Pathogens 2014, 3, 473–498. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Singh, B.N.; Deepak, D.; Rawat, A.K.; Singh, B.R. Colostrum hexasaccharide, a novel Staphylococcus aureus quorum-sensing inhibitor. Antimicrob. Agents Chemother. 2015, 59, 2169–2178. [Google Scholar] [CrossRef] [PubMed]
- Steindler, L.; Venturi, V. Detection of quorum-sensing N-acyl homoserine lactone signal molecules by bacterial biosensors. FEMS Microbiol. Lett. 2007, 266, 1–9. [Google Scholar] [CrossRef]
- Al Akeel, R.; Mateen, A.; Syed, R. An alanine-rich peptide attenuates quorum sensing-regulated virulence and biofilm formation in Staphylococcus aureus. J. AOAC Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Chen, A.; Shi, B.; Min, X.; Zhang, T.; Dong, Z.; Yang, H.; Chen, X.; Tian, Y.; Chen, Z. Preliminary study on the effect of brazilin on biofilms of Staphylococcus aureus. Exp. Ther. Med. 2018, 16, 2108–2118. [Google Scholar] [CrossRef]
- Brackman, G.; Hillaert, U.; Van Calenbergh, S.; Nelis, H.J.; Coenye, T. Use of quorum sensing inhibitors to interfere with biofilm formation and development in Burkholderia multivorans and Burkholderia cenocepacia. Res. Microbiol. 2009, 160, 144–151. [Google Scholar] [CrossRef]
- Zhang, Y.; Sass, A.; Van Acker, H.; Wille, J.; Verhasselt, B.; Van Nieuwerburgh, F.; Kaever, V.; Crabbé, A.; Coenye, T. Coumarin Reduces Virulence and Biofilm Formation in Pseudomonas aeruginosa by Affecting Quorum Sensing, Type III Secretion and C-di-GMP Levels. Front. Microbiol. 2018, 9, 1952. [Google Scholar] [CrossRef]
- Luo, J.; Dong, B.; Wang, K.; Cai, S.; Liu, T.; Cheng, X.; Lei, D.; Chen, Y.; Li, Y.; Kong, J.; et al. Baicalin inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model. PLoS ONE 2017, 12, e0176883. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, H.F.; Vos, M.C.; Ott, A.; van Belkum, A.; Voss, A.; Kluytmans, J.A.; van Keulen, P.H.; Vandenbroucke-Grauls, C.M.; Meester, M.H.; Verbrugh, H.A. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 2004, 364, 703–705. [Google Scholar] [CrossRef]
- Seybold, U.; Kourbatova, E.V.; Johnson, J.G.; Halvosa, S.J.; Wang, Y.F.; King, M.D.; Ray, S.M.; Blumberg, H.M. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA 300 genotype as a major cause of health care-associated blood stream infections. Clin. Infect. Dis. 2006, 42, 647–656. [Google Scholar] [CrossRef]
- Szaff, M.; Høiby, N. Antibiotic treatment of Staphylococcus aureus infection in cystic fibrosis. Acta Paediatr. 1982, 71, 821–826. [Google Scholar] [CrossRef]
- Goss, C.H.; Muhlebach, M.S. Review: Staphylococcus aureus and MRSA in cystic fibrosis. J. Cyst. Fibros. 2011, 10, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Cigana, C.; Bianconi, I.; Baldan, R.; De Simone, M.; Riva, C.; Sipione, B.; Rossi, G.; Cirillo, D.M.; Bragonzi, A. Staphylococcus aureus impacts Pseudomonas aeruginosa chronic respiratory disease in murine models. J. Infect. Dis. 2017, 217, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Sagel, S.; Gibson, R.; Emerson, J.; McNamara, S.; Burns, J.L.; Wagener, J.S.; Ramsey, B.W. Impact of Pseudomonas and Staphylococcus infection on inflammation and clinical status in young children with cystic fibrosis. J. Pediatr. 2009, 154, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Junge, S.; Gorlich, D.; den Reijer, M.; Wiedemann, B.; Tümmler, B.; Ellemunter, H.; Dübbers, A.; Küster, P.; Ballmann, M.; Koerner-Rettberg, C.; et al. Factors associated with worse lung function in cystic fibrosis patients with persistent Staphylococcus aureus. PLoS ONE 2016, 11, e0166220. [Google Scholar] [CrossRef] [PubMed]
- Ahlgren, H.G.; Benedetti, A.; Landry, J.S.; Bernier, J.; Matouk, E.; Radzioch, D.; Lands, L.C.; Rousseau, S.; Nguyen, D. Clinical outcomes associated with Staphylococcus aureus and Pseudomonas aeruginosa airway infections in adult cystic fibrosis patients. BMC Pulm. Med. 2015, 15, 67. [Google Scholar] [CrossRef]
- Dasenbrook, E.C.; Merlo, C.A.; Diener-West, M.; Lechtzin, N.; Boyle, M.-P. Persistent methicillin-resistant Staphylococcus aureus and rate of FEV1 decline in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2008, 178, 814–821. [Google Scholar] [CrossRef]
- Dasenbrook, E.C.; Checkley, W.; Merlo, C.A.; Konstan, M.W.; Lechtzin, N.; Boyle, M.P. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA 2010, 303, 2386–2392. [Google Scholar] [CrossRef]
- Wolter, D.J.; Emerson, J.C.; McNamara, S.; Buccat, A.M.; Qin, X.; Cochrane, E.; Houston, L.S.; Rogers, G.B.; Marsh, P.; Prehar, K.; et al. Staphylococcus aureus small-colony variants are independently associated with worse lung disease in children with cystic fibrosis. Clin. Infect. Dis. 2013, 57, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Cystic Fibrosis Foundation. Patient Registry: 2017 Annual Data Report; Cystic Fibrosis Foundation: Bethesda, MD, USA, 2018. [Google Scholar]
- UK Cystic Fibrosis Trust. UK CF Registry. In Annual Data Report 2016; Cystic Fibrosis Trust: London, UK, 2017. [Google Scholar]
- Weaver, L.T.; Green, M.R.; Nicholson, K.; Mills, J.; Heeley, M.E.; Kuzemko, J.A.; Austin, S.; Gregory, G.A.; Dux, A.E.; Davis, J.A. Prognosis in cystic fibrosis treated with continuous flucloxacillin from the neonatal period. Arch. Dis. Child. 1994, 70, 84–89. [Google Scholar] [CrossRef]
- Smyth, A.R.; Walters, S. Prophylactic anti-staphylococcal antibiotics for cystic fibrosis. Cochrane Database Syst. Rev. 2012, 12, CD001912. [Google Scholar] [CrossRef] [PubMed]
- Cystic Fibrosis Trust. Standard for the Clinical Care of Children and Adults with Cystic Fibrosis in the UK; Cystic Fibrosis Trust: London, UK, 2016. [Google Scholar]
- Andersen, C.; Kahl, B.C.; Olesen, H.V.; Jensen-Fangel, S.; Norskov-Lauritsen, N. Intravenous antibiotics given for 2 weeks do not eradicate persistent Staphylococcus aureus clones in cystic fibrosis patients. Clin. Microbiol. Infect. 2014, 20, O285–O291. [Google Scholar] [CrossRef] [PubMed]
- Kleerebezem, M.; Quadri, L.E.; Kuipers, O.P.; deVos, W.M. Quorum sensing by peptide pheromones and two-component signal-transduction systems in Gram-positive bacteria. Mol. Microbiol. 1997, 24, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.L.; Novick, R.P.; Kreiswirth, B.; Kornblum, J.; Schlievert, P. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J. Bacteriol. 1988, 170, 4365–4372. [Google Scholar] [CrossRef] [PubMed]
- Kornblum, J.; Kreiswirth, B.; Projan, S.J.; Ross, H.; Novick, R.P. Agr: A polycistronic locus regulating exoprotein synthesis in Staphylococcus aureus. In Molecular Biology of the Staphylococci; Novick, R.P., Ed.; VCH Publishers: New York, NY, USA, 1990; pp. 373–401. ISBN1 3527280820. ISBN2 9783527280827. [Google Scholar]
- Zhang, L.; Ji, G. Identification of a staphylococcal AgrB segment(s) responsible for group-specific processing of AgrD by gene swapping. J. Bacteriol. 2004, 186, 6706–6713. [Google Scholar] [CrossRef]
- Lina, G.; Jarraud, S.; Ji, G.; Greenland, T.; Pedraza, A.; Etienne, J.; Novick, R.P.; Vandenesch, F. Transmembrane topology and histidine protein kinase activity of AgrC, the agr signal receptor in Staphylococcus aureus. Mol. Microbiol. 1998, 28, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Koenig, R.L.; Ray, J.L.; Maleki, S.J.; Smeltzer, M.S.; Hurlburt, B.K. Staphylococcus aureus AgrA binding to the RNAIII-agr regulatory region. J. Bacteriol. 2004, 186, 7549–7555. [Google Scholar] [CrossRef] [PubMed]
- Queck, S.Y.; Jameson-Lee, M.; Villaruz, A.E.; Bach, T.H.; Khan, B.A.; Sturdevant, D.E.; Ricklefs, S.M.; Li, M.; Otto, M. RNAIII-independent target gene control by the agr quorum-sensing system: Insight into the evolution of virulence regulation in Staphylococcus aureus. Mol. Cell 2008, 32, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Fechter, P.; Caldelari, I.; Lioliou, E.; Romby, P. Novel aspects of RNA regulation in Staphylococcus aureus. FEBS Lett. 2014, 588, 2523–2529. [Google Scholar] [CrossRef] [PubMed]
- Pace, J.L.; Rupp, M.E.; Finch, R.G. Biofilms, Infection, and Antimicrobial Therapy; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Periasamy, S.; Joo, H.S.; Duong, A.C.; Bach, T.H.; Tan, V.Y.; Chatterjee, S.S.; Cheung, G.Y.; Otto, M. How Staphylococcus aureus biofilms develop their characteristic structure. Proc. Natl. Acad. Sci. USA 2012, 109, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Bubeck Wardenburg, J.; Bae, T.; Otto, M.; Deleo, F.R.; Schneewind, O. Poring over pores: Alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat. Med. 2007, 13, 1405–1406. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.L.; Eberhardt, K.J.; Chung, E.; Yeaman, M.R.; Sullam, P.M.; Ramos, M.; Bayer, A.S. Diminished virulence of a sar-/agr-mutant of Staphylococcus aureus in the rabbit model of endocarditis. J. Clin. Investig. 1994, 94, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Abdelnour, A.; Arvidson, S.; Bremell, T.; Ryden, C.; Tarkowski, A. The accessory gene regulator (agr) controls Staphylococcus aureus virulence in a murine arthritis model. Infect. Immun. 1993, 61, 3879–3885. [Google Scholar] [PubMed]
- Gillaspy, A.F.; Hickmon, S.G.; Skinner, R.A.; Thomas, J.R.; Nelson, C.L.; Smeltzer, M.S. Role of the accessory gene regulator (agr) in pathogenesis of staphylococcal osteomyelitis. Infect. Immun. 1995, 63, 3373–3380. [Google Scholar] [PubMed]
- Wright, J.S., III; Jin, R.; Novick, R.P. Transient interference with staphylococcal quorum sensing blocks abscess formation. Proc. Natl. Acad. Sci. USA 2005, 102, 1691–1696. [Google Scholar] [CrossRef]
- Chen, X.; Schauder, S.; Potier, N.; Van Dorsselaer, A.; Pelczer, I.; Bassler, B.L.; Hughson, F.M. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 2002, 415, 545–549. [Google Scholar] [CrossRef]
- Zhao, L.; Xue, T.; Shang, F.; Sun, H.; Sun, B. Staphylococcus aureus AI-2 quorum sensing associates with the KdpDE two-component system to regulate capsular polysaccharide synthesis and virulence. Infect. Immun. 2010, 78, 3506–3515. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhao, L.; Xue, T.; Sun, B. Staphylococcus aureus autoinducer-2 quorum sensing decreases biofilm formation in an icaR-dependent manner. BMC Microbiol. 2012, 12, 288. [Google Scholar] [CrossRef]
- Doherty, N.; Holden, M.T.; Qazi, S.N.; Williams, P.; Winzer, K. Functional analysis of luxS in Staphylococcus aureus reveals a role in metabolism but not quorum sensing. J. Bacteriol. 2006, 188, 2885–2897. [Google Scholar] [CrossRef] [PubMed]
- Essigmann, H.T.; Darkoh, C.; McHugh, E.E.; Brown, E.L. The Clostridium difficile quorum-sensing molecule alters the Staphylococcus aureus toxin expression profile. Int. J. Antimicrob. Agents 2017, 49, 391–393. [Google Scholar] [CrossRef]
- Silva, L.N.; Da Hora, G.C.A.; Soares, T.A.; Bojer, M.S.; Ingmer, H.; Macedo, A.J.; Trentin, D.S. Myricetin protects Galleria mellonella against Staphylococcus aureus infection and inhibits multiple virulence factors. Sci. Rep. 2017, 7, 2823. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.C.; Khoo, H.E. Biological effects of myricetin. Gen. Pharmacol. 1997, 29, 121–126. [Google Scholar] [CrossRef]
- Gao, X.J.; Wang, T.C.; Zhang, Z.C.; Cao, Y.G.; Zhang, N.S.; Guo, M.Y. Brazilin plays an anti-inflammatory role with regulating Toll-like receptor 2 and TLR 2 downstream pathways in Staphylococcus aureus-induced mastitis in mice. Int. Immunopharmacol. 2015, 27, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, N.P.; Panichayupakaranant, P. Antioxidant, antibacterial, and anti-inflammatory activities of standardized brazilin-rich Caesalpinia sappan extract. Pharm. Biol. 2015, 53, 1339–1343. [Google Scholar] [CrossRef]
- Merghni, A.; Noumi, E.; Hadded, O.; Dridi, N.; Panwar, H.; Ceylan, O.; Mastouri, M.; Snoussi, M. Assessment of the antibiofilm and antiquorum sensing activities of Eucalyptus globulus essential oil and its main component 1,8-cineole against methicillin-resistant Staphylococcus aureus strains. Microb. Pathog. 2018, 118, 74–80. [Google Scholar] [CrossRef]
- Sharifi, A.; Mohammadzadeh, A.; Zahraei Salehi, T.; Mahmoodi, P. Antibacterial, antibiofilm and antiquorum sensing effects of Thymus daenensis and Satureja hortensis essential oils against Staphylococcus aureus isolates. J. Appl. Microbiol. 2018, 124, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Bojer, M.S.; Lindemose, S.; Vestergaard, M.; Ingmer, H. Quorum sensing-regulated phenol-soluble modulins limit persister cell populations in Staphylococcus aureus. Front. Microbiol. 2018, 9, 255. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.; Månsson, M.; Bojer, M.S.; Gram, L.; Larsen, T.O.; Novick, R.P.; Frees, D.; Frøkiær, H.; Ingmer, H. Solonamide B inhibits quorum sensing and reduces Staphylococcus aureus mediated killing of human neutrophils. PLoS ONE 2014, 9, e84992. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.M.; Peng, P.; Baldry, M.; Perez-Gassol, I.; Christensen, S.B.; Vinther, J.M.O.; Ingmer, H.; Franzyk, H. Lactam hybrid analogues of solonamide B and autoinducing peptides as potent S. aureus AgrC antagonists. Eur. J. Med. Chem. 2018, 152, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.; Burton, N.; Cooper, R. Proteomic and genomic analysis of methicillin-resistant Staphylococcus aureus (MRSA) exposed to manuka honey in vitro demonstrated down-regulation of virulence markers. J. Antimicrob. Chemother. 2014, 69, 603–615. [Google Scholar] [CrossRef]
- Blair, S.E.; Cockcetin, N.N.; Harry, E.J.; Carter, D.A. The unusual antibacterial activity of medical-grade Leptospermum honey: Antibacterial spectrum, resistance and transcriptome analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 10, 1199–1208. [Google Scholar] [CrossRef]
- Daly, S.M.; Elmore, B.O.; Kavanaugh, J.S.; Triplett, K.D.; Figueroa, M.; Raja, H.A.; El-Elimat, T.; Crosby, H.A.; Femling, J.K.; Cech, N.B.; et al. ω-Hydroxyemodin limits Staphylococcus aureus quorum sensing-mediated pathogenesis and inflammation. Antimicrob. Agents. Chemother. 2015, 59, 2223–2235. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Gohda, F.; Kadoshima, T.; Fukuda, T.; Hanafusa, T.; Shojima, A.; Nakayama, J.; Bills, G.F.; Peterson, S. Avellanin C, an inhibitor of quorum-sensing signaling in Staphylococcus aureus, from Hamigera ingelheimensis. J. Antibiot. (Tokyo) 2015, 68, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Quave, C.L.; Lyles, J.T.; Kavanaugh, J.S.; Nelson, K.; Parlet, C.P.; Crosby, H.A.; Heilmann, K.P.; Horswill, A.R. Castanea sativa (European Chestnut) Leaf Extracts Rich in Ursene and Oleanene Derivatives Block Staphylococcus aureus Virulence and Pathogenesis without Detectable Resistance. PLoS ONE 2015, 10, e0136486. [Google Scholar] [CrossRef] [PubMed]
- Baldry, M.; Nielsen, A.; Bojer, M.S.; Zhao, Y.; Friberg, C.; Ifrah, D.; Glasser Heede, N.; Larsen, T.O.; Frøkiær, H.; Frees, D.; et al. Norlichexanthone reduces virulence gene expression and biofilm formation in Staphylococcus aureus. PLoS ONE 2016, 11, e0168305. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, T.; Wang, K.; Hou, C.; Cai, S.; Huang, Y.; Du, Z.; Huang, H.; Kong, J.; Chen, Y. Baicalein inhibits Staphylococcus aureus biofilm formation and the quorum sensing system in vitro. PLoS ONE 2016, 11, e0153468. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, T.H.; Warming, A.N.; Vejborg, R.M.; Moscoso, J.A.; Stegger, M.; Lorenzen, F.; Rybtke, M.; Andersen, J.B.; Petersen, R.; Andersen, P.S.; et al. A broad range quorum sensing inhibitor working through sRNA inhibition. Sci. Rep. 2017, 7, 9857. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Tang, H.; Lyles, J.T.; Pineau, R.; Mashwani, Z.U.; Quave, C.L. Antibacterial properties of medicinal plants from Pakistan against multidrug-resistant ESKAPE pathogens. Front. Pharmacol. 2018, 9, 815. [Google Scholar] [CrossRef] [PubMed]
- Kuo, D.; Yu, G.; Hoch, W.; Gabay, D.; Long, L.; Ghannoum, M.; Nagy, N.; Harding, C.V.; Viswanathan, R.; Shoham, M. Novel quorum-quenching agents promote methicillin-resistant Staphylococcus aureus (MRSA) wound healing and sensitize MRSA to β-lactam antibiotics. Antimicrob. Agents Chemother. 2015, 59, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Gui, Z.; Wang, H.; Ding, T.; Zhu, W.; Zhuang, X.; Chu, W. Azithromycin Reduces the Production of α-hemolysin and Biofilm Formation in Staphylococcus aureus. Indian J. Microbiol. 2014, 54, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Gizdavic-Nikolaidis, M.R.; Pagnon, J.C.; Ali, N.; Sum, R.; Davies, N.; Roddam, L.F.; Ambrose, M. Functionalized polyanilines disrupt Pseudomonas aeruginosa and Staphylococcus aureus biofilms. Colloids Surf. B Biointerfaces 2015, 136, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Balamurugan, P.; Vasudevan, S.; Jadav, S.; Princy, S.A. Antimicrobial and antibiofilm potential of acyclic amines and diamines against multi-drug resistant Staphylococcus aureus. Front. Microbiol. 2017, 8, 1767. [Google Scholar] [CrossRef] [PubMed]
- Brackman, G.; Breyne, K.; De Rycke, R.; Vermote, A.; Van Nieuwerburgh, F.; Meyer, E.; Van Calenbergh, S.; Coenye, T. The quorum sensing inhibitor hamamelitannin increases antibiotic susceptibility of Staphylococcus aureus biofilms by affecting peptidoglycan biosynthesis and eDNA eRelease. Sci. Rep. 2016, 6, 20321. [Google Scholar] [CrossRef] [PubMed]
- Vermote, A.; Brackman, G.; Risseeuw, M.D.; Vanhoutte, B.; Cos, P.; Van Hecke, K.; Breyne, K.; Meyer, E.; Coenye, T.; Van Calenbergh, S. Hamamelitannin Analogues that Modulate Quorum Sensing as Potentiators of Antibiotics against Staphylococcus aureus. Angew. Chem. Int. Ed. Engl. 2016, 55, 6551–6555. [Google Scholar] [CrossRef]
- Vermote, A.; Brackman, G.; Risseeuw, M.D.P.; Coenye, T.; Van Calenbergh, S. Novel hamamelitannin analogues for the treatment of biofilm related MRSA infections-A scaffold hopping approach. Eur. J. Med. Chem. 2017, 127, 757–770. [Google Scholar] [CrossRef]
- Vermote, A.; Brackman, G.; Risseeuw, M.D.; Cappoen, D.; Cos, P.; Coenye, T.; Van Calenbergh, S. Novel Potentiators for Vancomycin in the Treatment of Biofilm-Related MRSA Infections via a Mix and Match Approach. ACS Med. Chem. Lett. 2016, 8, 38–42. [Google Scholar] [CrossRef]
- Vijayakumar, K.; Ramanathan, T. Antiquorum sensing and biofilm potential of 5-Hydroxymethylfurfural against Gram positive pathogens. Microb. Pathog. 2018, 125, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.J.; Crowley, R.C.; Truman, A.; Clarke, S.R.; Cottam, J.A.; Jadhav, G.P.; Steele, V.R.; O’Shea, P.; Lindholm, C.; Cockayne, A.; et al. Targeting Staphylococcus aureus quorum sensing with nonpeptidic small molecule inhibitors. J. Med. Chem. 2014, 57, 2813–2819. [Google Scholar] [CrossRef] [PubMed]
- Sully, E.K.; Malachowa, N.; Elmore, B.O.; Alexander, S.M.; Femling, J.K.; Gray, B.M.; DeLeo, F.R.; Otto, M.; Cheung, A.L.; Edwards, B.S.; et al. Selective chemical inhibition of agr quorum sensing in Staphylococcus aureus promotes host defense with minimal impact on resistance. PLoS Pathog. 2014, 10, e1004174. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, J.P.; Daly, S.M.; Triplett, K.D.; Peabody, D.; Chackerian, B.; Hall, P.R. Development of a mimotope vaccine targeting the Staphylococcus aureus quorum sensing pathway. PLoS ONE 2014, 9, e111198. [Google Scholar] [CrossRef]
- Elmore, B.O.; Triplett, K.D.; Hall, P.R. Apolipoprotein B48, the Structural Component of Chylomicrons, Is Sufficient to Antagonize Staphylococcus aureus Quorum-Sensing. PLoS ONE 2015, 10, e0125027. [Google Scholar] [CrossRef] [PubMed]
- Da, F.; Yao, L.; Su, Z.; Hou, Z.; Li, Z.; Xue, X.; Meng, J.; Luo, X. Antisense locked nucleic acids targeting agrA inhibit quorum sensing and pathogenesis of community-associated methicillin-resistant Staphylococcus aureus. J. Appl. Microbiol. 2017, 122, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Tal-Gan, Y.; Ivancic, M.; Cornilescu, G.; Yang, T.; Blackwell, H.E. Highly Stable, Amide-Bridged Autoinducing Peptide Analogues that Strongly Inhibit the AgrC Quorum Sensing Receptor in Staphylococcus aureus. Angew. Chem. Int. Ed. Engl. 2016, 55, 8913–8917. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, J.K.; Tal-Gan, Y.; Cornilescu, G.; Tyler, K.A.; Blackwell, H.E. Simplified AIP-II Peptidomimetics Are Potent Inhibitors of Staphylococcus aureus AgrC Quorum Sensing Receptors. ChemBioChem 2017, 18, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Karathanasi, G.; Bojer, M.S.; Baldry, M.; Johannessen, B.A.; Wolff, S.; Greco, I.; Kilstrup, M.; Hansen, P.R.; Ingmer, H. Linear peptidomimetics as potent antagonists of Staphylococcus aureus agr quorum sensing. Sci. Rep. 2018, 8, 3562. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, P.; Praveen Krishna, V.; Bharath, D.; Lavanya, R.; Vairaprakash, P.; Princy, S.A. Staphylococcus aureus quorum regulator SarA targeted compound, 2-[(Methylamino)methyl]phenol inhibits biofilm and down-regulates virulence genes. Front. Microbiol. 2017, 8, 1290. [Google Scholar] [CrossRef]
- Bodey, G.P.; Bolivar, R.; Fainstein, V.; Jadeja, L. Infections Caused by Pseudomonas aeruginosa. Rev. Infect. Dis. 1983, 5, 279–313. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Available online: http://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/ (accessed on 5 March 2019).
- Pang, B.; Armbruster, C.E.; Foster, G.; Learman, B.S.; Gandhi, U.; Swords, W.E. Autoinducer 2 (AI-2) Production by nontypeable Haemophilus influenzae 86-028NP promotes expression of a predicted glycosyltransferase that is a determinant of biofilm maturation, prevention of dispersal, and persistence in vivo. Infect. Immun. 2018, 86, e00506-18. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Filho, L.V.R.F.; de Agujar Ferreira, F.; Reis, F.J.C.; de Britto, M.C.A.; Levy, C.E.; Clark, O.; Ribeiro, J.D.J. Pseudomonas aeruginosa infection in patients with cystic fibrosis: Scientific evidence regarding clinical impact, diagnosis, and treatment. Bras. Pneumol. 2013, 39, 495–512. [Google Scholar] [CrossRef] [PubMed]
- Sordé, R.; Pahissa, A.; Rello, J. Management of refractory Pseudomonas aeruginosa infection in cystic fibrosis. Infect. Drug Resist. 2011, 4, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.W.; Schurr, M.J.; Mudd, M.H.; Govan, J.R.; Holloway, B.W.; Deretic, V. Mechanism of conversion to mucoidy in Pseudomonas aeruginosa infecting cystic fibrosis patients. Proc. Natl. Acad. Sci. USA 1993, 90, 8377–8381. [Google Scholar] [CrossRef] [PubMed]
- Littlewood, J.M.; Miller, M.G.; Ghoneim, A.T.; Ramsden, C.H. Nebulised colomycin for early pseudomonal colonisation in cystic fibrosis. Lancet 1985, 1, 865. [Google Scholar] [CrossRef]
- Ratjen, F.; Döring, G.; Nikolaizik, W.H. Effect of inhaled tobramycin on early Pseudomonas aeruginosa colonisation in patients with cystic fibrosis. Lancet 2001, 358, 983–984. [Google Scholar] [CrossRef]
- Taccetti, G.; Campana, S.; Festini, F.; Mascherini, M.; Doring, G. Early eradication therapy against Pseudomonas aeruginosa in cystic fibrosis patients. Eur. Respir. J. 2005, 26, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Döring, G.; Conway, S.; Heijerman, H.; Hodson, M.; Høiby, N.; Smyth, A.; Touw, D.J. Antibiotic therapy against Pseudomonas aeruginosa: A European consensus. Eur. Respir. J. 2000, 16, 749–767. [Google Scholar] [CrossRef]
- Langton Hewer, S.C.; Smyth, A.R. Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis. Cochrane Database Syst. Rev. 2017, 4, CD004197. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.; Greenberg, E.P. A network of networks: Quorum-sensing gene regulation in Pseudomonas aeruginosa. Int. J. Med. Microbiol. 2006, 296, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Heeb, S.; Fletcher, M.P.; Chhabra, S.R.; Diggle, S.P.; Williams, P.; Camara, M. Quinolones: From antibiotics to autoinducers. FEMS Microbiol. Rev. 2011, 35, 247–274. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Wu, J.; Deng, Y.; Wang, J.; Wang, C.; Wang, J.; Chang, C.; Dong, Y.; Williams, P.; Zhang, L.H. A cell-cell communication signal integrates quorum sensing and stress response. Nat. Chem. Biol. 2013, 9, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Lépine, F.; Milot, S.; Déziel, E.; He, J.; Rahme, L.G. Electrospray/mass spectrometric identification and analysis of 4-hydroxy-2-alkylquinolines (HAQs) produced by Pseudomonas aeruginosa. J. Am. Soc. Mass Spectrom. 2004, 15, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Pesci, E.C.; Milbank, J.B.J.; Pearson, J.P.; McKnight, S.; Kende, A.S.; Greenberg, E.P.; Iglewski, B.H. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 1999, 96, 11229–11234. [Google Scholar] [CrossRef] [PubMed]
- McGrath, S.; Wade, D.S.; Pesci, E.C. Dueling quorum sensing systems in Pseudomonas aeruginosa control the production of the Pseudomonas quinolone signal (PQS). FEMS Microbiol. Lett. 2004, 230, 27–34. [Google Scholar] [CrossRef]
- McKnight, S.L.; Iglewski, B.H.; Pesci, E.C. The Pseudomonas quinolone signal regulates rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 2000, 182, 2702–2708. [Google Scholar] [CrossRef] [PubMed]
- Collier, D.N.; Anderson, L.S.; McKnight, L.; Noah, T.L.; Knowles, M.; Boucher, R.; Schwab, U.; Gilligan, P.; Pesci, E.C. A bacterial cell to cell signal in the lungs of cystic fibrosis patients. FEMS Microbiol. Lett. 2002, 215, 41–46. [Google Scholar] [CrossRef]
- Smith, R.S.; Iglewski, B.H. P. aeruginosa quorum-sensing systems and virulence. Curr. Opin. Microbiol. 2003, 6, 56–60. [Google Scholar] [CrossRef]
- Feng, L.; Xiang, Q.; Ai, Q.; Wang, Z.; Zhang, Y.; Lu, Q. Effects of quorum sensing systems on regulatory T cells in catheter-related Pseudomonas aeruginosa biofilm infection rat models. Mediat. Inflamm. 2016, 2016, 4012912. [Google Scholar] [CrossRef] [PubMed]
- Feltner, J.B.; Wolter, D.J.; Pope, C.E.; Groleau, M.C.; Smalley, N.E.; Greenberg, E.P.; Mayer-Hamblett, N.; Burns, J.; Déziel, E.; Hoffman, L.R.; et al. LasR variant cystic fibrosis isolates Reveal an adaptable quorum-sensing hierarchy in Pseudomonas aeruginosa. mBio 2016, 7, e01513-16. [Google Scholar] [CrossRef]
- Welsh, M.A.; Blackwell, H.E. Chemical genetics reveals environment-specific roles for quorum sensing circuits in Pseudomonas aeruginosa. Cell Chem. Biol. 2016, 23, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Barr, H.L.; Halliday, N.; Cámara, M.; Barrett, D.A.; Williams, P.; Forrester, D.L.; Simms, R.; Smyth, A.R.; Honeybourne, D.; Whitehouse, J.L.; et al. Pseudomonas aeruginosa quorum sensing molecules correlate with clinical status in cystic fibrosis. Eur. Respir. J. 2015, 46, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Ryall, B.; Carrara, M.; Zlosnik, J.E.; Behrends, V.; Lee, X.; Wong, Z.; Lougheed, K.E.; Williams, H.D. The mucoid switch in Pseudomonas aeruginosa represses quorum sensing systems and leads to complex changes to stationary phase virulence factor regulation. PLoS ONE 2014, 9, e96166. [Google Scholar] [CrossRef]
- Maisuria, V.B.; Los Santos, Y.L.; Tufenkji, N.; Déziel, E. Cranberry-derived proanthocyanidins impair virulence and inhibit quorum sensing of Pseudomonas aeruginosa. Sci. Rep. 2016, 6, 30169. [Google Scholar] [CrossRef] [PubMed]
- Suneby, E.G.; Herndon, L.R.; Schneider, T.L. Pseudomonas aeruginosa LasR·DNA binding is directly inhibited by quorum sensing antagonists. ACS Infect. Dis. 2017, 3, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Nizalapur, S.; Kimyon, O.; Biswas, N.N.; Gardner, C.R.; Griffith, R.; Rice, S.A.; Manefield, M.; Willcox, M.; Black, D.S.; Kumar, N. Design, synthesis and evaluation of N-aryl-glyoxamide derivatives as structurally novel bacterial quorum sensing inhibitors. Org. Biomol. Chem. 2016, 14, 680–693. [Google Scholar] [CrossRef] [PubMed]
- Nizalapur, S.; Kimyon, O.; Yee, E.; Bhadbhade, M.M.; Manefield, M.; Willcox, M.; Black, D.S.; Kumar, N. Synthesis and biological evaluation of novel acyclic and cyclic glyoxamide based derivatives as bacterial quorum sensing and biofilm inhibitors. Org. Biomol. Chem. 2017, 15, 5743–5755. [Google Scholar] [CrossRef] [PubMed]
- Chourasiya, S.S.; Kathuria, D.; Singh, S.; Sonawane, V.C.; Chakraborti, A.K.; Bharatam, P.V. Design, synthesis and biological evaluation of novel unsymmetrical azines as quorum sensing inhibitors. RSC Adv. 2015, 5, 80027–80038. [Google Scholar] [CrossRef]
- O’Reilly, M.C.; Blackwell, H.E. Structure-based design and biological evaluation of triphenyl scaffold-based hybrid compounds as hydrolytically stable modulators of a LuxR-type quorum sensing receptor. ACS Infect. Dis. 2016, 2, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.Y.; Kong, X.; Feng, P. Benzene ring substituted n-acyl homoserine lactone compounds as well as preparation method and application thereof. CN106749119A, 31 May 2017. [Google Scholar]
- Malladi, V.L.; Schneper, L.; Sobczak, A.J.; Mathee, K.; Wnuk, S.F. 2-methylthiopyrrolidines and their use for modulating bacterial quorum sensing. U.S. Patent No. 9,249,095; WO2012174511A1, 19 January 2016. [Google Scholar]
- Park, S.; Kim, H.S.; Ok, K.; Kim, Y.; Park, H.D.; Byun, Y. Design, synthesis and biological evaluation of 4-(alkyloxy)-6-methyl-2H-pyran-2-one derivatives as quorum sensing inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 2913–2917. [Google Scholar] [CrossRef] [PubMed]
- Amara, N.; Mashiach, R.; Amar, D.; Krief, P.; Spieser, S.A.; Bottomley, M.J.; Aharoni, A.; Meijler, M.M. Covalent inhibition of bacterial quorum sensing. J. Am. Chem. Soc. 2009, 131, 10610–10619. [Google Scholar] [CrossRef] [PubMed]
- Amara, N.; Gregor, R.; Rayo, J.; Dandela, R.; Daniel, E.; Liubin, N.; Willems, H.M.; Ben-Zvi, A.; Krom, B.P.; Meijler, M.M. Fine-tuning covalent inhibition of bacterial quorum sensing. ChemBioChem 2016, 17, 825–835. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.T.; Noto, J.G.; Nichols-O’Neill, L.; Perez, L.J. Potent irreversible inhibitors of LasR quorum sensing in Pseudomonas aeruginosa. ACS Med. Chem. Lett. 2015, 6, 162–167. [Google Scholar] [CrossRef]
- Chang, C.Y.; Krishnan, T.; Wang, H.; Chen, Y.; Yin, W.F.; Chong, Y.M.; Tan, L.Y.; Chong, T.M.; Chan, K.G. Non-antibiotic quorum sensing inhibitors acting against N-acyl homoserine lactone synthase as druggable target. Sci. Rep. 2014, 4, 7245. [Google Scholar] [CrossRef] [PubMed]
- Lidor, O.; Al-Quntar, A.; Pesci, E.C.; Steinberg, D. Mechanistic analysis of a synthetic inhibitor of the Pseudomonas aeruginosa LasI quorum-sensing signal synthase. Sci. Rep. 2015, 5, 16569. [Google Scholar] [CrossRef] [PubMed]
- Kamarudheen, N.; Rao, K.V.B. Fatty acyl compounds from marine Streptomyces griseoincarnatus strain HK12 against two major bio-film forming nosocomial pathogens; an in vitro and in silico approach. Microb. Pathog. 2019, 127, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Bassler, B.L.; Semmelhack, M.F.; Drescher, K.; Siryaporn, A.; Miller, L.C.; O’Loughlin, C.T. Molecules and Compositions that Inhibit Gram Negative Bacteria and Their Uses. U.S. Patent No. 9,751,851, 5 September 2017. [Google Scholar]
- Eibergen, N.R.; Moore, J.D.; Mattmann, M.E.; Blackwell, H.E. Potent and selective modulation of the RhlR quorum sensing receptor by using non-native ligands: An emerging target for virulence control in Pseudomonas aeruginosa. ChemBioChem 2015, 16, 2348–2356. [Google Scholar] [CrossRef] [PubMed]
- Welsh, M.A.; Eibergen, N.R.; Moore, J.D.; Blackwell, H.E. Small molecule disruption of quorum sensing cross-regulation in Pseudomonas aeruginosa causes major and unexpected alterations to virulence phenotypes. J. Am. Chem. Soc. 2015, 137, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Maurer, C.K.; Kirsch, B.; Steinbach, A.; Hartmann, R.W. Overcoming the unexpected functional inversion of a PqsR antagonist in Pseudomonas aeruginosa: An in vivo potent antivirulence agent targeting pqs quorum sensing. Angew. Chem. Int. Ed. 2014, 53, 1109–1112. [Google Scholar] [CrossRef] [PubMed]
- Starkey, M.; Lepine, F.; Maura, D.; Bandyopadhaya, A.; Lesic, B.; He, J.; Kitao, T.; Righi, V.; Milot, S.; Tzika, A.; et al. Identification of anti-virulence compounds that disrupt quorum-sensing regulated acute and persistent pathogenicity. PLoS Pathog. 2014, 10, e1004321. [Google Scholar] [CrossRef] [PubMed]
- Maura, D.; Rahme, L.G. Pharmacological Inhibition of the Pseudomonas aeruginosa MvfR Quorum-Sensing System interferes with biofilm formation and potentiates antibiotic-mediated biofilm disruption. Antimicrob. Agents Chemother. 2017, 61, e01362-17. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Sharma, I.; Pratihar, D.; Hudson, L.L.; Maura, D.; Guney, T.; Rahme, L.G.; Pesci, E.C.; Coleman, J.P.; Tan, D.S. Designed Small-Molecule Inhibitors of the Anthranilyl-CoA Synthetase PqsA Block Quinolone Biosynthesis in Pseudomonas aeruginosa. ACS Chem. Biol. 2016, 11, 3061–3067. [Google Scholar] [CrossRef] [PubMed]
- Weidel, E.; Negri, M.; Empting, M.; Hinsberger, S.; Hartmann, R.W. Composing compound libraries for hit discovery--rationality-driven preselection or random choice by structural diversity? Future Med. Chem. 2014, 6, 2057–2072. [Google Scholar] [CrossRef]
- Sahner, J.H.; Empting, M.; Kamal, A.; Weidel, E.; Groh, M.; Borger, C.; Hartmann, R.W. Exploring the chemical space of ureidothiophene-2-carboxylic acids as inhibitors of the quorum sensing enzyme PqsD from Pseudomonas aeruginosa. Eur. J. Med. Chem. 2015, 96, 14–21. [Google Scholar] [CrossRef]
- Allegretta, G.; Weidel, E.; Empting, M.; Hartmann, R.W. Catechol-based substrates of chalcone synthase as a scaffold for novel inhibitors of PqsD. Eur. J. Med. Chem. 2015, 90, 351–359. [Google Scholar] [CrossRef]
- Thomann, A.; de Mello Martins, A.G.; Brengel, C.; Empting, M.; Hartmann, R.W. Application of dual inhibition concept within looped autoregulatory systems toward antivirulence agents against Pseudomonas aeruginosa I infections. ACS Chem. Biol. 2016, 11, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Hinsberger, S.; de Jong, J.C.; Groh, M.; Haupenthal, J.; Hartmann, R.W. Benzamidobenzoic acids as potent PqsD inhibitors for the treatment of Pseudomonas aeruginosa infections. Eur. J. Med. Chem. 2014, 76, 343–351. [Google Scholar] [CrossRef]
- Prothiwa, M.; Englmaier, F.; Böttcher, T. Competitive Live-Cell Profiling Strategy for discovering inhibitors of the quinolone biosynthesis of Pseudomonas aeruginosa. J. Am. Chem. Soc. 2018, 140, 14019–14023. [Google Scholar] [CrossRef] [PubMed]
- Rampioni, G.; Falcone, M.; Heeb, S.; Frangipani, E.; Fletcher, M.P.; Dubern, J.F.; Visca, P.; Leoni, L.; Cámara, M.; Williams, P. Unravelling the Genome-Wide contributions of specific 2-Alkyl-4-Quinolones and PqsE to Quorum Sensing in Pseudomonas aeruginosa. PLoS Pathog. 2016, 12, e1006029. [Google Scholar] [CrossRef]
- Zender, M.; Witzgall, F.; Drees, S.L.; Weidel, E.; Maurer, C.K.; Fetzner, S.; Blankenfeldt, W.; Empting, M.; Hartmann, R.W. Dissecting the multiple roles of PqsE in Pseudomonas aeruginosa virulence by discovery of small tool compounds. ACS Chem. Biol. 2016, 11, 1755–1763. [Google Scholar] [CrossRef]
- Maura, D.; Drees, S.L.; Bandyopadhaya, A.; Kitao, T.; Negri, M.; Starkey, M.; Lesic, B.; Milot, S.; Déziel, E.; Zahler, R.; et al. Polypharmacology approaches against the Pseudomonas aeruginosa MvfR regulon and their application in blocking virulence and antibiotic tolerance. ACS Chem. Biol. 2017, 12, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Allegretta, G.; Maurer, C.K.; Eberhard, J.; Maura, D.; Hartmann, R.W.; Rahme, L.; Empting, M. In-depth profiling of MvfR-regulated small molecules in Pseudomonas aeruginosa after Quorum Sensing Inhibitor treatment. Front. Microbiol. 2017, 8, 924. [Google Scholar] [CrossRef]
- Aleksić, I.; Šegan, S.; Andrić, F.; Zlatović, M.; Moric, I.; Opsenica, D.M.; Senerovic, L. long-chain 4-aminoquinolines as quorum sensing inhibitors in Serratia marcescens and Pseudomonas aeruginosa. ACS Chem. Biol. 2017, 12, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Soukarieh, F.; Vico Oton, E.; Dubern, J.F.; Gomes, J.; Halliday, N.; de Pilar Crespo, M.; Ramírez-Prada, J.; Insuasty, B.; Abonia, R.; Quiroga, J.; et al. In silico and in vitro-guided identification of inhibitors of alkylquinolone-dependent quorum sensing in Pseudomonas aeruginosa. Molecules 2018, 23, 257. [Google Scholar] [CrossRef]
- Fong, J.; Yuan, M.; Jakobsen, T.H.; Mortensen, K.T.; Delos Santos, M.M.S.; Chua, S.L.; Yang, L.; Tan, C.H.; Nielsen, T.E.; Givskov, M. Disulfide bond-containing ajoene analogues as novel quorum sensing inhibitors of Pseudomonas aeruginosa. J. Med. Chem. 2017, 60, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Goswami, M.; Espinasse, A.; Carlson, E.E. Disarming the virulence arsenal of Pseudomonas aeruginosa by blocking two-component system signaling. Chem. Sci. 2018, 9, 7332–7337. [Google Scholar] [CrossRef]
- Kasper, S.H.; Bonocora, R.P.; Wade, J.T.; Musah, R.A.; Cady, N.C. Chemical inhibition of kynureninase reduces Pseudomonas aeruginosa quorum sensing and virulence factor expression. ACS Chem. Biol. 2016, 11, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Vanlaere, E.; Lipuma, J.J.; Baldwin, A.; Henry, D.; De Brandt, E.; Mahenthiralingam, E.; Speert, D.; Dowson, C.; Vandamme, P. Burkholderia latens sp. nov., Burkholderia diffusa sp. nov., Burkholderia arboris sp. nov., Burkholderia seminalis sp. nov. and Burkholderia metallica sp. nov., novel species within the Burkholderia cepacia complex. Int. J. Syst. Evol. Microbiol. 2008, 58, 1580–1590. [Google Scholar] [CrossRef] [PubMed]
- De Smet, B.; Mayo, M.; Peeters, C.; Zlosnik, J.E.; Spilker, T.; Hird, T.J.; LiPuma, J.J.; Kidd, T.J.; Kaestli, M.; Ginther, J.L.; et al. Burkholderia stagnalis sp. nov. and Burkholderia territorii sp. nov., two novel Burkholderia cepacia complex species from environmental and human sources. Int. J. Syst. Evol. Microbiol. 2015, 65, 2265–2271. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.S.; Aw, Y.K.; Lee, L.H.; Yule, C.M.; Cheow, Y.L.; Lee, S.M. Burkholderia paludis sp. nov., an antibiotic-siderophore producing novel Burkholderia cepacia complex species, isolated from Malaysian tropical peat swamp soil. Front. Microbiol. 2016, 7, 2046. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.F.; King, G.M. Volcanic soils as sources of novel CO-oxidizing Paraburkholderia and Burkholderia: Paraburkholderia hiiakae sp. nov., Paraburkholderia metrosideri sp. nov., Paraburkholderia paradisi sp. nov., Paraburkholderia peleae sp. nov., and Burkholderia alpina sp. nov. a member of the Burkholderia cepacia complex. Front. Microbiol. 2017, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Mahenthiralingam, E.; Urban, T.A.; Goldberg, J.B. The multifarious, multireplicon Burkholderia cepacia complex. Nat. Rev. Microbiol. 2005, 3, 144–156. [Google Scholar] [CrossRef]
- Burns, J.L. Antibiotic resistance of Burkholderia spp. In Burkholderia: Molecular Microbiology and Genomics; Coenye, T., Vandamme, P., Eds.; Horizon Bioscience: Norfolk, VA, USA, 2007; ISBN 978-1-904933-41-0. [Google Scholar]
- Scoffone, V.C.; Chiarelli, L.R.; Trespidi, G.; Mentasti, M.; Riccardi, G.; Buroni, S. Burkholderia cenocepacia Infections in Cystic Fibrosis Patients: Drug Resistance and Therapeutic Approaches. Front. Microbiol. 2017, 8, 1592. [Google Scholar] [CrossRef] [PubMed]
- Bodilis, J.; Denet, E.; Brothier, E.; Graindorge, A.; Favre-Bonté, S.; Nazaret, S. Comparative Genomics of Environmental and Clinical Burkholderia cenocepacia Strains Closely Related to the Highly Transmissible Epidemic ET12 Lineage. Front. Microbiol. 2018, 9, 383. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Dodd, M.E.; Webb, A.K. Burkholderia cepacia: Current clinical issues, environmental controversies and ethical dilemmas. Eur. Respir. J. 2001, 17, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Drevinek, P.; Mahenthiralingam, E. Burkholderia cenocepacia in cystic fibrosis: Epidemiology and molecular mechanisms of virulence. Clin. Microbiol. Infect. 2010, 16, 821–830. [Google Scholar] [CrossRef]
- Loutet, S.A.; Valvano, M.A. A decade of Burkholderia cenocepacia virulence determinant research. Infect. Immun. 2010, 78, 4088–4100. [Google Scholar] [CrossRef]
- Waters, C.M.; Bassler, B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef] [PubMed]
- Lutter, E.; Lewenza, S.; Dennis, J.J.; Visser, M.B.; Sokol, P.A. Distribution of quorum-sensing genes in the Burkholderia cepacia complex. Infect. Immun. 2001, 69, 4661–4666. [Google Scholar] [CrossRef] [PubMed]
- Malott, R.J.; Baldwin, A.; Mahenthiralingam, E.; Sokol, P.A. Characterization of the CciIR quorum-sensing system in Burkholderia cenocepacia. Infect. Immun. 2005, 73, 4982–4992. [Google Scholar] [CrossRef] [PubMed]
- Malott, R.J.; O’Grady, E.P.; Toller, J.; Inhulsen, S.; Eberl, L.; Sokol, P.A. A Burkholderia cenocepacia orphan LuxR homolog is involved in quorum-sensing regulation. J. Bacteriol. 2009, 191, 2447–2460. [Google Scholar] [CrossRef] [PubMed]
- Ryan, G.T.; Wei, Y.; Winans, S.C. A LuxR-type repressor of Burkholderia cenocepacia inhibits transcription via antiactivation and is inactivated by its cognate acylhomoserine lactone. Mol. Microbiol. 2013, 87, 94–111. [Google Scholar] [CrossRef] [PubMed]
- Malott, R.J.; Sokol, P.A. Expression of the BviIR and CepIR quorum-sensing systems of Burkholderia vietnamiensis. J. Bacteriol. 2007, 189, 3006–3016. [Google Scholar] [CrossRef]
- Chapalain, A.; Groleau, M.C.; Le Guillouzer, S.; Miomandre, A.; Vial, L.; Milot, S.; Déziel, E. Interplay between 4-Hydroxy-3-Methyl-2-Alkylquinoline and N-Acyl-Homoserine Lactone Signaling in a Burkholderia cepacia Complex Clinical Strain. Front. Microbiol. 2017, 8, 1021. [Google Scholar] [CrossRef]
- Boon, C.; Deng, Y.; Wang, L.H.; He, Y.; Xu, J.L.; Fan, Y.; Pan, S.Q.; Zhang, L.H. A novel DSF-like signal from Burkholderia cenocepacia interferes with Candida albicans morphological transition. ISME J. 2008, 2, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, F.; Scoffone, V.C.; Chiarelli, L.R.; Fumagalli, M.; Buroni, S.; Riccardi, G.; Forneris, F. The Crystal Structure of Burkholderia cenocepacia DfsA Provides Insights into Substrate Recognition and Quorum Sensing Fatty Acid Biosynthesis. Biochemistry 2016, 55, 3241–3250. [Google Scholar] [CrossRef]
- Waldron, E.J.; Snyder, D.; Fernandez, N.L.; Sileo, E.; Inoyama, D.; Freundlich, J.S.; Waters, C.M.; Cooper, V.S.; Neiditch, M.B. Structural basis of DSF recognition by its receptor RpfR and its regulatory interaction with the DSF synthase RpfF. PLoS Biol. 2019, 17, e3000123. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Cui, C.; Ye, Q.; Kan, J.; Fu, S.; Song, S.; Huang, Y.; He, F.; Zhang, L.H.; Jia, Y.; et al. Burkholderia cenocepacia integrates cis-2-dodecenoic acid and cyclic dimeric guanosine monophosphate signals to control virulence. Proc. Natl. Acad. Sci. USA 2017, 114, 13006–13011. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, Y.; Yang, L.; Twomey, K.B.; Sass, A.; Tolker-Nielsen, T.; Mahenthiralingam, E.; Dow, J.M.; Ryan, R.P. A sensor kinase recognizing the cell–cell signal BDSF (cis-2-dodecenoic acid) regulates virulence in Burkholderia cenocepacia. Mol. Microbiol. 2010, 77, 1220–1236. [Google Scholar] [CrossRef] [PubMed]
- Diggle, S.P.; Lumjiaktase, P.; Dipilato, F.; Winzer, K.; Kunakorn, M.; Barrett, D.A.; Chhabra, S.R.; Cámara, M.; Williams, P. Functional genetic analysis reveals a 2-alkyl-4-quinolone signaling system in the human pathogen Burkholderia pseudomallei and related bacteria. Chem. Biol. 2006, 13, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Vial, L.; Lepine, F.; Milot, S.; Groleau, M.C.; Dekimpe, V.; Woods, D.E.; Déziel, E. Burkholderia pseudomallei, B. thailandensis, and B. ambifaria produce 4-hydroxy-2-alkylquinoline analogues with a methyl group at the 3 position that is required for quorum-sensing regulation. J. Bacteriol. 2008, 190, 5339–5352. [Google Scholar] [CrossRef] [PubMed]
- Jenul, C.; Sieber, S.; Daeppen, C.; Mathew, A.; Lardi, M.; Pessi, G.; Hoepfner, D.; Neuburger, M.; Linden, A.; Gademann, K.; et al. Biosynthesis of fragin is controlled by a novel quorum sensing signal. Nat Commun 2018, 9, 1297. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, E.P.; Viteri, D.F.; Malott, R.J.; Sokol, P.A. Reciprocal regulation by the CepIR and CciIR quorum sensing systems in Burkholderia cenocepacia. BMC Genom. 2009, 10, 441. [Google Scholar] [CrossRef] [PubMed]
- Udine, C.; Brackman, G.; Bazzini, S.; Buroni, S.; Van Acker, H.; Pasca, M.R.; Riccardi, G.; Coenye, T. Phenotypic and genotypic characterisation of Burkholderia cenocepacia J2315 mutants affected in homoserine lactone and diffusible signal factor-based quorum sensing systems suggests interplay between both types of systems. PLoS ONE 2013, 8, e55112. [Google Scholar] [CrossRef]
- Schmid, N.; Suppiger, A.; Steiner, E.; Pessi, G.; Kaever, V.; Fazli, M.; Tolker-Nielsen, T.; Jenal, U.; Eberl, L. High intracellular c-di-GMP levels antagonize quorum sensing and virulence gene expression in Burkholderia cenocepacia H111. Microbiology 2017, 163, 754–764. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, E.P.; Viteri, D.F.; Sokol, P.A. A unique regulator contributes to quorum sensing and virulence in Burkholderia cenocepacia. PLoS ONE 2012, 7, e37611. [Google Scholar] [CrossRef]
- Huber, B.; Riedel, K.; Kothe, M.; Givskov, M.; Molin, S.; Eberl, L. Genetic analysis of functions involved in the late stages of biofilm development in Burkholderia cepacia H111. Mol. Microbiol. 2002, 46, 411–426. [Google Scholar] [CrossRef]
- Aubert, D.F.; O’grady, E.P.; Hamad, M.A.; Sokol, P.A.; Valvano, M.A. The Burkholderia cenocepacia sensor kinase hybrid AtsR is a global regulator modulating quorum-sensing signalling. Environ. Microbiol. 2013, 15, 372–385. [Google Scholar] [CrossRef]
- Michalska, K.; Chhor, G.; Clancy, S.; Jedrzejczak, R.; Babnigg, G.; Winans, S.C.; Joachimiak, A. RsaM: A transcriptional regulator of Burkholderia spp. with novel fold. FEBS J. 2014, 281, 4293–4306. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, H.; De Canck, E.; Van Nieuwerburgh, F.; Sass, A.; Deforce, D.; Nelis, H.J.; Coenye, T. The BCESM genomic region contains a regulator involved in quorum sensing and persistence in Burkholderia cenocepacia J2315. Future Microbiol. 2014, 9, 845–860. [Google Scholar] [CrossRef] [PubMed]
- Merry, C.R.; Perkins, M.; Mu, L.; Peterson, B.K.; Knackstedt, R.W.; Weingart, C.L. Characterization of a novel two-component system in Burkholderia cenocepacia. Curr. Microbiol. 2015, 70, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Yang, C.; Song, S.; Fu, S.; Sun, X.; Yang, L.; He, F.; Zhang, L.H.; Zhang, Y.; Deng, Y. A novel two-component system modulates quorum sensing and pathogenicity in Burkholderia cenocepacia. Mol. Microbiol. 2018, 108, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Subsin, B.; Chambers, C.E.; Visser, M.B.; Sokol, P.A. Identification of genes regulated by the cepIR quorum-sensing system in Burkholderia cenocepacia by high-throughput screening of a random promoter library. J. Bacteriol. 2007, 189, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Schmid, N.; Pessi, G.; Deng, Y.; Aguilar, C.; Carlier, A.L.; Grunau, A.; Omasits, U.; Zhang, L.H.; Ahrens, C.H.; Eberl, L. The AHL-and BDSF-dependent quorum sensing systems control specific and overlapping sets of genes in Burkholderia cenocepacia H111. PLoS ONE 2012, 7, e49966. [Google Scholar] [CrossRef] [PubMed]
- McKeon, S.A.; Nguyen, D.T.; Viteri, D.F.; Zlosnik, J.E.; Sokol, P.A. Functional quorum sensing systems are maintained during chronic Burkholderia cepacia complex infections in patients with cystic fibrosis. J. Infect. Dis. 2011, 203, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.B.; Majumdar, S.; Hani, E.; Sokol, P.A. Importance of the ornibactin and pyochelin siderophore transport systems in Burkholderia cenocepacia lung infections. Infect. Immun. 2004, 72, 2850–2857. [Google Scholar] [CrossRef] [PubMed]
- Inhülsen, S.; Aguilar, C.; Schmid, N.; Suppiger, A.; Riedel, K.; Eberl, L. Identification of functions linking quorum sensing with biofilm formation in Burkholderia cenocepacia H111. Microbiologyopen 2012, 1, 225–242. [Google Scholar] [CrossRef]
- Uehlinger, S.; Schwager, S.; Bernier, S.P.; Riedel, K.; Nguyen, D.T.; Sokol, P.A.; Eberl, L. Identification of specific and universal virulence factors in Burkholderia cenocepacia strains by using multiple infection hosts. Infect. Immun. 2009, 77, 4102–4110. [Google Scholar] [CrossRef]
- Brackman, G.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob. Agents Chemother. 2011, 55, 2655–2661. [Google Scholar] [CrossRef] [PubMed]
- Brackman, G.; Risseeuw, M.; Celen, S.; Cos, P.; Maes, L.; Nelis, H.J.; Van Calenbergh, S.; Coenye, T. Synthesis and evaluation of the quorum sensing inhibitory effect of substituted triazolyldihydrofuranones. Bioorg. Med. Chem. 2012, 20, 4737–4743. [Google Scholar] [CrossRef] [PubMed]
- Scoffone, V.C.; Chiarelli, L.R.; Makarov, V.; Brackman, G.; Israyilova, A.; Azzalin, A.; Forneris, F.; Riabova, O.; Savina, S.; Coenye, T.; et al. Discovery of new diketopiperazines inhibiting Burkholderia cenocepacia quorum sensing in vitro and in vivo. Sci. Rep. 2016, 6, 32487. [Google Scholar] [CrossRef] [PubMed]
- Buroni, S.; Scoffone, V.C.; Fumagalli, M.; Makarov, V.; Cagnone, M.; Trespidi, G.; De Rossi, E.; Forneris, F.; Riccardi, G.; Chiarelli, L.R. Investigating the Mechanism of Action of Diketopiperazines Inhibitors of the Burkholderia cenocepacia Quorum Sensing Synthase CepI: A Site-Directed Mutagenesis Study. Front. Pharmacol. 2018, 9, 836. [Google Scholar] [CrossRef] [PubMed]
- Slachmuylders, L.; Van Acker, H.; Brackman, G.; Sass, A.; Van Nieuwerburgh, F.; Coenye, T. Elucidation of the mechanism behind the potentiating activity of baicalin against Burkholderia cenocepacia biofilms. PLoS ONE 2018, 13, e0190533. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Qian, L.; Cao, L.; Tan, H.; Huang, Y.; Xue, X.; Shen, Y.; Zhou, S. Virtual screening for novel quorum sensing inhibitors to eradicate biofilm formation of Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2008, 79, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Huber, B.; Eberl, L.; Feucht, W.; Polster, J. Influence of polyphenols on bacterial biofilm formation and quorum-sensing. Z. Nat. C 2003, 58, 879–884. [Google Scholar] [CrossRef]
- Bugni, T.S.; Ireland, C.M. Marine-derived fungi: A chemically and biologically diverse group of microorganisms. Nat. Prod. Rep. 2004, 21, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Tommonaro, G.; Abbamondi, G.R.; Iodice, C.; Tait, K.; De Rosa, S. Diketopiperazines produced by the halophilic archaeon, Haloterrigena hispanica, activate AHL bioreporters. Microb. Ecol. 2012, 63, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Lin, Q.; Geske, G.D.; Blackwell, H.E. New and unexpected insights into the modulation of LuxR-type quorum sensing by cyclic dipeptides. ACS Chem. Biol. 2009, 4, 1051–1059. [Google Scholar] [CrossRef]
- Adegoke, A.A.; Stenström, T.A.; Okoh, A.I. Stenotrophomonas maltophilia as an emerging ubiquitous pathogen: Looking beyond contemporary antibiotic therapy. Front. Microbiol. 2017, 8, 2276. [Google Scholar] [CrossRef] [PubMed]
- Salsgiver, E.L.; Fink, A.K.; Knapp, E.A.; LiPuma, J.J.; Olivier, K.N.; Marshall, B.C.; Saiman, L. Changing epidemiology of the respiratory bacteriology of patients with cystic fibrosis. Chest 2016, 149, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Barsky, E.E.; Williams, K.A.; Priebe, G.P.; Sawicki, G.S. Incident Stenotrophomonas maltophilia infection and lung function decline in cystic fibrosis. Pediatric Pulmonol. 2017, 52, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.; Waters, V. Antibiotic treatment for Stenotrophomonas maltophilia in people with cystic fibrosis. Cochrane Database Syst. Rev. 2016, 7, CD009249. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.B. Antibiotic resistance in the opportunistic pathogen Stenotrophomonas maltophilia. Front. Microbiol. 2015, 6, 658. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Eberl, L.; Hartmann, A. The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environ. Microbiol. 2005, 7, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Huedo, P.; Yero, D.; Martínez-Servat, S.; Estibariz, I.; Planell, R.; Martínez, P.; Ruyra, A.; Roher, N.; Roca, I.; Vila, J.; Daura, X.; et al. Two different rpf clusters distributed among a population of Stenotrophomonas maltophilia clinical strains display differential diffusible signal factor production and virulence regulation. J. Bacteriol. 2014, 196, 2431–2442. [Google Scholar] [CrossRef]
- Huedo, P.; Yero, D.; Martinez-Servat, S.; Ruyra, À.; Roher, N.; Daura, X.; Gibert, I. Decoding the genetic and functional diversity of the DSF quorum-sensing system in Stenotrophomonas maltophilia. Front. Microbiol. 2015, 6, 761. [Google Scholar] [CrossRef]
- Pompilio, A.; Crocetta, V.; DeNicola, S.; Verginelli, F.; Fiscarelli, E.; Di Bonaventura, G. Cooperative pathogenicity in cystic fibrosis: Stenotrophomonas maltophilia modulates Pseudomonas aeruginosa virulence in mixed biofilm. Front. Microbiol. 2015, 6, 951. [Google Scholar] [CrossRef]
- Martínez, P.; Huedo, P.; Martinez-Servat, S.; Planell, R.; Ferrer-Navarro, M.; Daura, X.; Yero, D.; Gilbert, I. Stenotrophomonas maltophilia responds to exogenous AHL signals through the LuxR solo SmoR (Smlt1839). Front. Cell. Infect. Microbiol. 2015, 5, 41. [Google Scholar] [CrossRef]
- Hudaiberdiev, S.; Choudhary, K.S.; Vera Alvarez, R.; Gelencsér, Z.; Ligeti, B.; Lamba, D.; Pongor, S. Census of solo LuxR genes in prokaryotic genomes. Front. Cell. Infect. Microbiol. 2015, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Kavita, K.; Prabhakaran, R.; Jha, B. Cis-9-octadecenoic acid from the rhizospheric bacterium Stenotrophomonas maltophilia BJ01 shows quorum quenching and anti-biofilm activities. Biofouling 2013, 29, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Sriram, K.B.; Cox, A.J.; Clancy, R.L.; Slack, M.P.E.; Cripps, A.W. Nontypeable Haemophilus influenzae and chronic obstructive pulmonary disease: A review for clinicians. Crit. Rev. Microbiol. 2018, 44, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S.; Saiman, L. Pulmonary infections in patients with cystic fibrosis. Semin. Respir. Infect. 2002, 17, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Starner, T.D.; Zhang, N.; Kim, G.; Apicella, M.A.; McCray, P.B., Jr. Haemophilus influenzae forms biofilms on airway epithelia: Implications in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2006, 174, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Swords, W.E. Nontypeable Haemophilus influenzae biofilms: Role in chronic airway infections. Front. Cell. Infect. Microbiol. 2012, 2, 97. [Google Scholar] [CrossRef] [PubMed]
- Starner, T.D.; Shrout, J.D.; Parsek, M.R.; Appelbaum, P.C.; Kim, G. Subinhibitory concentrations of azithromycin decrease nontypeable Haemophilus influenzae biofilm formation and diminish established biofilms. Antimicrob. Agents Chemother. 2008, 52, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, C.E.; Pang, B.; Murrah, K.; Juneau, R.A.; Perez, A.C.; Weimer, K.E.; Swords, W.E. RbsB (NTHI_0632) mediates quorum signal uptake in nontypeable Haemophilus influenzae strain 86-028NP. Mol. Microbiol. 2011, 82, 836–850. [Google Scholar] [CrossRef]
- Rickard, A.H.; Palmer, R.J., Jr.; Blehert, D.S.; Campagna, S.R.; Semmelhack, M.F.; Egland, P.G.; Bassler, B.L.; Kolenbrander, P.E. Autoinducer 2: A concentration-dependent signal for mutualistic bacterial biofilm growth. Mol. Microbiol. 2006, 60, 1446–1456. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Unal, C.M.; Singh, B.; Fleury, C.; Singh, K.; Chávez de Paz, L.; Svensäter, G.; Riesbeck, K. QseC controls biofilm formation of non-typeable Haemophilus influenzae in addition to an AI-2-dependent mechanism. Int. J. Med. Microbiol. 2012, 302, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Steele, K.H.; O’Connor, L.H.; Burpo, N.; Kohler, K.; Johnston, J.-W. Characterization of a ferrous iron-responsive two-component system in nontypeable Haemophilus influenzae. J. Bacteriol. 2012, 194, 6162–6173. [Google Scholar] [CrossRef] [PubMed]
- Novotny, L.A.; Clements, J.D.; Goodman, S.D.; Bakaletz, L.O. Transcutaneous immunization with a band-aid prevents experimental otitis media in a polymicrobial model. Clin. Vaccine Immunol. 2017, 24, e00563-16. [Google Scholar] [CrossRef] [PubMed]
- Mokrzan, E.M.; Novotny, L.A.; Brockman, K.L.; Bakaletz, L.O. Antibodies against the majority subunit (PilA) of the type IV pilus of nontypeable Haemophilus influenzae disperse Moraxella catarrhalis from a dual-species biofilm. mBio 2018, 9, e02423-18. [Google Scholar] [CrossRef] [PubMed]
- Muhlebach, M.S.; Zorn, B.T.; Esther, C.R.; Hatch, J.E.; Murray, C.P.; Turkovic, L.; Ranganathan, S.C.; Boucher, R.C.; Stick, S.M.; Wolfgang, M.C. Initial acquisition and succession of the cystic fibrosis lung microbiome is associated with disease progression in infants and preschool children. PLoS Pathog. 2018, 14, e1006798. [Google Scholar] [CrossRef] [PubMed]
- Fleshner, M.; Olivier, K.N.; Shaw, P.A.; Adjemian, J.; Strollo, S.; Claypool, R.J.; Folio, L.; Zelazny, A.; Holland, S.M.; Prevots, D.R. Mortality among patients with pulmonary non-tuberculous mycobacteria disease. Int. J. Tuberc. Lung Dis. 2016, 20, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Skolnik, K.; Kirkpatrick, G.; Quon, B.S. Nontuberculous mycobacteria in cystic fibrosis. Curr. Treat. Options Infect. Dis. 2016, 8, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Qvist, T.; Taylor-Robinson, D.; Waldmann, E.; Olesen, H.V.; Hansen, C.R.; Mathiesen, I.H.; Høiby, N.; Katzenstein, T.L.; Smyth, R.L.; Diggle, P.J.; et al. Comparing the harmful effects of nontuberculous mycobacteria and Gram negative bacteria on lung function in patients with cystic fibrosis. J. Cyst. Fibros. 2016, 15, 380–385. [Google Scholar] [CrossRef]
- Waters, V.; Ratjen, F. Antibiotic treatment for nontuberculous mycobacteria lung infection in people with cystic fibrosis. Cochrane Database Syst. Rev. 2016, 12, CD010004. [Google Scholar] [CrossRef] [PubMed]
- Polkade, A.V.; Mantri, S.S.; Patwekar, U.J.; Jangid, K. Quorum sensing: An under-explored phenomenon in the phylum Actinobacteria. Front. Microbiol. 2016, 7, 131. [Google Scholar] [CrossRef]
- Santos, C.L.; Correia-Neves, M.; Moradas-Ferreira, P.; Mendes, M.V. A walk into the LuxR regulators of Actinobacteria: Phylogenomic distribution and functional diversity. PLoS ONE 2012, 7, e46758. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.M.; Petchiappan, A.; Chatterji, D. Quorum sensing and biofilm formation in Mycobacteria: Role of c-di-GMP and methods to study this second messenger. IUBMB Life 2014, 66, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Simões, M. Antimicrobial strategies effective against infectious bacterial biofilms. Curr. Med. Chem. 2011, 18, 2129–2145. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.G.; Guterres, K.B.; Bonez, P.C.; da Silva Gundel, S.; Aggertt, V.A.; Siqueira, F.S.; Ourique, A.F.; Wagnerd, R.; Klein, B.; Santos, R.C.V.; et al. Antibiofilm activity of nanoemulsions of Cymbopogon flexuosus against rapidly growing mycobacteria. Microb. Pathog. 2017, 113, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Flores, V.D.; Siqueira, F.D.; Mizdal, C.R.; Bonez, P.C.; Agertt, V.A.; Stefanello, S.T.; Rossi, G.G.; Campos, M.M. Antibiofilm effect of antimicrobials used in the therapy of mycobacteriosis. Microb. Pathog. 2016, 99, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, F.D.S.; Rossi, G.G.; Machado, A.K.; Alves, C.F.S.; Flores, V.C.; Somavilla, V.D.; Agertt, V.A.; Siqueira, J.D.; Dias, R.S.; Copetti, P.M.; et al. Sulfamethoxazole derivatives complexed with metals: A new alternative against biofilms of rapidly growing mycobacteria. Biofouling 2018, 34, 893–911. [Google Scholar] [CrossRef] [PubMed]
- García-Contreras, R.; Maeda, T.; Wood, T.K. Resistance to quorum-quenching compounds. Appl. Environ. Microbiol. 2013, 79, 6840–6846. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C.; Wood, T.K.; Kumar, P. Evolution of resistance to quorum-sensing inhibitors. Microb. Ecol. 2014, 68, 13–23. [Google Scholar] [CrossRef]
- Sass, A.; Slachmuylders, L.; Van Acker, H.; Vandenbussche, I.; Ostyn, L.; Bové, M.; Crabbé, A.; Chiarelli, L.R.; Buroni, S.; Van Nieuwerburgh, F.; et al. Various Evolutionary Trajectories Lead to Loss of the Tobramycin-Potentiating Activity of the Quorum-Sensing Inhibitor Baicalin Hydrate in Burkholderia cenocepacia Biofilms. Antimicrob. Agents Chemother. 2019, 63, e02092-18. [Google Scholar] [CrossRef]
- García-Contreras, R. Is Quorum Sensing Interference a Viable Alternative to Treat Pseudomonas aeruginosa Infections? Front. Microbiol. 2016, 7, 1454. [Google Scholar] [CrossRef]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scoffone, V.C.; Trespidi, G.; Chiarelli, L.R.; Barbieri, G.; Buroni, S. Quorum Sensing as Antivirulence Target in Cystic Fibrosis Pathogens. Int. J. Mol. Sci. 2019, 20, 1838. https://doi.org/10.3390/ijms20081838
Scoffone VC, Trespidi G, Chiarelli LR, Barbieri G, Buroni S. Quorum Sensing as Antivirulence Target in Cystic Fibrosis Pathogens. International Journal of Molecular Sciences. 2019; 20(8):1838. https://doi.org/10.3390/ijms20081838
Chicago/Turabian StyleScoffone, Viola Camilla, Gabriele Trespidi, Laurent R. Chiarelli, Giulia Barbieri, and Silvia Buroni. 2019. "Quorum Sensing as Antivirulence Target in Cystic Fibrosis Pathogens" International Journal of Molecular Sciences 20, no. 8: 1838. https://doi.org/10.3390/ijms20081838
APA StyleScoffone, V. C., Trespidi, G., Chiarelli, L. R., Barbieri, G., & Buroni, S. (2019). Quorum Sensing as Antivirulence Target in Cystic Fibrosis Pathogens. International Journal of Molecular Sciences, 20(8), 1838. https://doi.org/10.3390/ijms20081838