Green Tea Seed Oil Suppressed Aβ1–42-Induced Behavioral and Cognitive Deficit via the Aβ-Related Akt Pathway
Abstract
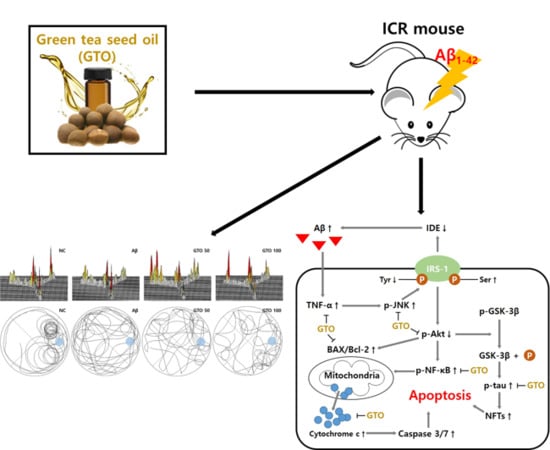
:1. Introduction
2. Results
2.1. Identification of Main Compounds Using UPLC Q-TOF/MS2
2.2. Cell Viability and Intracellular ROS
2.3. Y-Maze Test
2.4. Passive Avoidance Test
2.5. Morris Water Maze Test
2.6. Antioxidant System in Brain Tissue
2.7. Cholinergic System in Brain Tissue
2.8. Mitochondrial Function in Brain Tissue
2.9. Protein Expression via the Aβ-Related JNK/Akt Pathway
2.10. Protein Expression via the Aβ-Related Akt/Apoptosis Pathway
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Sample Preparation
4.3. Identification of Main Compounds Using UPLC Q-TOF/MS2
4.4. Cell Culture and Treatment
4.5. Cell Viability
4.6. Intracellular ROS
4.7. Animals and Experimental Design
4.8. Y-Maze Test
4.9. Passive Avoidance Test
4.10. Morris Water Maze Test
4.11. Preparation of Brain Tissue
4.12. Measurement of MDA
4.13. Measurement of SOD Contents
4.14. Measurement of Reduced GSH
4.15. Measurement of ACh
4.16. Measurement of AChE Activity
4.17. Mitochondrial Extraction in Brain Tissue
4.18. Measurement of Mitochondrial ROS
4.19. Measurement of MMP
4.20. Western Blotting
4.21. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| APP | Amyloid precursor protein |
| NFTs | neurofibrillar tangles |
| p-tau | hyperphosphorylated tau |
| GTO | green tea seed oil |
| ROS | reactive oxygen stress |
| MDA | malondialdehyde |
| SOD | superoxide dismutase |
| GSH | glutathione |
| ACh | acetylcholine |
| AChE | acetylcholinesterase |
| MMP | mitochondrial membrane potential |
| Aβ | amyloid beta |
| TNF-α | tumor necrosis factor-alpha |
| JNK | c-Jun N-terminal kinase |
| IRS-1pSer | phosphorylation of serine residues of IRS-1 |
| Akt | protein kinase B |
| BAX | Bcl-2-associated X protein |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
References
- Glenner, G.G. Alzheimer’s disease. The commonest form of amyloidosis. Arch. Pathol. Lab. Med. 1983, 107, 281–282. [Google Scholar] [PubMed]
- Rozemuller, A.J.; van Gool, W.A.; Eikelenboom, P. The neuroinflammatory response in plaques and amyloid angiopathy in Alzheimer’s disease: Therapeutic implications. Current drug targets. CNS Neurol. Disord.-Drug Targets 2005, 4, 223–233. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, H.; Kim, J.; Hong, S.; Kim, H.; Jo, T.; Park, Y.; Lee, C.; Kim, Y.; Lee, S. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 2010, 649, 210–217. [Google Scholar] [CrossRef]
- Karran, E.; Mercken, M.; De Strooper, B. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011, 10, 698. [Google Scholar] [CrossRef] [PubMed]
- Kontush, A. Apolipoprotein Aβ: Black sheep in a good family. Brain pathol. 2004, 14, 433–447. [Google Scholar] [CrossRef]
- Youdim, K.A.; Joseph, J.A. A possible emerging role of phytochemicals in improving age-related neurological dysfunctions: A multiplicity of effects. Free Radic. Biol. Med. 2001, 30, 583–594. [Google Scholar] [CrossRef]
- Santini, A.; Tenore, G.C.; Novellino, E. Nutraceuticals: A paradigm of proactive medicine. Eur. J. Pharm. Sci. 2017, 96, 53–61. [Google Scholar] [CrossRef]
- Rautiainen, S.; Manson, J.E.; Lichtenstein, A.H.; Sesso, H.D. Dietary supplements and disease prevention—A global overview. Nat. Rev. Endocrinol. 2016, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of nutraceuticals in human health. J. Food Sci. Technol.-Mysore. 2012, 49, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Flôres, M.F.; Martins, A.; Schimidt, H.L.; Santos, F.W.; Izquierdo, I.; Mello-Carpes, P.B.; Carpes, F.P. Effects of green tea and physical exercise on memory impairments associated with aging. Neurochem. Int. 2014, 78, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.S.; Jeong, Y.S.; Oh, S.M.; Kim, C.G.; Cho, Y.S.; Hwang, I.G. Catechin contents of green tea produced in Korea. J. Korean Soc. Food Sci. Nutr. 2018, 47, 852–862. [Google Scholar] [CrossRef]
- Hibasami, H.; Komiya, T.; Achiwa, Y.; Ohnishi, K.; Kojima, T.; Nakanishi, K.; Hara, Y. Induction of apoptosis in human stomach cancer cells by green tea catechins. Oncol. Rep. 1998, 5, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Schimidt, H.L.; Vieira, A.; Altermann, C.; Martins, A.; Sosa, P.; Santos, F.W.; Carpes, F.P. Memory deficits and oxidative stress in cerebral ischemia–reperfusion: Neuroprotective role of physical exercise and green tea supplementation. Neurobiol. Learn. Mem. 2014, 114, 242–250. [Google Scholar] [CrossRef]
- Kang, B.; Chang, H.; Na, Y.J.; Kang, Y.G.; Kim, B.G.; Park, J.S. Extract of enzyme-hydrolyzed green tea seed as potent melanin synthesis inhibitor. Bull. Korean Chem. Soc. 2014, 34, 2199–2202. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, J.Y.; Jeong, E.T.; Choi, T.H.; Yoon, T.M. Preservative effect of Camellia sinensis (L.) Kuntze seed extract in soy sauce and its mutagenicity. Food Res. Int. 2018, 105, 982–988. [Google Scholar] [CrossRef]
- Guo, X.Y.; Liu, D.; Ye, M.; Han, J.; Deng, S.; Ma, X.C.; Che, Q.M. Structural characterization of minor metabolites and pharmacokinetics of ganoderic acid C2 in rat plasma by HPLC coupled with electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2013, 75, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Fabre, N.; Rustan, I.; de Hoffmann, E.; Quetin-Leclercq, J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Distribution of C-and O-glycosyl flavonoids, (3-hydroxy-3-methylglutaryl) glycosyl flavanones and furocoumarins in Citrus aurantium L. juice. Food Chem. 2011, 124, 576–582. [Google Scholar] [CrossRef]
- Shi, P.; He, Q.; Song, Y.; Qu, H.; Cheng, Y. Characterization and identification of isomeric flavonoid O-diglycosides from genus Citrus in negative electrospray ionization by ion trap mass spectrometry and time-of-flight mass spectrometry. Anal. Chim. Acta 2007, 598, 110–118. [Google Scholar] [CrossRef]
- Engels, C.; Gräter, D.; Esquivel, P.; Jiménez, V.M.; Gänzle, M.G.; Schieber, A. Characterization of phenolic compounds in jocote (Spondias purpurea L.) peels by ultra high-performance liquid chromatography/electrospray ionization mass spectrometry. Food Res. Int. 2012, 46, 557–562. [Google Scholar] [CrossRef]
- Van Hoyweghen, L.; De Bosscher, K.; Haegeman, G.; Deforce, D.; Heyerick, A. In vitro inhibition of the transcription factor NF-κB and cyclooxygenase by Bamboo extracts. Phytother. Res. 2014, 28, 224–230. [Google Scholar] [CrossRef]
- Behl, C.; Moosmann, B. Oxidative nerve cell death in Alzheimers disease and stroke: Antioxidants as neuroprotective compounds. Biol. Chem. 2002, 383, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Mecocci, P.; MacGarvey, U.; Beal, M.F. Oxidative damage to mitochondrial DNA is increased in Alzheimer’s disease. Ann. Neurol. 1994, 36, 747–751. [Google Scholar] [CrossRef]
- Coyle, J.T.; Puttfarcken, P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993, 262, 689–695. [Google Scholar] [CrossRef]
- Galasko, D.; Montine, T.J. Biomarkers of oxidative damage and inflammation in Alzheimer’s disease. Biomark. Med. 2010, 4, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, J.W.; Lee, H.; Yoo, H.S.; Yun, Y.P.; Oh, K.W.; Hong, J.T. Inhibitory effect of green tea extract on β-amyloid-induced PC12 cell death by inhibition of the activation of NF-κB and ERK/p38 MAP kinase pathway through antioxidant mechanisms. Mol. Brain Res. 2005, 140, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Heo, H.J.; Kim, D.O.; Shin, S.C.; Kim, M.J.; Kim, B.G.; Shin, D.H. Effect of antioxidant flavanone, naringenin, from Citrus junos on neuroprotection. J. Agric. Food Chem. 2004, 52, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Dellu, F.; Mayo, W.; Cherkaoui, J.; Le Moal, M.; Simon, H. A two-trial memory task with automated recording: Study in young and aged rats. Brain Res. 1992, 588, 132–139. [Google Scholar] [CrossRef]
- Okuyama, S.; Kotani, Y.; Yamamoto, K.; Sawamoto, A.; Sugawara, K.; Sudo, M.; Furukawa, Y. The peel of Citrus kawachiensis (kawachi bankan) ameliorates microglial activation, tau hyper-phosphorylation, and suppression of neurogenesis in the hippocampus of senescence-accelerated mice. Biosci. Biotechnol. Biochem. 2018, 82, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; Tait, A.R.; Kitts, D.D. Flavonoid composition of orange peel and its association with antioxidant and anti-inflammatory activities. Food Chem. 2017, 218, 15–21. [Google Scholar] [CrossRef]
- Lee, S.S.; Baek, Y.S.; Eun, C.S.; Yu, M.H.; Baek, N.I.; Chung, D.K.; Yang, S.A. Tricin derivatives as anti-inflammatory and anti-allergic constituents from the aerial part of Zizania latifolia. Biosci. Biotechnol. Biochem. 2015, 79, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, S.; Joghataei, M.; Mohseni, S.; Baluchnejadmojarad, T.; Bagheri, M.; Khamse, S.; Roghani, M. Naringenin improves learning and memory in an Alzheimer’s disease rat model: Insights into the underlying mechanisms. Eur. J. Pharmacol. 2015, 764, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, S.; Gallicchio, M.; Lorenz, P.; Duckstein, S.M.; Potenza, N.; Galasso, S.; Marciano, S.; Fiorentino, A.; Stintzing, F.C.; Monaco, P. Neuroprotective potential of Laurus nobilis antioxidant polyphenol-enriched leaf extracts. Chem. Res. Toxicol. 2014, 27, 611–626. [Google Scholar] [CrossRef] [PubMed]
- Mufson, E.J.; Counts, S.E.; Perez, S.E.; Ginsberg, S.D. Cholinergic system during the progression of Alzheimer’s disease: Therapeutic implications. Expert Rev. Neurother. 2008, 8, 1703–1718. [Google Scholar] [CrossRef] [PubMed]
- Reyes, A.E.; Chacón, M.A.; Dinamarca, M.C.; Cerpa, W.; Morgan, C.; Inestrosa, N.C. Acetylcholinesterase-Aβ complexes are more toxic than Aβ fibrils in rat hippocampus: Effect on rat β-amyloid aggregation, laminin expression, reactive astrocytosis, and neuronal cell loss. Am. J. Pathol. 2004, 164, 2163–2174. [Google Scholar] [CrossRef]
- Ćilerdžić, J.L.; Sofrenić, I.V.; Tešević, V.V.; Brčeski, I.D.; Duletić-Laušević, S.N.; Vukojević, J.B.; Stajić, M.M. Neuroprotective potential and chemical profile of alternatively cultivated Ganoderma lucidum basidiocarps. Chem. Biodiversity 2018, 15, 1800036. [Google Scholar] [CrossRef]
- Park, S.K.; Ha, J.S.; Kim, J.M.; Kang, J.Y.; Lee, D.S.; Guo, T.J.; Heo, H.J. Antiamnesic effect of broccoli (Brassica oleracea var. italica) leaves on amyloid beta (Aβ)1–42-induced learning and memory impairment. J. Agric. Food Chem. 2016, 64, 3353–3361. [Google Scholar]
- Wong-Riley, M.T. Cytochrome oxidase: An endogenous metabolic marker for neuronal activity. Trends Neurosci. 1989, 12, 94–101. [Google Scholar] [CrossRef]
- Reddy, P.H. Amyloid precursor protein-mediated free radicals and oxidative damage: Implications for the development and progression of Alzheimer’s disease. J. Neurochem. 2006, 96, 1–13. [Google Scholar] [CrossRef]
- Buxbaum, J.D.; Thinakaran, G.; Koliatsos, V.; O’Callahan, J.; Slunt, H.H.; Price, D.L.; Sisodia, S.S. Alzheimer amyloid protein precursor in the rat hippocampus: Transport and processing through the perforant path. J. Neurosci. 1998, 18, 9629–9637. [Google Scholar] [CrossRef]
- Glabe, C.G.; Kayed, R. Common structure and toxic function of amyloid oligomers implies a common mechanism of pathogenesis. Neurology 2006, 66, 74–78. [Google Scholar] [CrossRef]
- Mattson, M.P.; Gary, D.S.; Chan, S.L.; Duan, W. Perturbed endoplasmic reticulum function, synaptic apoptosis and the pathogenesis of Alzheimer’s disease. Biochem. Soc. Symp. 2001, 67, 151–162. [Google Scholar] [CrossRef]
- Kumar, A.; Prakash, A.; Dogra, S. Naringin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress induced by D-galactose in mice. Food Chem. Toxicol. 2010, 48, 626–632. [Google Scholar] [CrossRef]
- Das, U.N. Long-chain polyunsaturated fatty acids in the growth and development of the brain and memory. Nutrition 2003, 19, 62–65. [Google Scholar] [CrossRef]
- Dragicevic, N.; Smith, A.; Lin, X.; Yuan, F.; Copes, N.; Delic, V.; Bradshaw, P.C. Green tea epigallocatechin-3-gallate (EGCG) and other flavonoids reduce Alzheimer’s amyloid-induced mitochondrial dysfunction. J. Alzheimers Dis. 2011, 26, 507–521. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, Q.; del Mar Contreras, M.; Wang, L. Wang, Profiling and quantification of phenolic compounds in Camellia seed oils: Natural tea polyphenols in vegetable oil. Food Res. Int. 2017, 102, 184–194. [Google Scholar] [CrossRef]
- Bomfim, T.R.; Forny-Germano, L.; Sathler, L.B.; Brito-Moreira, J.; Houzel, J.C.; Decker, H.; Holscher, C. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease–associated Aβ oligomers. J. Clin. Investig. 2012, 122, 1339–1353. [Google Scholar] [CrossRef]
- De Felice, F.G. Alzheimer’s disease and insulin resistance: Translating basic science into clinical applications. J. Clin. Investig. 2013, 123, 531–539. [Google Scholar] [CrossRef]
- Ali, T.; Yoon, G.H.; Shah, S.A.; Lee, H.Y.; Kim, M.O. Osmotin attenuates amyloid beta-induced memory impairment, tau phosphorylation and neurodegeneration in the mouse hippocampus. Sci. Rep. 2015, 5, 11708. [Google Scholar] [CrossRef]
- Okuyama, S.; Yamamoto, K.; Mori, H.; Toyoda, N.; Yoshimura, M.; Amakura, Y.; Furukawa, Y. Auraptene in the peels of Citrus kawachiensis (Kawachi Bankan) ameliorates lipopolysaccharide-induced inflammation in the mouse brain. Evid.-based Complement Altern. Med. 2014, 2014, 408503. [Google Scholar] [CrossRef]
- Rahigude, A.; Bhutada, P.; Kaulaskar, S.; Aswar, M.; Otari, K. Participation of antioxidant and cholinergic system in protective effect of naringenin against type-2 diabetes-induced memory dysfunction in rats. Neuroscience 2012, 226, 62–72. [Google Scholar] [CrossRef]
- Heo, H.J.; Cho, H.Y.; Hong, B.; Kim, H.K.; Kim, E.K.; Kim, B.G.; Shin, D.H. Protective effect of 4′, 5-dihydroxy-3′, 6, 7-trimethoxyflavone from Artemisia asiatica against Aβ-induced oxidative stress in PC12 cells. Amyloid 2001, 8, 194–201. [Google Scholar] [CrossRef]
- Laursen, S.E.; Belknap, J.K. Intracerebroventricular injections in mice: Some methodological refinements. J. Pharmacol. Toxicol. Methods 1986, 16, 355–357. [Google Scholar]
- Van der Borght, K.; Havekes, R.; Bos, T.; Eggen, B.J.; Van der Zee, E.A. Exercise improves memory acquisition and retrieval in the Y-maze task: Relationship with hippocampal neurogenesis. Behav. Neurosci. 2007, 121, 324–334. [Google Scholar] [CrossRef]
- Newman, J.P.; Kosson, D.S. Passive avoidance learning in psychopathic and nonpsychopathic offenders. J. Abnorm. Psychol. 1986, 95, 252–256. [Google Scholar] [CrossRef]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Liu, F.; Ng, T.B. Effect of pineal indoles on activities of the antioxidant defense enzymes superoxide dismutase, catalase, and glutathione reductase, and levels of reduced and oxidized glutathione in rat tissues. Biochem. Cell Biol. 2000, 78, 447–453. [Google Scholar] [CrossRef]
- Vincent, D.; Segonzac, G.; Vincent, M.C. Colorimetric determination of acetylcholine by the Hestrin hydroxylamine reaction and its application in pharmacy. Ann. Pharm. Fr. 1958, 16, 179–185. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Brown, M.R.; Geddes, J.W.; Sullivan, P.G. Brain region-specific, age-related, alterations in mitochondrial responses to elevated calcium. J. Bioenerg. Biomembr. 2004, 36, 401–406. [Google Scholar] [CrossRef] [PubMed]









| No. | RT a (min) | Parent Ion b (m/z) | MS2 Ions c (m/z) | Compound |
|---|---|---|---|---|
| 1 | 3.31 | 741 | 271, 459, 579, 595, 621 | Narirutin 4′-glucoside |
| 2 | 3.33 | 565 | 271, 301 | Quercetin-3-O-pentosyl-pentoside |
| 3 | 4.11 | 271 | 199, 151, 177 | Naringenin |
| 4 | 4.29 | 329 | 139, 171, 183, 211, 229 | 9,12,13-THODE |
| 5 | 4.50 | 517 | 455, 499 | Ganoderic acid C2 |
| 6 | 4.95 | 501 | 393, 421, 423, 439, 483 | Ganolucidic acid B |
| Parameters | Groups | |||
|---|---|---|---|---|
| NC | Aβ | GTO 50 | GTO 100 | |
| MDA | 40.88 ± 2.97 c | 54.52 ± 5.03 a | 47.62 ± 4.80 b | 44.93 ± 5.84 bc |
| SOD | 44.18 ± 3.96 a | 30.73 ± 6.30 b | 35.99 ± 10.88 ab | 38.85 ± 4.23 ab |
| reduced GSH | 2.79 ± 0.33 ab | 2.02 ± 0.29 b | 2.14 ± 0.49 b | 3.22 ± 0.45 a |
| Parameters | Groups | |||
|---|---|---|---|---|
| NC | Aβ | GTO 50 | GTO 100 | |
| ROS | 100.00 ± 19.83 b | 140.41 ± 20.24 a | 130.52 ± 2.88 ab | 106.47 ± 13.36 ab |
| MMP | 100.00 ± 5.12 a | 68.74 ± 6.17 b | 79.02 ± 7.31 ab | 80.04 ± 16.16 ab |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.M.; Park, S.K.; Kang, J.Y.; Park, S.B.; Yoo, S.K.; Han, H.J.; Cho, K.H.; Kim, J.C.; Heo, H.J. Green Tea Seed Oil Suppressed Aβ1–42-Induced Behavioral and Cognitive Deficit via the Aβ-Related Akt Pathway. Int. J. Mol. Sci. 2019, 20, 1865. https://doi.org/10.3390/ijms20081865
Kim JM, Park SK, Kang JY, Park SB, Yoo SK, Han HJ, Cho KH, Kim JC, Heo HJ. Green Tea Seed Oil Suppressed Aβ1–42-Induced Behavioral and Cognitive Deficit via the Aβ-Related Akt Pathway. International Journal of Molecular Sciences. 2019; 20(8):1865. https://doi.org/10.3390/ijms20081865
Chicago/Turabian StyleKim, Jong Min, Seon Kyeong Park, Jin Yong Kang, Su Bin Park, Seul Ki Yoo, Hye Ju Han, Kyoung Hwan Cho, Jong Cheol Kim, and Ho Jin Heo. 2019. "Green Tea Seed Oil Suppressed Aβ1–42-Induced Behavioral and Cognitive Deficit via the Aβ-Related Akt Pathway" International Journal of Molecular Sciences 20, no. 8: 1865. https://doi.org/10.3390/ijms20081865
APA StyleKim, J. M., Park, S. K., Kang, J. Y., Park, S. B., Yoo, S. K., Han, H. J., Cho, K. H., Kim, J. C., & Heo, H. J. (2019). Green Tea Seed Oil Suppressed Aβ1–42-Induced Behavioral and Cognitive Deficit via the Aβ-Related Akt Pathway. International Journal of Molecular Sciences, 20(8), 1865. https://doi.org/10.3390/ijms20081865